Introduction
Systemic chemotherapy is one of the primary
modalities used to improve the survival of patients with metastatic
urothelial cancer (UC). Cisplatin-based chemotherapy remains the
standard treatment strategy for patients with metastatic UC;
however, despite the administration of various novel combination
regimens, the overall response rate varies between 36–69%. Another
limiting factor associated with the currently available
chemotherapeutic regimens is their level of toxicity. As such, the
treatment of metastatic UC with cytotoxic chemotherapy has reached
a therapeutic plateau, and the identification of novel treatment
modalities is urgently required (1).
Cisplatin is an effective antitumor agent owing to
its ability to induce intra- and inter-strand DNA cross-links
(2). Cisplatin-based combination
chemotherapy is currently the primary treatment strategy for
patients with advanced bladder cancer, however, its clinical use as
an anti-cancer agent is predominantly limited by its association
with a high incidence of chemoresistance (3,4). One of
the known mechanisms underlying chemoresistance is alteration of
the apoptotic signaling pathways and a resultant decreased
apoptotic response (5). For example,
B-cell lymphoma-2 (Bcl-2) is a significant anti-apoptotic protein,
responsible for regulation of the mitochondrial apoptotic signaling
pathway. Overexpression of Bcl-2 is known to block apoptosis by
preventing translocation of Bcl-2-associated X protein to the
mitochondrial membrane and reducing programmed cell death (3). Bcl-2 is frequently upregulated in
multiple types of cancer, including bladder cancer (6). Furthermore, upregulated Bcl-2 protein
appears to be crucial in the development of cisplatin resistance
(3,7).
To overcome this resistance, improvements in systemic combined
chemotherapeutic regimes and the development of novel treatment
regimens are essential. For example, combining cisplatin with other
agents is known to enhance its efficiency. These combinations may
result in decreased levels of anti-apoptotic protein expression and
upregulation of pro-apoptotic protein expression by shifting the
balance between cell death and survival (7). Various studies have demonstrated that
proteasome inhibition is the key regulator of intracellular protein
degradation (8,9). This inhibition promotes the degradation
of anti-apoptotic proteins while preventing the degradation of
pro-apoptotic proteins, resulting in increased accumulation of
pro-apoptotic proteins within the cells, and subsequent cell growth
inhibition and programmed cell death in numerous malignant cell
types (8,9).
Bortezomib is a potent, selective and reversible
inhibitor of the 26S proteasome, comprised of a complex
multi-subunit protease that controls the degradation of short-lived
regulatory proteins involved in various cellular processes,
including apoptosis (9,10). Previous studies have identified that
the bortezomib-induced apoptotic mechanism is the key regulator in
the balance of pro- and anti-apoptotic Bcl-2 family proteins
(9,11). Bortezomib treatment upregulates the
expression of pro-apoptotic proteins, including Bcl-2-like 11 (Bim)
and Bcl-2-interacting killer (Bik), and downregulates the
expression of anti-apoptotic Bcl-2 family proteins, for example
Bcl-2 (11).
The present study examined the anti-proliferative
effects of cisplatin and bortezomib, applied alone or in
combination, on the human T24 urinary bladder carcinoma cell line.
Following treatment with cisplatin and bortezomib alone or in
combination, the activation of caspase-3, -8 and -9, and the
expression levels of anti-apoptotic [Bcl-2 and Bcl-extra large
(Bcl-xL)] and pro-apoptotic (Bim and Bik) proteins were
investigated. Furthermore, the current study aimed to establish
whether the synergistic effects of combined cisplatin and
bortezomib treatment may offer a potential therapeutic approach to
overcome cisplatin resistance.
Materials and methods
Cell lines and chemicals
The human T24 urinary bladder carcinoma cell line
was obtained from the American Type Culture Collection (Manassas,
VA, USA). The T24 cells were cultured in McCoy's 5A medium
containing L-glutamine, 10% fetal bovine serum, 100 U/ml penicillin
and 100 mg/ml streptomycin (Thermo Fisher Scientific, Inc.,
Waltham, MA, USA), and incubated in a humidified atmosphere of 5%
CO2 at a temperature of 37°C. Cisplatin was obtained
from Sigma-Aldrich (St. Louis, MO, USA) and bortezomib was obtained
from BioVision, Inc. (Milpitas, CA, USA)
Cell viability assay
The antitumor effects of single and combined
cisplatin and bortezomib treatment on the viability of T24 cells
were determined by performing cell proliferation water-soluble
tetrazolium salt-1 (WST-1) assays (Roche Diagnostics GmbH,
Mannheim, Germany). Half maximal inhibitory concentration values
for cisplatin and bortezomib were determined by treating cells with
cisplatin (0, 0.5, 2.5, 5, 10 and 20 µM) and/or bortezomib (0, 1,
5, 7.5, 10, 25, 50, 100, 200 and 300 nM). The control cells were
treated only with cell culture medium. Briefly, in each well of a
96-well plate, 5×103 cells were seeded in 200 µl medium
and treated with cisplatin, bortezomib or a combination of the two
agents for 24 h. WST-1 solution (10 µl) was added to each well and
absorbance was measured after 3 h at a wavelength of 450 nm using
an ELISA reader (Spectramax® M3; Molecular Devices LLC, Sunnyvale,
CA, USA) following the incubation period.
Active caspase-3 level
Caspase-3 protein activity was measured using a
luminescence assay, according to the manufacturer's instructions
[PathScan® Cleaved Caspase-3 (Asp175) Sandwich ELISA kit; Cell
Signaling Technology, Inc. (Danvers, MA, USA)]. Considering that
the caspase family of proteases have key effector roles in
apoptosis in mammalian cells, the aim was to detect the
pro-apoptotic effects of treatment with cisplatin and bortezomib
alone or in combination for 24 h. Briefly, addition of the reagent
to the wells induced cell lysis, followed by caspase cleavage of
the substrate and generation of a luminescent signal by luciferase
that was proportional to the level of caspase activity. Caspase-3
activity was quantified by reading the absorbance at a wavelength
of 450 nm using a microplate ELISA reader.
Protein extraction and western blot
analysis
Western blot analysis of Bcl-2, Bcl-xL, Bim, Bik,
caspase-8 and caspase-9 was performed as previously described
(12). Briefly, cells were lysed in
lysis buffer (Cell Signaling Technology, Inc.) containing 1 mM
phenylmethanesulfonylfluoride (Sigma-Aldrich) prior to treatment
with the specified concentrations of cisplatin and/or bortezomib for
24 h. Equal quantities of protein were loaded and separated by 12%
SDS-PAGE then transferred to a polyvinylidene difluoride membrane
(Thermo Fisher Scientific, Inc.). Following blocking with 5% w/v
non-fat milk or 5% w/v bovine serum albumin in Tris-buffered saline
with 0.1% Tween 20 (TBST-T), the membrane was incubated overnight
at 4°C with rabbit anti-human Bcl-2 (catalog no. PA5-27094), Bcl-xL
(catalog no. PA5-17805), Bim (catalog no. PA5-11385), Bik (catalog
no. PA5-20249), caspase-8 (catalog no. PA5-20118) and caspase-9
(catalog no. PA5-19904) (Thermo Fisher Scientific, Inc.) polyclonal
antibodies, as well as rabbit anti-human β-actin monoclonal
antibody (catalog no. 4970; Cell Signaling Technology, Inc.) as the
loading control. All primary antibodies were diluted 1:1,000. This
process was followed by incubation with goat anti-rabbit
horseradish peroxidase (HRP)-conjugated secondary antibody (catalog
no. 31210; dilution, 1:5,000; Thermo Fisher Scientific, Inc.) for 2
h at room temperature. Proteins were visualized using a Kodak Gel
Logic 2200 imaging system (Kodak, Rochester, NY, USA) with
Luminata™ Crescendo Western HRP substrate (EMD Millipore,
Billerica, MA, USA).
Statistical analysis
Each data point was measured in three independent
experiments. Cell viability was analyzed by performing one-way
analysis of variance and multiple comparison analyses were
performed using SPSS software (version 15.0; SPSS, Inc., Chicago,
IL, USA). P<0.01 was considered to indicate a statistically
significant difference and the results are expressed as the mean ±
standard deviation.
Results
Combined treatment with cisplatin and
bortezomib enhances inhibition of T24 cell proliferation
Optimal doses of cisplatin alone, bortezomib alone,
and cisplatin and bortezomib combined were determined by performing
a WST-1 assay. To identify the effects of exposure to cisplatin and
bortezomib alone and in combination, T24 cells were treated with
various concentrations of cisplatin (0–20 µM) and bortezomib (0–300
nM) for 24 h. The most effective and least toxic doses of cisplatin
(Fig. 1A) and bortezomib (Fig. 1B) alone were determined to be 10 µM
and 10 nM, respectively. Furthermore, the most effective doses of
cisplatin and bortezomib during their combined use were 7.5 µM and
5 nM, respectively (Fig. 1C). These
values indicated that the two agents synergistically enhance cell
proliferation inhibition.
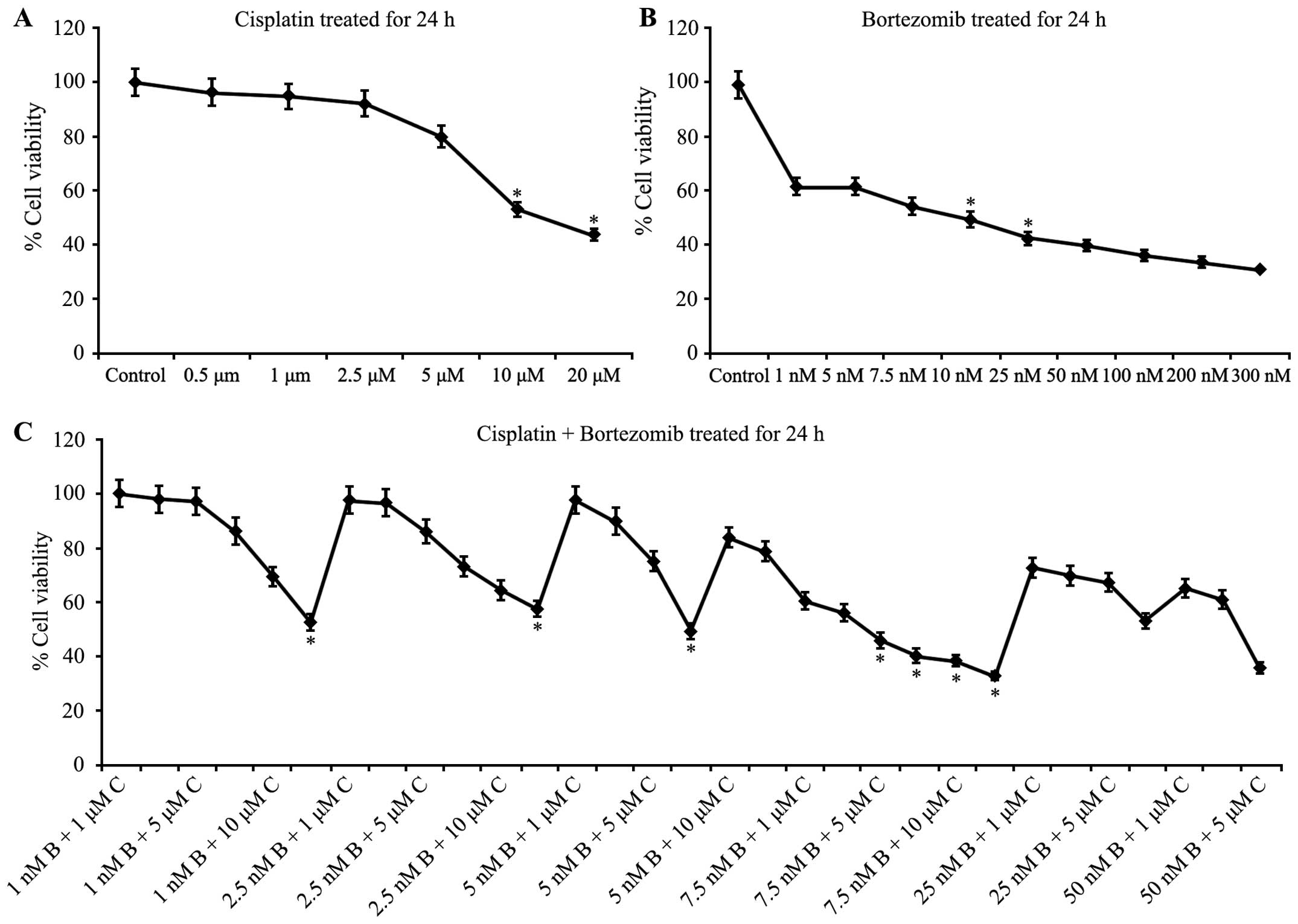 | Figure 1.Combined treatment with cisplatin and
bortezomib inhibits cell proliferation of T24 cells. Graphical
representation of water soluble tetrazolium salt assay, indicating
percentage change in cell viability in T24 cells treated with (A)
cisplatin (0, 0.5, 1, 2.5, 5, 10 and 20 mM), (B) bortezomib (0, 1,
5, 7.5, 10, 25, 50, 100, 200 and 300 nM) and (C) cisplatin plus
bortezomib for 24 h. Data points represent the mean ± standard
deviation of triplicate experiments. *IC50 value. C,
cisplatin; B, bortezomib. |
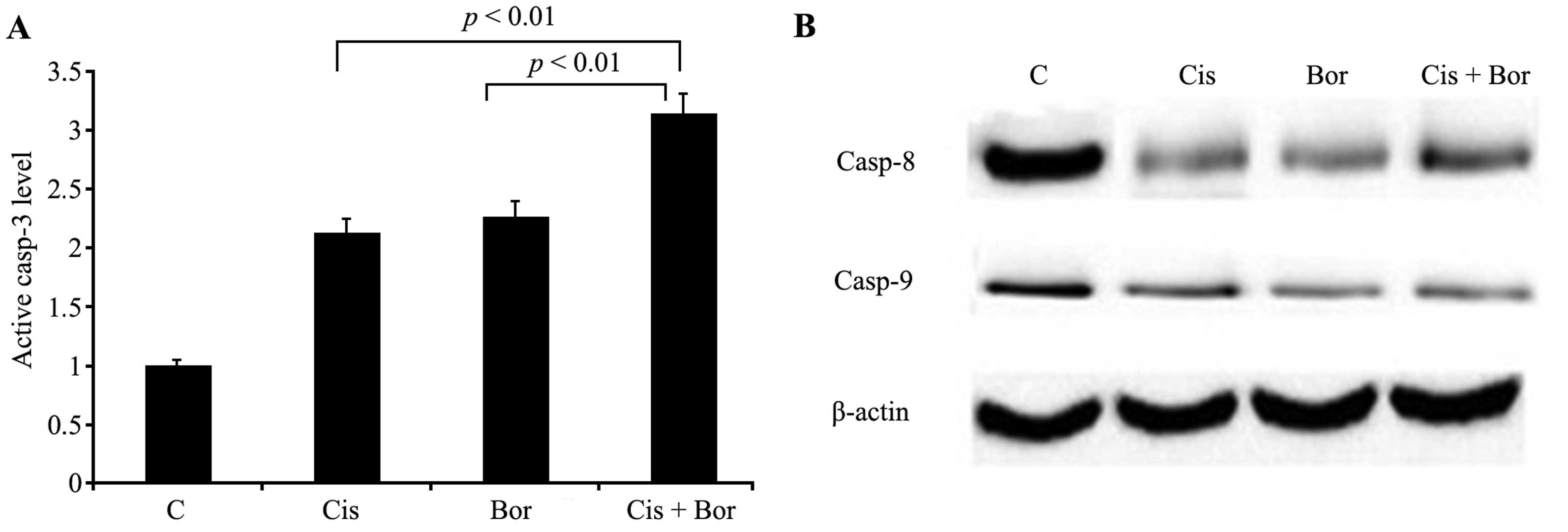
Combined treatment with cisplatin and
bortezomib enhances caspase-3, -8 and -9 activity
Treatment of T24 cells with 10 µM cisplatin, 10 nM
bortezomib and cisplatin plus bortezomib (7.5 µM and 5 nM) resulted
in 2.1-, 2.3- and 3.2-fold increases in caspase-3 activation,
respectively. The cause of these significant increases was
suggested to be the triggering of cell apoptosis (P<0.01;
Fig. 2A). This apoptosis was
associated with more effective induction caspase-3 activation by
cisplatin and bortezomib combination treatment of T24 cells,
compared with that of the administration of the two agents alone.
Furthermore, enhanced activation of caspase-8 and -9 were detected
when the cells were treated with cisplatin and bortezomib in
combination, in agreement with the effects on caspase-3 activation
(Fig. 2B).
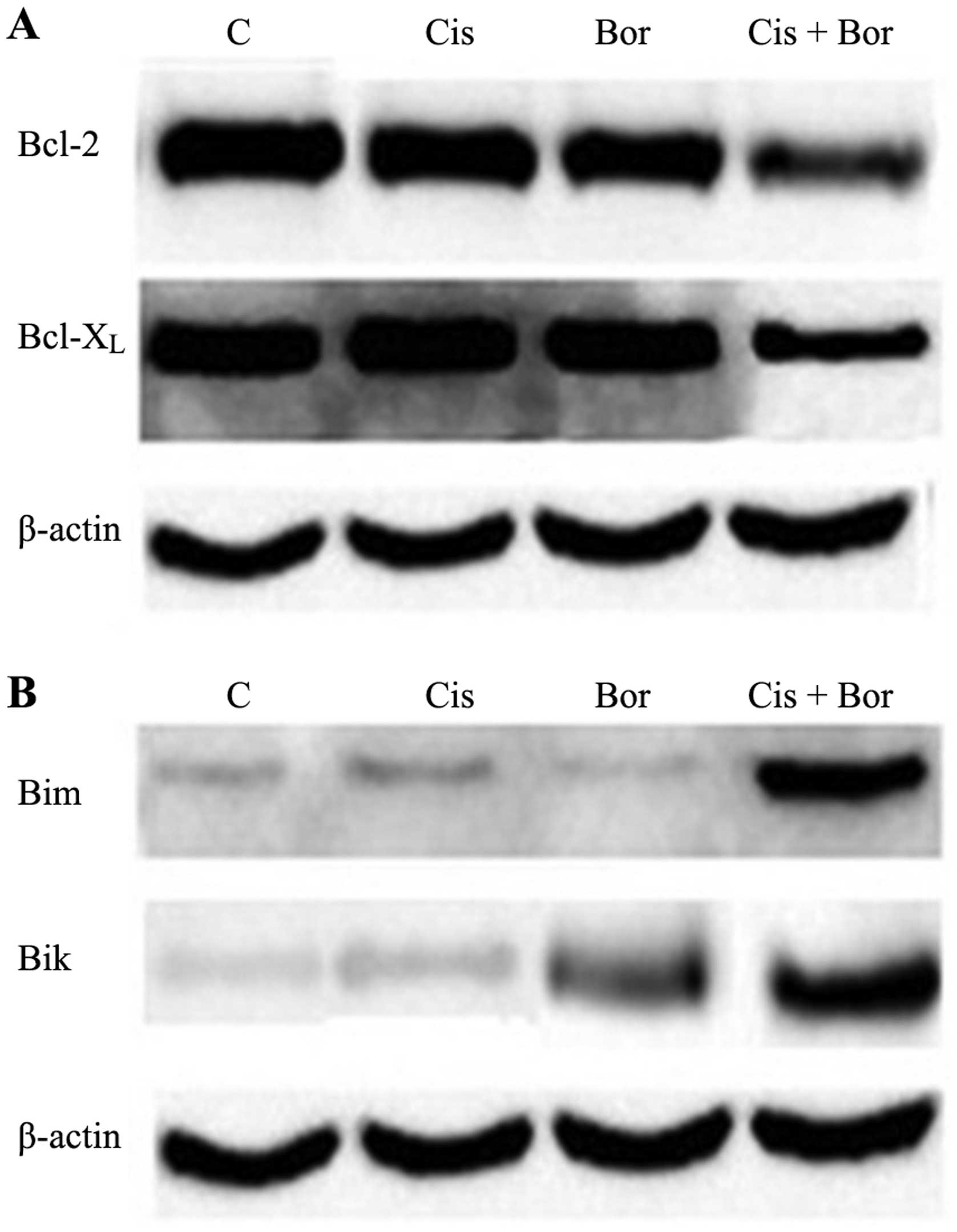
Combined treatment with cisplatin and
bortezomib alters the balance of Bcl-2 family protein expression
levels
The present study also investigated the effect of
cisplatin and bortezomib treatment on the expression levels of the
pro-apoptotic and anti-apoptotic proteins of the Bcl-2 family.
Overexpression of Bcl-2 protein is associated with the development
of cisplatin resistance, with Bcl-2 and Bcl-xL protein expression
levels known to be high in cisplatin-resistant T24 cells. Western
blot analysis revealed that the expression levels of Bcl-2 and
Bcl-xL were not markedly altered following treatment of T24 cells
with cisplatin or bortezomib alone, compared with those of the
control. However, combined administration of these agents resulted
in markedly decreased expression of Bcl-2 and Bcl-xL at the protein
level (Fig. 3A). Furthermore, it was
demonstrated that, following treatment with bortezomib alone, only
the expression of pro-apoptotic protein Bik increased. However,
combined administration of cisplatin and bortezomib led to an
increase in the expression of pro-apoptotic proteins Bik and Bim
(Fig. 3B). These results indicated
that combined administration of cisplatin and bortezomib may
synergistically induce apoptosis. In addition, exposure to a
lower-dose drug combination resulted in an anti-proliferative
effect.
Discussion
Preclinical studies employing bladder cancer cells
have demonstrated that combined therapy with conventional agents
may result in greater tumor growth inhibition than that observed
following therapy with either agent alone, without inducing
significant increases in toxicity (13–15). The
proteasome inhibitor bortezomib is a promising novel agent in the
treatment of bladder cancer; however, inducible cytoprotective
mechanisms may limit its potential efficacy (15). In previous studies, bortezomib has
been observed to enhance the activity of cisplatin, particularly in
cisplatin-resistant cells (6,8,16–18). The present study demonstrated for the
first time that cisplatin and bortezomib combined treatment induced
inhibition of cell proliferation by intrinsic and extrinsic
apoptotic signaling pathways in the T24 human bladder cancer cell
line. Exposure to a lower-dose drug combination resulted in a
significant anti-proliferative effect. The results revealed that
cisplatin plus bortezomib combination treatment was more potent
than the administration of either agent alone.
The development of resistance to treatment is one of
the major limitations to successful cisplatin-based chemotherapy
regimes, frequently resulting in poor clinical prognoses.
Chemoresistance has previously been associated with the failure of
cisplatin to induce apoptosis (3,19), the
most common response of cells to chemotherapeutic agents. To date,
two major apoptotic pathways have been identified in mammalian
cells. The first involves caspase-8, which is activated by membrane
death receptor-mediated extrinsic signaling pathways, while the
second involves mitochondria-dependent intrinsic signaling
pathways, characterized by the activation of caspase-9 by
cytochrome c release into the cytosol and subsequent
apoptosome formation. These extrinsic and intrinsic apoptotic
signaling pathways converge at the level of caspase-3 activation
(20). The current study demonstrated
that the extrinsic death receptor- and intrinsic
mitochondria-dependent signaling pathways were activated following
combinatorial treatment of T24 cells with cisplatin and bortezomib.
Furthermore, caspases-3, -8 and -9 only exhibited marked activation
following combined treatment. Thus, increased caspase activation
indicates that combined treatment with these agents induces cell
apoptosis. Bortezomib appeared to enhance apoptosis and inhibit
proliferation in T24 cells, as well as enhance the
growth-inhibitory effects of cisplatin. The current results
corroborate those of previous studies performed in various other
cell types evaluating the effect of bortezomib treatment on caspase
activation and apoptosis (21,22).
Although caspase activation has a key role in the
mechanism of apoptosis, the primary controllers underlying caspase
activation are the Bcl-2 family proteins (23). Bcl-2 protein family members function
as key regulators of cellular apoptosis and are important
determinants of cellular sensitivity or resistance to
chemotherapeutic agents (7), with
overexpression of Bcl-2 known to block apoptosis. An association
between Bcl-2 upregulation and cisplatin resistance was previously
reported in a cisplatin-resistant subclone of the human T24 bladder
cancer cell line (3,6). Therefore, Bcl-2 may be a significant
target for the prevention of resistance to cisplatin treatment. It
is known that the expression levels of pro-apoptotic and
anti-apoptotic Bcl-2 family proteins are directly regulated by
proteasome inhibition. Therefore, the expression levels of Bcl-2
family proteins may change in response to inhibitors of the
ubiquitin proteasome system (24,25).
Western blot analyses performed in the present study identified no
noticeable alterations in the expression levels of Bcl-2 and Bcl-xL
following treatment with cisplatin and bortezomib alone, when
compared with those of the control. However, the expression levels
of Bcl-2 and Bcl-xL were markedly decreased following combination
treatment. Furthermore, it was demonstrated that only the
expression of pro-apoptotic protein Bik was increased following
treatment with bortezomib alone. By contrast, combination treatment
resulted in increased expression levels of the two pro-apoptotic
proteins Bik and Bim. This accumulation of Bik and Bim indicated
the synergistic action of cisplatin and bortezomib on the induction
of apoptosis.
In conclusion, the results of the current study
demonstrated the potential of bortezomib and cisplatin combination
therapy in the treatment of bladder cancer. This effect occurred
via the efficient activation of intrinsic and extrinsic apoptotic
signaling pathways in T24 cells, by modulation of the balance among
Bcl-2 family proteins towards apoptosis. This synergistic effect of
combined agents may offer a novel approach to overcome cisplatin
resistance, however, additional research using in vivo
models is required.
References
|
1
|
Latini DM, Lerner SP, Wade SW, Lee DW and
Quale DZ: Bladder cancer detection, treatment and outcomes:
Opportunities and challenges. Urology. 75:334–339. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
da Silva GN, de Castro Marcondes JP, de
Camargo EA, da Silva Passos Júnior GA, Sakamoto-Hojo ET and
Salvadori DM: Cell cycle arrest and apoptosis in TP53 subtypes of
bladder carcinoma cell lines treated with cisplatin and
gemcitabine. Exp Biol Med. 235:814–824. 2010. View Article : Google Scholar
|
|
3
|
Yu HM and Wang TC: Mechanism of cisplatin
resistance in human urothelial carcinoma cells. Food Chem Toxicol.
50:1226–1237. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Galluzzi L, Senovilla L, Vitale I, Michels
J, Martins I, Kepp O, Castedo M and Kroemer G: Molecular mechanisms
of cisplatin resistance. Oncogene. 31:1869–1883. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Köberle B, Tomicic MT, Usanova S and Kaina
B: Cisplatin resistance: Preclinical findings and clinical
implications. Biochim Biophys Acta. 1806:172–182. 2010.PubMed/NCBI
|
|
6
|
Cho HJ, Kim JK, Kim KD, et al:
Upregulation of Bcl-2 is associated with cisplatin-resistance via
inhibition of Bax translocation in human bladder cancer cells.
Cancer Lett. 237:56–66. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li C, Li R, Grandis JR and Johnson DE:
Bortezomib induces apoptosis via Bim and Bik up-regulation and
synergizes with cisplatin in the killing of head and neck squamous
cell carcinoma cells. Mol Cancer Ther. 7:1647–1655. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Al-Eisawi Z, Beale P, Chan C, Yu JQ and
Huq F: Carboplatin and oxaliplatin in sequenced combination with
bortezomib in ovarian tumour models. J Ovarian Res. 6:782013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen D, Frezza M, Schmitt S, Kanwar J and
Dou QP: Bortezomib as the first proteasome inhibitor anticancer
drug: Current status and future perspectives. Curr Cancer Drug
Targets. 11:239–253. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hutter G, Rieken M, Pastore A, Weigert O,
Zimmermann Y, Weinkauf M, Hiddemann W and Dreyling M: The
proteasome inhibitor bortezomib targets cell cycle and apoptosis
and acts synergistically in a sequence dependent way with
chemotherapeutic agents in mantle cell lymphoma. Ann Hematol.
91:847–856. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yang TM, Barbone D, Fennell DA and
Broaddus VC: Bcl-2 family proteins contribute to apoptotic
resistance in lung cancer multicellular spheroids. Am J Respir Cell
Mol Biol. 41:14–23. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Konac E, Varol N, Yilmaz A, Menevse S and
Sozen S: DNA methyltransferase inhibitor-mediated apoptosis in the
Wnt/β-catenin signal pathway in a renal cell carcinoma cell line.
Exp Biol Med (Maywood). 238:1009–1016. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kamat AM, Karashima T, Davis DW, Lashinger
L, Bar-Eli M, Millikan R, Shen Y, Dinney CP and McConkey DJ: The
proteasome inhibitor bortezomib synergizes with gemcitabine to
block the growth of human 253JB-V bladder tumors in vivo. Mol
Cancer Ther. 3:279–290. 2004.PubMed/NCBI
|
|
14
|
Papageorgiou A, Kamat A, Benedict WF,
Dinney C and McConkey DJ: Combination therapy with IFN-alpha plus
bortezomib induces apoptosis and inhibits angiogenesis in human
bladder cancer cells. Mol Cancer Ther. 5:3032–3041. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Qi W, White MC, Choi W, Guo C, Dinney C,
McConkey DJ and Siefker-Radtke A: Inhibition of inducible heat
shock protein-70 (hsp72) enhances bortezomib-induced cell death in
human bladder cancer cells. PLoS One. 8:e695092013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yerlikaya A, Altıkat S, Irmak R, Cavga FZ,
Kocacan SA and Boyaci I: Effect of bortezomib in combination with
cisplatin and 5 fluorouracil on 4T1 breast cancer cells. Mol Med
Rep. 8:277–281. 2013.PubMed/NCBI
|
|
17
|
Fribley AM, Evenchik B, Zeng Q, Park BK,
Guan JY, Zhang H, Hale TJ, Soengas MS, Kaufman RJ and Wang CY:
Proteasome inhibitor PS-341 induces apoptosis in
cisplatin-resistant squamous cell carcinoma cells by induction of
Noxa. J Biol Chem. 281:31440–31447. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Brozovic A, Ambriović-Ristov A and Osmak
M: The relationship between cisplatin-induced reactive oxygen
species, glutathione, and BCL-2 and resistance to cisplatin. Crit
Rev Toxicol. 40:347–359. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rabik CA and Dolan ME: Molecular
mechanisms of resistance and toxicity associated with platinating
agents. Cancer Treat Rev. 33:9–23. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hyman BT and Yuan J: Apoptotic and
non-apoptotic roles of caspases in neuronal physiology and
pathophysiology. Nat Rev Neurosci. 13:395–406. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kim SY, Song X, Zhang L, Bartlett DL and
Lee YJ: Role of Bcl-xL/Beclin-1 in interplay between apoptosis and
autophagy in oxaliplatin and bortezomib-induced cell death. Biochem
Pharmacol. 88:178–188. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Krętowski R, Borzym-Kluczyk M and
Cechowska-Pasko M: Efficient induction of apoptosis by proteasome
inhibitor: Bortezomib in the human breast cancer cell line
MDA-MB-231. Mol Cell Biochem. 389:177–185. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Elkholi R, Floros KV and Chipuk JE: The
role of BH3-only proteins in tumor cell development, signaling, and
treatment. Genes Cancer. 2:523–537. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fennell DA, Chacko A and Mutti L: BCL-2
family regulation by the 20S proteasome inhibitor bortezomib.
Oncogene. 27:1189–1197. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Neutzner A, Li S, Xu S and Karbowski M:
The ubiquitin/proteasome system-dependent control of mitochondrial
steps in apoptosis. Semin Cell Dev Biol. 23:499–508. 2012.
View Article : Google Scholar : PubMed/NCBI
|