Introduction
Pulmonary hamartoma is the most common benign tumor
of the lung, accounting for 6% of all solitary pulmonary nodules
(1). The majority of hamartomas are
identified incidentally, with the peak incidence occurring in
patients in their sixth decade (2).
Hamartomas are rarely symptomatic, but in symptomatic cases the
hamartoma is associated with hemoptysis or cough. Originally,
hamartomas were considered to be congenital lesions, but pulmonary
hamartomas are currently considered to be true neoplasms by the
majority of investigators. Consistent with this concept are the
observations that hamartomas are often identified in the elderly
and that the lesions grow slowly. The typical radiographic
appearance of a hamartoma is that of a smooth or slightly lobulated
peripheral solitary pulmonary nodule. The presence of fat or
popcorn-like calcifications may enable the confident diagnosis of a
hamartoma, but these findings are not usually identified.
The radiological diagnosis of hamartoma is usually
based on computed tomography (CT) findings, particularly the
detection of popcorn-like calcifications and fat (3). 18F-fluorodeoxyglucose
(18F-FDG) positron emission tomography (PET)/CT
investigations may be of use when neither calcification nor fat is
identified on CT scans (4). The
present study reports the case of a 77-year-old female patient that
was diagnosed with a pulmonary hamartoma that demonstrated atypical
imaging findings on two imaging modalities.
Case report
An asymptomatic 77-year-old female was admitted to
The 117th hospital of PLA (Hangzhou, China) for the investigation
of a lesion in the left upper lobe of the lung that had been
present for 3 years.
The patient had undergone a routine health
examination in The 117th hospital of PLA on January 29, 2007. The
patient was asymptomatic and no abnormal findings were detected in
the examination, with the exception of a 1.2-cm solitary pulmonary
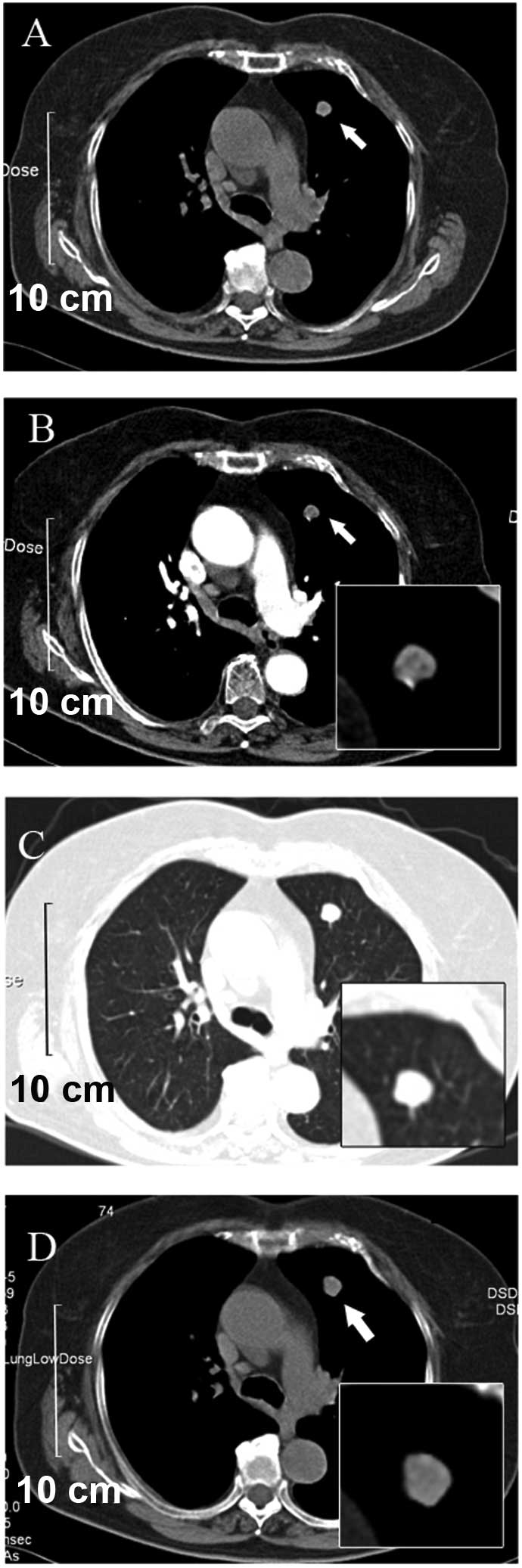
nodule that was identified on a CT scan. The scan revealed a round
non-homogeneous parenchymal neoformation in the anterior segment of
the left upper lobe of the lung, with a clear border (Fig. 1A). The contrast-enhanced CT that was
performed four days later revealed that the neoformation contained
hypodense regions with possible involvement of the vessel. In
addition, non-homogeneous enhancement during the arterial phase was
demonstrated. There was no indication of lobulation and
spiculation. Neither the mediastinal nor hilar lymph nodes were
enlarged (Fig. 1B and C).
Since there was no evidence of a malignant neoplasm,
it was decided continue to follow-up the present patient. The
following CT scan was performed two years subsequent to the first
presentation (March 9, 2009). The CT scan revealed that the
non-homogeneous oval mass had increased in size, to ~1.7 cm in
diameter, with a regular margin and no calcification (Fig. 2). The results of physical examination
and laboratory tests (serum tumor markers detection, including AFP,
CEA and CA125, performed on November 6, 2010) were negative and the
appearance of the radiology results were non-specific. Therefore,
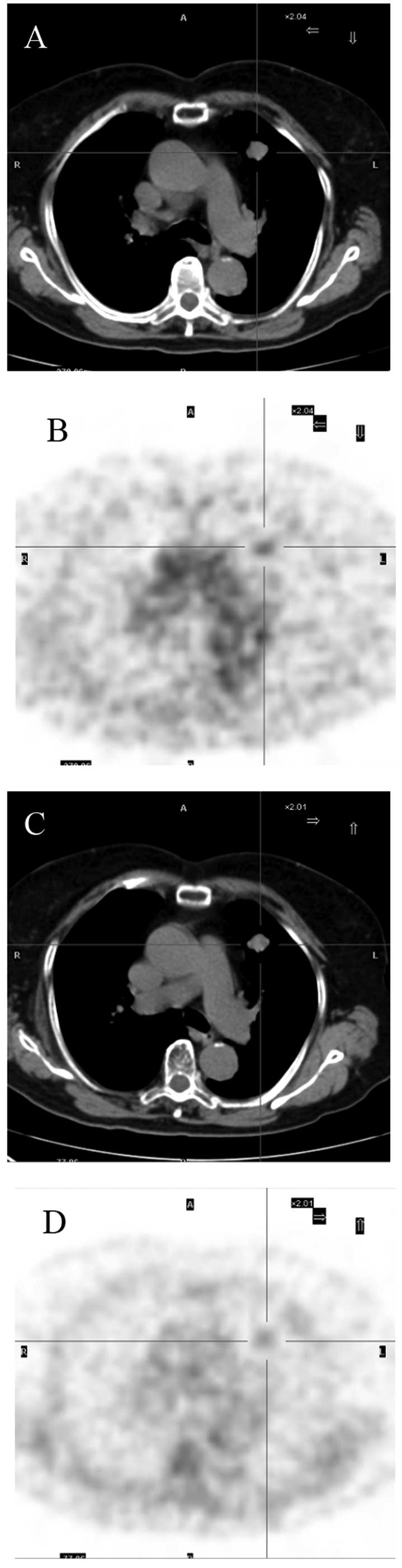
PET/CT was performed on November 8, 2010. PET/CT revealed a
slightly lobulated mass in the left upper lobe of the lung that was
~1.9 cm in diameter and well defined, with involvement of the
mediastinal pleura. Slight uptake of 18F-FDG was also
detected. The maximum standardized uptake value (SUVmax)
was found to be 2.44. Delayed scanning demonstrated that the lesion
possessed diminishing metabolism, with an SUVmax of 1.64
(Fig. 3). Review of the laboratory
tests, including those for serum tumor markers, remained within the
normal range.
The lesion was suspected to be a pulmonary benign
tumor or primary lung cancer, such as well-differentiated
adenocarcinoma, but the diagnosis was unable to be confirmed
pre-operatively. Since the patient requested removal of the lesion,
it was resected by video-assisted thoracic surgery. Gross
examination of the resected specimen revealed that the tumor
measured 2.5×2.0 cm, and was a white, multilobular, flexible mass
without an envelope. The margin of the lesion and peripheral lung
tissue was clear and there was no area demonstrating infiltrative
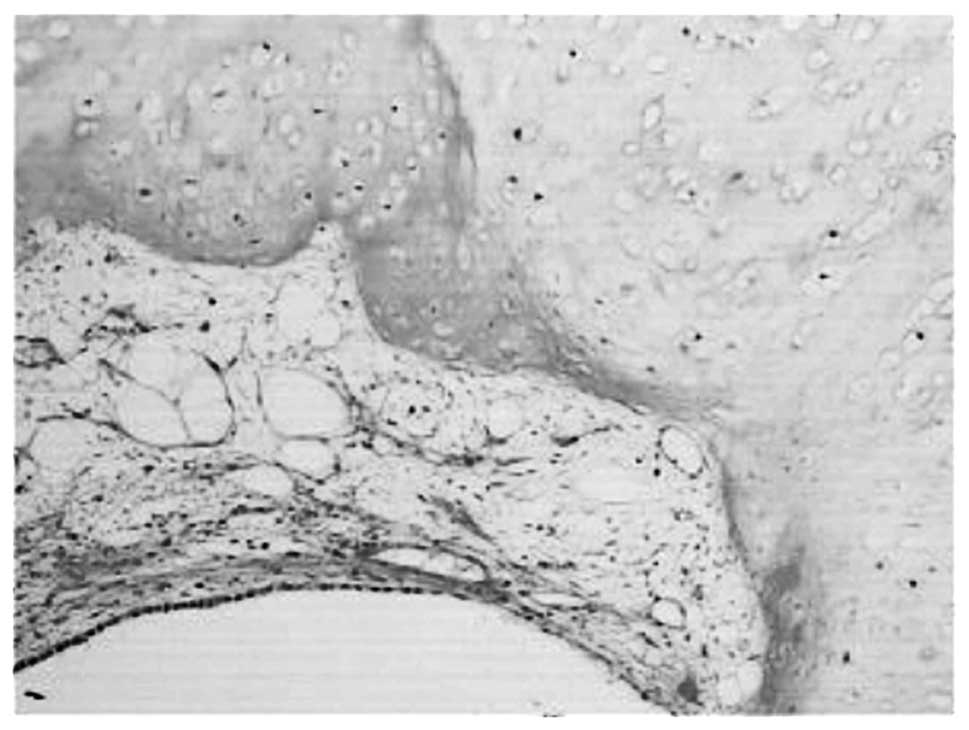
growth. Histologically, the lesion was composed of fat, cartilage
and smooth muscle, covered by normal bronchial epithelial cells
(Fig. 3). On the basis of these
findings, the tumor was diagnosed as a hamartoma. Six months
subsequent to the surgical procedure, the patient is completely
well, with no evidence of recurrence.
Discussion
Hamartomas are the third most common solitary
pulmonary nodule, following granuloma and carcinoma, and usually
account for 6–8% of localized parenchymal masses treated by
thoracotomy (1). Individuals aged
60–79 years old develop hamartomas with the highest frequency
(2). The majority of the patients
with pulmonary hamartoma are free of symptoms, and the tumor is
found incidentally on chest X-ray examination. On X-ray
examination, a pulmonary hamartoma usually presents as a sharply
demarcated coin lesion with slow growth, occasionally with
calcification, but this is not diagnostic since calcifications may
appear in carcinomas.
The radiological diagnosis of a pulmonary hamartoma
has frequently been made using CT. In particular, CT densitometry
with an advanced narrow collimated technique has been accepted as
one of the best sensitive methods from eliminated partial volume
averaging in detecting intranodular fat (−40 to −120 Hounsfield
units), providing a highly predictive diagnosis of a pulmonary
hamartoma or characteristic popcorn calcifications (1,2). However,
hamartomas are extremely challenging to diagnose when the lesions
possess neither fat nor calcification in 50% of pulmonary
hamartomas, at most (1). This results
in a diagnostic challenge, as the hamartomas cannot be
differentiated from a primary or secondary lung cancer or other
benign nodules. In the present study, the patient was asymptomatic
and the tumor was identified incidentally on a CT scan. The mean
size of hamartomas increased between 1.2 and 1.9 cm in three years,
with an irregular margin and no calcification or fat identified on
CT. The lesion was suspected to be a benign or primary lung cancer,
such as well-differentiated adenocarcinoma, but the diagnosis was
unable to be confirmed pre-operatively. 18FDG PET/CT
studies may aid diagnosis when neither calcification nor fat is
demonstrated on CT studies.
The use of an 18F-FDG-PET scan is based
on the principle that cancer cells demonstrate an increased glucose
uptake and higher rate of glycolysis compared with non-cancerous
cells. In a meta-analysis reported by Gould et al, a
sensitivity of 97% and a specificity of 78% were determined for the
differentiation of malignant from benign pulmonary lesions using an
18F-FDG-PET scan (4).
However, 18F-FDG-PET possesses a limited capacity for
the detection of tumors with low glycolytic activity, including
small tumors, bronchioloalveolar cell carcinoma and carcinoid
tumors (5). As a result, the use of
18F-FDG-PET scans for the identification of hamartomas
is limited.
To the best of our knowledge, there are only two
published studies that investigated the 18F-FDG-PET scan
findings in hamartomas. Teramoto et al reported that the
uptake of 18F-FDG did not increase in pulmonary
hamartomas (6). By contrast, in the
case reported by Himpe et al, only slightly elevated uptake
with an SUVmax of 3.3 and SUVmean of 1.7 was
identified (7). Consistent with the
findings of Himpe et al, the present patient possessed a
lesion with an SUVmax of 2.44. Delayed scanning
demonstrated that the lesion possessed diminishing metabolism, with
an SUVmax of 1.64. The mechanism of slightly enhanced
uptake of 18F-FDG in hamartomas remains unknown. Benign
and slow-growing tumors usually demonstrate low glucose metabolism.
Therefore, familiarity with this false positive finding would aid
the interpretation of 18F-FDG-PET scan results on
solitary pulmonary nodules.
The clinical features are usually benign and the
prognosis subsequent to surgical excision is usually excellent.
However, certain studies consider hamartomas to be a potentially
low-grade malignancy, since a small number of cases of squamous
cell carcinoma, adenocarcinoma or sarcoma arising from pulmonary
hamartoma have been reported (8). In
addition, a previous study reported that synchronous or
metachronous lung cancer with hamartoma in an adjacent region
(9). If a benign tumor is strongly
suspected, observation of the patient is reasonable. However, if
the lesion has increased in size more rapidly than usual, in
addition to being large and symptomatic, patients should undergo
resection, unless surgery is contraindicated due to poor pulmonary
function, comorbidities, or withholding of consent. Furthermore, if
malignant causes cannot be excluded and patients agree to undergo
resection, the mass may be excised for diagnosis and intent to
cure. In the present patient, although the nodule was strongly
suspected to be benign, a malignant cause could not be completely
excluded and the patient agreed to undergo resection to obtain a
diagnosis. Therefore, the nodule was removed using thoracoscopic
enucleation. The findings of the present case add further support
to the continued use of 18F-FDG-PET scan in hamartomas
References
|
1
|
Siegelman SS, Khouri NF, Scott WW Jr, et
al: Pulmonary hamartoma: CT findings. Radiology. 160:313–317. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gjevre JA, Myers JL and Prakash UB:
Pulmonary hamartomas. Mayo Clin Proc. 71:14–20. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Potente G, Macori F, Caimi M, Mingazzini P
and Volpino P: Noncalcified pulmonary hamartomas: computed
tomography enhancement patterns with histologic correlation. J
Thorac Imaging. 14:101–104. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gould MK, Maclean CC, Kuschner WG, Rydzak
CE and Owens DK: Accuracy of positron emission tomography for
diagnosis of pulmonary nodules and mass lesions: a meta-analysis.
JAMA. 285:914–924. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chang JM, Lee HJ, Goo JM, et al: False
positive and false negative FDG-PET scans in various thoracic
diseases. Korean J Radiol. 7:57–69. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Teramoto K and Suzumura Y: Multiple
pulmonary hamartomas penetrating the visceral pleura: Report of a
case. Surg Today. 37:1087–1089. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Himpe U, Deroose CM, Leyn PD, Verbeken E
and Vansteenkiste J: Unexpected slight fluorodeoxyglucose-uptake on
positron emission tomography in a pulmonary hamartoma. J Thorac
Oncol. 4:107–108. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lee BJ, Kim HR, Cheon GJ, Koh JS, Kim CH
and Lee JC: Squamous cell carcinoma arising from pulmonary
hamartoma. Clin Nucl Med. 36:130–131. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mahouachi R, Ben Abdelkrim I, Chtourou A,
et al: Lung cancer and chondromatous hamartoma: A case report.
Tunis Med. 83:789–791. 2005.(In French). PubMed/NCBI
|