Introduction
Laryngeal cancer is one of the most common and
lethal head and neck carcinomas, worldwide (1). More than 90% of laryngeal cancer is
pathologically identified as laryngeal squamous cell carcinoma
(LSCC) (2). Despite numerous advances
in the diagnosis and treatment of this disease, the overall
survival rate has changed little over recent decades, in part due
to a lack of reliable biomarkers (3).
Therefore, investigation of the molecular mechanisms underlying the
development and progression of LSCC, may help to identify novel
molecular targets for the treatment and diagnosis of LSCC.
MicroRNAs (miRNAs) are a novel type of biomarker,
and are potential therapeutic targets for various diseases,
including cancer (4). They belong to
a class of small non-coding RNAs, and regulate expression of their
targets through inhibition of the translation or the degradation of
their corresponding mRNA targets. Approximately 30% of mRNAs are
predicted to be targeted by miRNAs (5). A number of studies have demonstrated
that specific miRNAs are aberrantly expressed in different types of
cancer, such as leukemia, breast cancer and colorectal cancer
(6–8).
These miRNAs are involved in tumorigenesis, either as
proto-oncogenes or as tumor suppressors, depending on their targets
(9).
Several studies have shown that aberrant expression
of miR-23a occurs in a variety of types of cancer, indicating that
it is involved in oncogenesis. Notably, miR-23a may produce
opposite effects in different types of cancer. For example, miR-23a
is downregulated in oral squamous cell carcinoma (OSCC), acute
promyelocytic leukemia and colon cancer (10–12). By
contrast, miR-23a is overexpressed in acute lymphoblastic leukemia,
glioblastoma and hepatocellular carcinoma (13–15). Li
et al (16) reported that
miR-23a is a candidate biomarker of laryngeal cancer, following
their analysis of DNA microarrays-based microRNA expression
profiles. However, the mechanisms underlying the effects of miR-23a
in laryngeal cancer remain to be elucidated.
Recently, apoptotic protease activating factor-1
(APAF-1) was confirmed as a target of miR-23a (17–19).
APAF-1 is frequently downregulated in a number of types of
cancer, such as colorectal and lung cancer, which indicates that it
participates in tumorigenesis (20–21). A
previous study by our group, demonstrated that APAF-1 is
downregulated in laryngeal carcinoma (22). In addition to loss of heterozygosity,
it was also shown that promoter methylation decreases APAF-1
expression in human leukemia, thereby indicating a second
inactivation mechanism of APAF-1 in cancer (23).
In the present study, the association between
miR-23a and APAF-1 expression in LSCC was analyzed, and the
binding of miR-23a to APAF-1 was assayed. The functions of
miR-23a and APAF-1 in laryngeal cancer cell proliferation
and apoptosis were also evaluated.
Materials and methods
Patient tissues, cell culture and
nucleotide sequences
Tissue specimens, which included tumor tissues in
addition to paired normal adjacent tissues from 82 patients with
LSCC recruited from the Otolaryngology department of the No. 463
Hospital of PLA, were collected after patients had provided
informed consent. Pathological diagnosis of the specimens was
performed by a pathologist. Laryngeal cancer tissues were
immediately frozen at −80°C, following removal from the patients.
Hep2 human laryngeal cancer and HEK293 human embryonic kidney cell
lines were obtained from the Cell Biology Institute of Shanghai,
Chinese Academy of Science (Shanghai, China) and were maintained in
RPMI 1640 (Gibco Life Technologies, Los Angeles, CA, USA) with 10%
fetal bovine serum (Hyclone, Logan, UT, USA), 100 units/ml
penicillin and 100 µg/ml streptomycin (Beyotime Institute of
Biotechnology, Haimen, China) in a humidified atmosphere at 37°C
with 5% CO2. All nucleotide sequences used in the study
are shown in Table I. Approval for
the study was received from the ethical board of China Medical
University (Shenyang, China).
 | Table I.Nucleotide sequences. |
Table I.
Nucleotide sequences.
| Name | Sequence |
|---|
| miR-23a mimic |
5′-AUCACAUUGCCAGGGAUUUCC-3′ |
| miR-23a
inhibitor |
5′-GGAAAUCCCUGGCAAUGUGAU-3′ |
| NC mimic |
5′-UUCUCCGAACGUGUCACGUTT-3′ |
| NC inhibitor |
5′-CAGUACUUUUGUGUAGUACAA-3′ |
| NC |
5′-GGCUACGUCCAGGAGCGCA CC-3′ |
| siAPAF-1 |
5′-GACGUCUGCAACUCAUUAATT-3′ |
| miRNA-23a (reverse
transcription primer) |
5′-CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGGGAAATCC-3′ |
| miRNA-23a (F) |
5′-ACACTCCAGCTGGGATCACATTGCCAGGGATTT-3′ |
| miRNA-23a (R) |
5′-TGGTGTCGTGGAGTCG-3′ |
| U6 (F) |
5′-CTCGCTTCGGCAGCACA-3′ |
| U6 (R) |
5′-AACGCTTCACGAATTTGCGT-3′ |
| APAF-1
(F) |
5′-CCTCTCATTTGCTGATGTCG-3′ |
| APAF-1
(R) |
5′-TCACTGCAGATTTTCACCAGA-3′ |
| GAPDH (F) |
5′-ATCATCAGCAATGCCTCC-3′ |
| GAPDH (R) |
5′-CATCACGCCACAGTTTCC-3′ |
Small RNAs, plasmids and gene
transfection
Small RNAs including an miR-23a mimic and inhibitor,
negative control miRNAs and small interfering RNA (siRNA) were
obtained from GenePharma (Shanghai, China). Dual-Luciferase miRNA
Target Expression Vectors (GV272-APAF-1-3′UTR and
GV272-APAF-1-3′UTR-mut) were also obtained from GenePharma
(Shanghai, China). Gene transfection was performed in Hep2 and/or
HEK293 cells, with small RNAs and/or plasmids, at a final
concentration of 50 pmol, using Lipofectamine 2000™ (Invitrogen
Life Technologies Carlsbad, CA, USA) according to the
manufacturer's instructions.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from the corresponding
tissues and cell lines using TRIzol® (Takara Bio, Inc., Dalian,
China), according to the manufacturer's instructions. miRNA was
separated using an miRcute miRNA isolation kit (Tiangen, Beijing,
China). Concentrations of miRNA and total RNA were measured by
reading the absorbance at an optical density (OD) of 260/280
nm.
In order to detect the expression of miR-23a and
APAF-1 mRNA in LSCC tissues and cell lines, RT-qPCR was conducted
using the ABI 7500 Real Time PCR system (Applied Biosystems, Foster
City, USA). To amplify the mature miR-23a, RT was performed using
the One Step PrimeScript miRNA cDNA Synthesis kit (Takara Bio,
Inc.), according to the manufacturer's instructions and qPCR was
conducted using SYBR® Premix Ex Taq™ II (Takara Bio, Inc.). U6
small nuclear RNA (snRNA) was used for normalization. The thermal
cycling conditions for miR-23a and U6 snRNA consisted of 95°C for
30 sec, 40 cycles of 95°C for 5 sec and 60°C for 34 sec. For the
detection of APAF-1 mRNA expression, RT was performed
using the cDNA Synthesis kit (Takara Bio, Inc.) according to the
manufacturer's instructions and qPCR was conducted using SYBR®
Premix Ex Taq™ II (Takara Bio, Inc.). GAPDH was used for
normalization. The conditions for amplifying APAF-1 and
GAPDH mRNA were 95°C for 30 min, 40 cycles of 95°C for 5 sec
and 60°C for 34 sec. ΔCt was calculated by subtracting the Ct of U6
or GAPDH mRNA from that of the mRNA of interest. ΔΔCt was then
calculated by subtracting the ΔCt of the negative control from the
ΔCt of the samples. The fold change in miR-23a and APAF-1 mRNA was
calculated according to the equation, 2−ΔΔCt.
Western blotting
Proteins were extracted from LSCC tissues and cell
lines, using a protein extraction reagent (Beyotime, Shanghai,
China) and protein concentration was measured using the BCA Protein
Assay kit (Beyotime, Shanghai, China). Protein (50 µg) from each
sample was separated on an 8% SDS-PAGE gel (Beyotime Institute of
Biotechnology) and transferred to a PVDF membrane (Beyotime
Institute of Biotechnology). The membrane was then blocked with 5%
non-fat milk and incubated with rabbit monoclonal anti-APAF-1
(ab32372, 1:500 dilution; Abcam, Cambridge, USA) and mouse
monoclonal anti-α-tubulin (BM1452; 1:500 dilution; Boster, Wuhan,
China) for normalization followed by by incubation at 37°C for 60
min with horseradish peroxidase-conjugated antibody (1:2,000
dilution; ZhongShan, Beijing, China). The membrane was stained with
ECL Plus (Beyotime Institute of Biotechnology), according to the
manufacturer's instructions and exposed to a film (Fuji,
Japan).
Luciferase reporter assay
HEK293 cells, seeded in 96-well plates in
triplicate, were cotransfected with GV272-APAF-1-3′UTR or
GV272-APAF-1-3′UTR-mut, and miRNA-23a mimic or non-relative control
RNA duplex, using Lipofectamine 2000 (Invitrogen LIfe Technologies,
Carlsbad, CA, USA) according to the manufacturer's instructions.
The pRL-TK (Promega Corporation, Madison, WI, USA) was used for
normalization. Cells were collected 24 h after transfection.
Luciferase activity was measured using a dual-luciferase reporter
assay kit (Promega Corporation) and recorded using a
Chemiluminescence meter (Promega Corporation).
Cell proliferation assay
Hep2 cells were grown in 6-well plates to ~60%
confluency and transiently transfected as described for the HEK293
cells. Following transfection, 2–3×103 Hep2 cells were
seeded into 96-well plates in triplicate. Cells were then cultured
for 1, 2, 3, 4 or 5 days. Absorbance at 490 nm was measured,
following incubation of the cells with 100 µl of sterile MTT dye
(0.5 mg/ml, Sigma, Ronkonkoma, NY, USA) for 4 h at 37°C and 150 µl
DMSO for 15 min. The cell growth curve was constructed using the
values at OD490 nm as ordinate axis.
Colony formation assay
At 12 h post-transfection, 3–5×103 Hep2
cells were seeded into 60-mm Petri dishes in triplicate and
maintained in RPMI 1640 (GIBCO, Los Angeles, USA) with 10% fetal
bovine serum. After 14 d, colonies were fixed with methanol for 30
min, stained with hematoxylin for 20 min, and visualized under a
microscope (Olympus BX5, Olympus Corporation, Tokyo, Japan).
Colonies was counted and calculated in relation to the values
obtained from the mock and scramble-treated controls.
Apoptosis assay
Hep2 cells were grown in 6-well plates to ~60%
confluence and transiently transfected with corresponding small
RNAs using Lipofectamine 2000. Cells were digested and collected at
48 h post-transfection, and washed twice with PBS. Cells were then
stained with Annexin V-EGFP, according to the manufacturer's
instructions (KeyGEN, Nanjing, China) and apoptotic cells were
quantified using flow cytometry (FACS calibur, Becton-Dickinson,
Franklin Lakes, USA).
Statistical analysis
Data are presented as the mean ± standard deviation.
Differences were assessed by one-way analysis of variance and
Student's unpaired t-test, using SPSS 17.0 (SPSS Inc.,
Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
miR-23a and APAF-1 are involved in
LSCC development
The results from the RT-qPCR assay, demonstrated
that miR-23 was upregulated in 72.8% (59 of 82) cases of laryngeal
cancer and the results of statistical analysis showed that miR-23a
expression was significantly higher in LSCC tissues than that in
adjacent normal tissues (Fig. 1),
suggesting that miR-23a is involved in laryngeal oncogenesis.
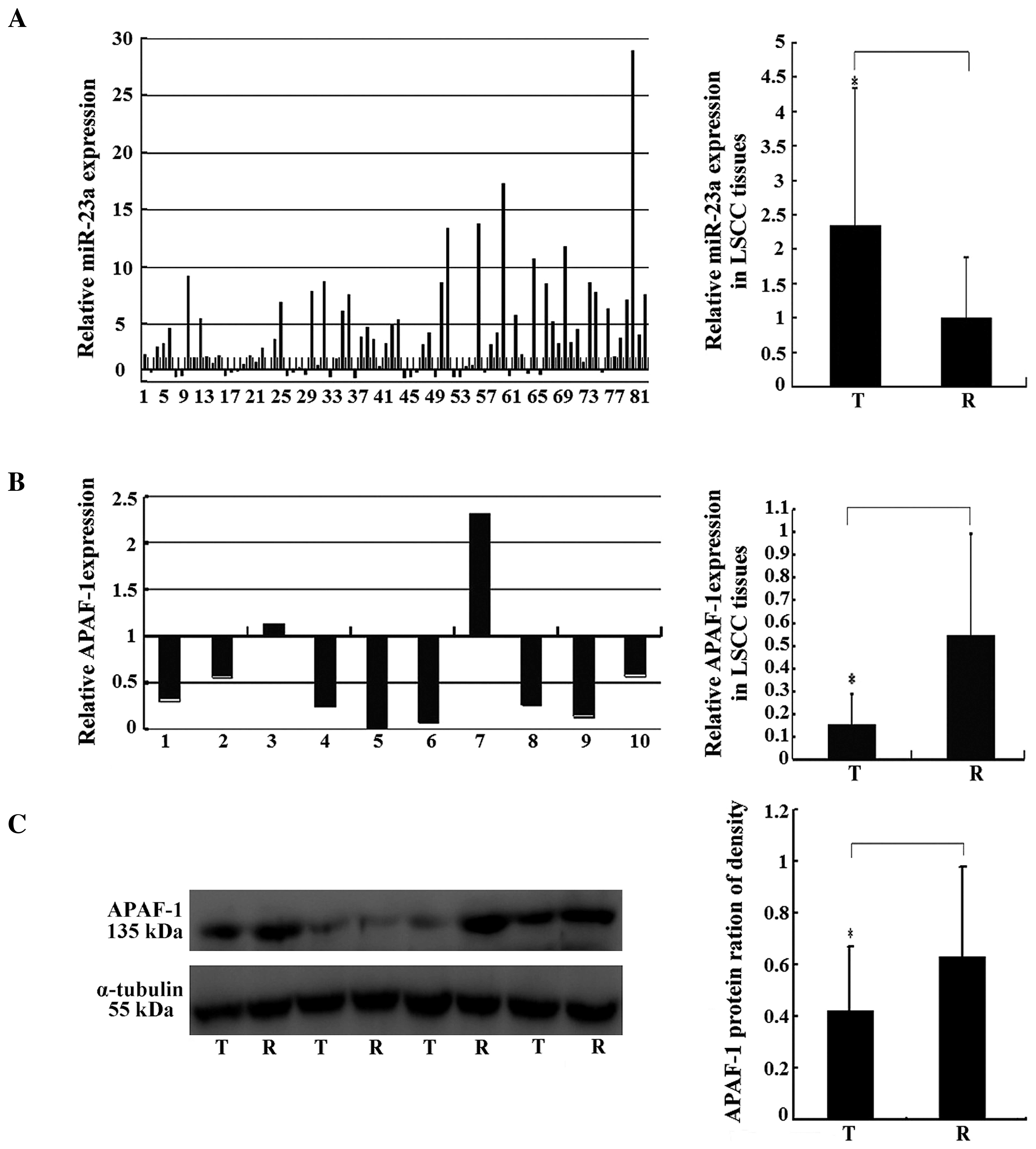 | Figure 1.miR-23a and APAF-1 gene
expression in LSCC. (A) miR-23a expression in 82 pairs of LSCC
tissues, analyzed by RT-qPCR. The y-axis indicates the ratio of
relative miR-23a expression in cancer tissues to that in paired
normal adjacent tissues. The relative expression was calculated as
the ratio of miR-23a to the internal control, using the equation
RQ=2−ΔΔCT in each sample. The x-axis represents the
number of the paired samples used in the study. (B) Relative mRNA
expression levels of APAF-1 in the miRNA-23a-upregulated
LSCC tissues, analyzed by RT-qPCR. The y-axis indicates the ratio
of relative APAF-1 mRNA expression in cancer tissues to that in
paired normal adjacent tissues. The relative expression was
calculated as the ratio of APAF-1 to the internal control
using the equation RQ=2−ΔΔCT in each sample. The x-axis
represents the number of the paired samples used in the study. (C)
Relative protein expression levels of APAF-1 in the
miRNA-23a-upregulated LSCC tissues, analyzed by western blotting.
α-tubulin was used as the internal control. All data are expressed
as the mean ± standard deviation of three independent experiments.
*P<0.05. miRNA, microRNA; APAF-1, apoptotic protease
activating factor 1; LSCC, laryngeal squamous cell carcinoma;
RT-qPCR, reverse transcription-quantitative polymerase chain
reaction; T, tumor sample; R, paired normal adjacent sample. |
In order to investigate the association between
miR-23a and APAF-1 expression in LSCC, 10 pairs of LSCC
tissues, in which miR-23a was upregulated, were randomly selected,
and APAF-1 expression in these samples was evaluated.
RT-qPCR and western blotting results showed that APAF-1 expression
was significantly downregulated at the mRNA and protein levels in
cancer tissues, compared with that in the normal controls (Fig. 1B and C). The results of statistical
analysis, demonstrated that miR-23a expression was negatively
correlated with APAF-1 expression in LSCC tissues
(Table II).
 | Table II.Correlation between miRNA-23a and
APAF-1 expression in laryngeal cancer tissues. |
Table II.
Correlation between miRNA-23a and
APAF-1 expression in laryngeal cancer tissues.
| Statistical
parameter | APAF-1 mRNA
(n=10) | APAF-1
protein (n=10) |
|---|
| R-value | −0.697 | −0.633 |
| P-value | 0.025 | 0.049 |
APAF-1 mRNA is a direct target of
miR-23a
As illustrated in Fig.
2A, cotransfection of the APAF-1 3′ untranslated region
(UTR) luciferase reporter and the miRNA-23a mimic into the HEK293
cells, resulted in a significant reduction in luciferase activity
in comparison with the control groups (P<0.01). These results
confirmed the hypothesis that miR-23a binds to the APAF-1
3′UTR. Western blotting and RT-qPCR results indicated that miR-23a
significantly decreased APAF-1 expression at the mRNA and
protein levels in Hep2 cells (Fig. 2B and
C). APAF-1 expression was also significantly inhibited
by APAF-1-specific siRNA, at the mRNA and protein
levels in Hep2 cells (Fig. 2B and C).
These results suggest that miR-23a negatively regulates
APAF-1 expression, by binding the 3′UTR nucleotides of this
gene in laryngeal cancer tissues.
miR-23a and siAPAF-1 promote Hep2 cell
proliferation and inhibit apoptosis
miR-23a expression was significantly higher and
lower than that in the control group, in the miR-23a mimic and
inhibitor groups, respectively, suggesting that transfection was
successful (Fig. 3A). The MTT assay
results indicated that the miR-23a mimic and inhibitor,
significantly increased and decreased Hep2 cell viability,
respectively, compared with the control group (Fig. 3B). In order to determine the effects
of miR-23a on long-term and independent growth activity, a colony
formation assay was performed. Colony formation assay results
demonstrated that Hep2 cells transfected with the miR-23a-mimic or
miR-23a-inhibitor exhibited significantly higher and lower
colony-forming ability, respectively, compared with the controls
(Fig. 3C). The flow cytometry assay
results indicated that the early apoptotic rate was significantly
increased in the miR 23a inhibitor group compared with the control
group. However, no significant difference was observed between the
miR-23a mimic group and the control group (Fig. 3D). In addition, the late apoptotic
rate was significantly increased in the miR-23a inhibitor group and
reduced in the miR-23a mimic group when compared with the controls,
respectively (Fig. 3E). However, no
significant differences in early or late apoptosis were detected in
the miR-23a mimic group compared with the control group (Fig. 3D and E). It was hypothesized that
there may be an abundant expression of internal miR-23a in human
Hep2 cells (Fig. 3A). In a similar
manner to the effect of miR-23a on Hep2 cells, knockdown of the
APAF-1 gene significantly promoted cell viability (Fig. 3B) and colony formation (Fig. 3C), and inhibited early and late
apoptosis in Hep2 cells (Fig. 3D and
E), suggesting that miR-23a functions in Hep2 cells, at least
in part via downregulation of APAF-1 expression.
 | Figure 3.Regulation of miR-23a and siAPAF-1 in
Hep2 human laryngeal cancer cell proliferation and apoptosis. (A)
miR-23a expression levels of Hep2 cells in different groups.
Following transfection of the Hep2 cells by different small RNAs,
including miR-23a mimics, miR-23a inhibitor, mimics NC, inhibitor
NC and siAPAF-1, the miR-23a expression was measured using RT-qPCR.
(B) Effects of miR-23a and siAPAF-1 on Hep2 cell proliferation.
Hep2 cells were transfected with the various small RNAs and cell
proliferation was detected using an MTT assay. (C) Effects of
miR-23a and siAPAF-1 on Hep2 cell colony formation. Hep2 cells were
transfected with the various small RNAs and the colony-forming
ability was detected using a colony formation assay. (D) and (E)
Effects of miR-23a and siAPAF-1 on early and late apoptosis in the
Hep2 cells. Hep2 cells were transfected with the various small RNAs
and then stained by Annexin V-EGFP, according to the manufacturer's
instructions. The apoptotic cells in the different groups were
monitored using a flow cytometer. Data are presented as the mean ±
standard deviation from three independent experiments. *P<0.05.
miRNA, microRNA; siAPAF-1, small interfering RNA specific to
apoptotic protease activating factor 1; RT-qPCR, reverse
transcription-quantitative polymerase chain reaction; NC, normal
control; lipo, Lipofectamine. |
Discussion
As outlined in the introduction, miR-23a is
aberrantly upregulated or downregulated in a number of types of
cancer, indicating that it is involved in oncogenesis.
In the present study, miR-23a was found to be
significantly overexpressed in laryngeal cancer tissues compared
with normal controls, suggesting that it acts as an oncogene in the
development of LSCC. In addition, APAF-1 was shown to be
downregulated in LSCC tissues compared with the control tissues,
and a negative correlation between miR-23a and APAF-1
expression was demonstrated in LSCC tissues. The present study also
confirmed that APAF-1 is a direct target of miR-23a.
Furthermore, miR-23a inhibited APAF-1 expression at
the mRNA and protein levels in Hep2 cells, indicating that the
degradation of APAF-1 mRNA, which may be mediated by
miR-23a, contributes to the decreased expression levels of
APAF-1 observed in LSCC.
As two of the ten hallmarks of cancer, sustaining
proliferation and resisting cell death, are known to be important
in carcinogenesis (24–25). Studies have shown that miRNAs are
involved in the regulation of cancer cell proliferation and
apoptosis (26–27).
The present study demonstrated that miR-23a
significantly promoted Hep2 cell proliferation, while its antisense
inhibitor partially reversed this effect. It was hypothesized that
this enhanced proliferation may be due to an effect on cell cycle
control or to the inhibition of apoptosis. However, the miR-23a
inhibitor significantly increased early apoptosis in Hep2 cells,
and it is suggested that low levels of apoptosis, are, in part,
responsible for the high level of proliferation observed in Hep2
cells. The intrinsic apoptotic pathway is also termed the
mitochondrial apoptotic pathway, and responds to intracellular
signals, such as DNA damage (28).
APAF-1 is a key regulator of the mitochondrial apoptotic
pathway and of the central element of the multimeric apoptosome
formed by procaspase 9, cytochrome c, and thus, is itself
involved in the initiation and progression of cancer (29).
The study also demonstrated that silencing of
APAF-1 significantly increased proliferation, and decreased
early and late apoptosis in Hep2 cells. It was also shown that
miR-23a significantly inhibited APAF-1 expression in Hep2
cells, suggesting that a high level of miR-23a partially represses
APAF-1 expression, leading to increased early apoptosis in
LSCC. In accordance with these results, miR-23a has been shown to
promote glioma cell growth and to suppress cell apoptosis, by
targeting APAF1 (18).
In conclusion, miR-23a is involved in the
development of LSCC, acting as a pro-proliferative and
antiapoptotic regulator, at least in part through direct targeting
of the APAF-1 3′UTR. Whether miR-23a also regulates cancer
cell proliferation via other targets, requires further
investigation. Future studies by this group will also focus on the
clinical application of miR-23a as a biomarker in the diagnosis and
treatment of laryngeal carcinoma.
Acknowledgements
This study was supported by the National Natural
Science Foundations of China (grant nos. 81172577, 81372876 and
81301767).
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Morshed K, Polz-Dacewicz M, Szymański M
and Polz D: Short-fragment PCR assay for highly sensitive
broad-spectrum detection of human papillomaviruses in laryngeal
squamous cell carcinoma and normal mucosa: Clinico-pathological
evaluation. Eur Arch Otorhinolaryngol. 265 (Suppl 1):S89–S96. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang W, Lin P, Han C, Cai W, Zhao X and
Sun B: Vasculogenic mimicry contributes to lymph node metastasis of
laryngeal squamous cell carcinoma. J Exp Clin Cancer Res.
29:602010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li J, Tan S, Kooger R, Zhang C and Zhang
Y: MicroRNAs as novel biological targets for detection and
regulation. Chem Soc Rev. 43:506–517. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bhatt K, Mi QS and Dong Z: MicroRNAs in
kidneys: Biogenesis, regulation and pathophysiological roles. Am J
Physiol Renal Physiol. 300:F602–F610. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Calin GA, Liu CG, Sevignani C, et al:
MicroRNA profiling reveals distinct signatures in B cell chronic
lymphocytic leukemias. Proc Natl Acad Sci USA. 101:11755–11760.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bhaumik D, Scott GK, Schokrpur S, Patil
CK, Campisi J and Benz CC: Expression of microRNA-146 suppresses
NF-kappaB activity with reduction of metastatic potential in breast
cancer cells. Oncogene. 27:5643–5647. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Michael MZ, O'Connor SM, van Holst
Pellekaan NG, Young GP and James RJ: Reduced accumulation of
specific microRNAs in colorectal neoplasia. Mol Cancer Res.
1:882–891. 2003.PubMed/NCBI
|
|
9
|
Zhang B, Pan X, Cobb GP and Anderson TA:
microRNAs as oncogenes and tumor suppressors. Dev Biol. 302:1–12.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kozaki K, Imoto I, Mogi S, Omura K and
Inazawa J: Exploration of tumor-suppressive microRNAs silenced by
DNA hypermethylation in oral cancer. Cancer Res. 68:2094–2105.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Saumet A, Vetter G, Bouttier M, et al:
Transcriptional repression of microRNA genes by PML-RARA increases
expression of key cancer proteins in acute promyelocytic leukemia.
Blood. 113:412–421. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xi Y, Shalgi R, Fodstad O, Pilpel Y and Ju
J: Differentially regulated micro-RNAs and actively translated
messenger RNA transcripts by tumor suppressor p53 in colon cancer.
Clin Cancer Res. 12:2014–2024. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mi S, Lu J, Sun M, et al: MicroRNA
expression signatures accurately discriminate acute lymphoblastic
leukemia from acute myeloid leukemia. Proc Natl Acad Sci USA.
104:19971–19976. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ciafrè SA, Galardi S, Mangiola A, et al:
Extensive modulation of a set of microRNAs in primary glioblastoma.
Biochem Biophys Res Commun. 334:1351–1358. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Huang S, He X, Ding J, et al: Upregulation
of miR-23a approximately 27a approximately 24 decreases
transforming growth factor-beta-induced tumor-suppressive
activities in human hepatocellular carcinoma cells. Int J Cancer.
123:972–978. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li L, Zhang ZM, Liu Y, et al: DNA
microarrays-based microRNA expression profiles derived from
formalin-fixed paraffin-embedded tissue blocks of squammous cell
carcinoma of larynx. Zhonghua Bing Li Xue Za Zhi. 39:391–395.
2010.(In Chinese). PubMed/NCBI
|
|
17
|
Chen Q, Xu J, Li L, et al: MicroRNA-23a/b
and microRNA-27a/b suppress Apaf-1 protein and alleviate
hypoxia-induced neuronal apoptosis. Cell Death Dis. 5:e11322014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lian S, Shi R, Bai T, et al:
Anti-miRNA-23a oligonucleotide suppresses glioma cells growth by
targeting apoptotic protease activating factor-1. Curr Pharm Des.
19:6382–6389. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shang J, Yang F, Wang Y, et al:
MicroRNA-23a antisense enhances 5-fluorouracil chemosensitivity
through APAF-1/caspase-9 apoptotic pathway in colorectal cancer
cells. J Cell Biochem. 115:772–784. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Huerta S, Heinzerling JH,
Anguiano-Hernandez YM, et al: Modification of gene products
involved in resistance to apoptosis in metastatic colon cancer
cells: Roles of Fas, Apaf-1, NF kappaB, IAPs, Smac/DIABLO and AIF.
J Surg Res. 142:184–194. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zang YS, Zhong YF, Fang Z, Li B and An J:
MiR-155 inhibits the sensitivity of lung cancer cells to cisplatin
via negative regulation of APAF-1 expression. Cancer Gene Ther.
19:773–778. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Huang DF, Fu WN, Shang C, Xu ZM, Li ZG and
Sun KL: Expression and promoter methylation of Apaf-1 gene in
laryngeal squamous cell carcinoma. Yi Chuan Xue Bao. 31:1327–1331.
2004.(In Chinese). PubMed/NCBI
|
|
23
|
Fu WN, Bertoni F, Kelsey SM, McElwaine SM,
Cotter FE, Newland AC and Jia L: Role of DNA methylation in the
suppression of Apaf-1 protein in human leukaemia. Oncogene.
22:451–455. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hanahan D and Weinberg RA: The hallmarks
of cancer. Cell. 100:57–70. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nakano H, Yamada Y, Miyazawa T and Yoshida
T: Gain-of-function microRNA screens identify miR-193a regulating
proliferation and apoptosis in epithelial ovarian cancer cells. Int
J Oncol. 42:1875–1882. 2013.PubMed/NCBI
|
|
27
|
Christensen LL, Holm A, Rantala J, et al:
Functional screening identifies miRNAs influencing apoptosis and
proliferation in colorectal cancer. PLoS One. 9:e967672014.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Taylor RC, Cullen SP and Martin SJ:
Apoptosis: controlled demolition at the cellular level. Nat Rev Mol
Cell Biol. 9:231–241. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Campioni M, Santini D, Tonini G, Murace R,
Dragonetti E, Spugnini EP and Baldi A: Role of Apaf-1, a key
regulator of apoptosis, in melanoma progression and
chemoresistance. Exp Dermatol. 14:811–818. 2005. View Article : Google Scholar : PubMed/NCBI
|

















