Introduction
Lung cancer is a leading cause of cancer-associated
mortality worldwide (1). Targeted
therapy has been developed and is widely used for the treatment of
non-small cell lung cancer (NSCLC), particularly in patients
harboring an activating epidermal growth factor receptor (EGFR)
mutation (2). The IRESSA Pan-Asia
Study trial found that patients with sensitizing EGFR mutations who
received gefitinib had increased progression-free survival (24.9%)
when compared with patients treated with carboplatin-paclitaxel
(6.7%), as well as an increased response rate (71.2 vs. 47.3%)
(3). Based on these results, the Food
and Drug Administration (FDA) approved erlotinib for the treatment
of patients with locally advanced or metastatic NSCLC after
progression on at least one prior chemotherapy regimen. With the
wide use of EGFR tyrosine kinase inhibitors (TKIs) in patients with
NSCLC, an increasing number of side effects have been observed,
including skin rash, diarrhea, stomatitis and eyelash trichomegaly
(4,5).
The adverse events most frequently reported by erlotinib-treated
patients are rash (56.0%) and diarrhea (62.0%) (6). Although erlotinib exhibits a number of
side effects, it presents significant clinical benefits in the
treatment of non-small cell lung cancer (NSCLC), particularly in
patients with an EGFR mutation (6).
Recently, the FDA approved the use of erlotinib as first-line
therapy in patients with exon 19 deletions or exon 21 (L858R)
substitution mutations (6). Eyelash
trichomegaly, which was first identified by Gray et al
(7) in 1944, is characterized by the
increased length, thickness, stiffness, curling and pigmentation of
the eyelashes. EGFR TKI-associated eyelash trichomegaly has been
rarely reported and its incidence remains unknown (4). In this study, a Chinese female patient
with NSCLC that developed eyelash trichomegaly following the
administration of erlotinib is presented. To the best our
knowledge, this is the first case of trichomegaly associated with
erlotinib treatment to be reported in the literature. As lung
cancer physicians administer EGFR TKIs with increasing frequency,
this untoward effect requires attention.
Case report
In May 2011, a 65-year-old Chinese female with no
history of smoking presented at the Second People's Hospital of
Yibin (Yibin, China) with isolated coughing that had persisted for
several months. A chest computed tomography (CT) scan revealed a
2.8×3 cm mass on the upper lobe of the right lung and widespread
bilateral pulmonary nodules consistent with metastases (Fig. 1A and B). A bone scan revealed the
presence of multiple metastases (pelvis and spine). However, no
evidence of metastases was detected in a brain magnetic resonance
imaging scan. Subsequently, a CT-guided fine-needle aspirate biopsy
of the lung lesion was performed. Immunohistology revealed that the
tumor cells were positive for cytokeratin 7, thyroid transcription
factor 1, NapsinA and EGFR, and a diagnosis of adenocarcinoma was
determined. Polymerase chain reaction-based DNA sequencing of the
EGFR gene revealed an activating mutation (L858R) in exon 21
of the EGFR gene. The patient declined to undergo systemic
chemotherapy treatment due to concerns regarding possible side
effects. In May 2011, the patient was administered erlotinib
monotherapy, at a single daily dose of 150 mg. Within 4 weeks of
treatment, a significant symptomatic improvement in exercise
tolerance was observed and almost complete resolution of coughing.
Radiological imaging demonstrated a partial response to the
treatment (Fig. 1C and D). During the
6-month erlotinib treatment, the patient experienced a moderate
rash on her face, chest and back, which was treated with topical
clindamycin. In addition, after 5 months of treatment, the
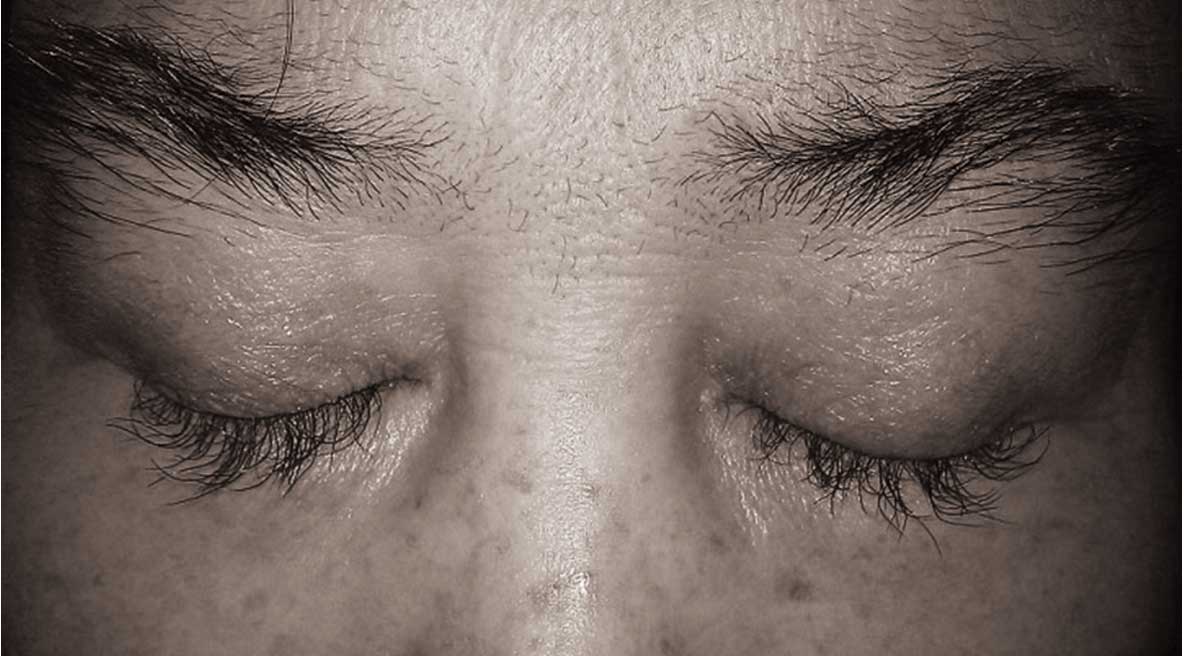
patient's eyelashes were overgrowing, showing increased length and
thickness (trichomegaly) which caused visual disturbance, and
necessitated trimming (Fig. 2).
Despite the initial response to erlotinib, an
assessment performed in November 2011 revealed progression of the
cancer and, after approximately 2 weeks, the patient succumbed to
the disease.
The current study was approved by the Ethics
Committee of The Second People's Hospital of Yibin. Written
informed consent was obtained from the patient's family.
Discussion
Eyelash trichomegaly is defined as the increased
length, thickness, stiffness, curling and pigmentation of the
eyelashes (7). This condition was
initially identified in patients with certain congenital syndromes,
including the Oliver-McFarlane syndrome (8), Cornelia de Lange syndrome (9) or familial hypertrichosis (10). Acquired trichomegaly is also
associated with specific drugs (11,12) and
other diseases, including vernal keratoconjunctivitis and atopic
dermatitis (13,14). However, eyelash trichomegaly is not a
drug-limiting side effect.
Recently, with the wide use of erlotinib in NSCLC
patients, an increased number of cutaneous adverse effects,
including acneiform rash or pruritus, have been observed (6). Erlotinib-associated eyelash trichomegaly
has been reported only in a small number of case reports (4,5,15–17). To
the best of our knowledge, erlotinib-induced trichomegaly has not
been previously reported in Chinese patients. Additionally, the
pathogenesis of these symptoms associated with the administration
of erlotinib is largely unknown. A previous study proposed that
systemic inhibition of EGFRs with erlotinib not only affects the
apoptosis and proliferation of cancerous cells, but is also able to
affect the progression of hair follicles from the anagen to the
telogen phase (18). This leads to an
aberrant anagen phase and subsequently to abnormal hair growth
(18), which can stimulate the
formation of a disorganized hair follicles.
The EGFR, a transmembrane glycoprotein, is a member
of the tyrosine kinase growth factor receptor family (19) and is expressed in the vast majority of
patients with NSCLC. The most common EGFR mutations in patients
with NSCLC include a deletion in exon 19 (E19del) and a mutation in
exon 21 (L858R) (20); these two
mutations are predictive of the NSCLC patient response and survival
following EGFR TKI treatment (21,22). A
prospective head-to-head phase 3 study has demonstrated an overall
response rate of 83% with a median progression-free survival of
13.1 months following first-line erlotinib therapy in Chinese
patients with advanced NSCLC, who have tumor harboring an
activating EGFR mutation (exon 19 deletion, 52%; L858R mutation,
48%) (23). In addition, other
factors, such as the cutaneous rash severity, have been established
as positive predictive markers of the clinical benefits following
treatment (24). Similarly, the
patient of the current study presented an activating mutation
(L858R) in exon 21 and experienced a moderate rash on her face,
chest and back; however, the patient did not present similar
progression-free survival benefits. Considering the present case
along with similar cases previously reported in the literature
(5,16,25) led to
several interesting observations. Patients who manifested
trichomegaly also exhibited a poor progression-free survival (3–7
months), and disease progression occurred after 1–3 months of
trichomegaly. Therefore, eyelash trichomegaly may correlate with
the resistance of lung cancer to EGFR inhibitors.
Although trichomegaly is not a drug-limiting side
effect, it can obscure vision and has been reported to cause
corneal problems, including erosions (26), irritation (15,26),
infection and ulcers (17). Trimming
and epilation of the elongated eyelashes are the most common and
safe therapeutic options used by patients experiencing
drug-associated eyelash trichomegaly (4,15,17,26). In
conclusion, trichomegaly is a rare, EGFR TKI-associated effect.
Oncologists should be aware of this potential sequela, for which
referral to an ophthalmologist or dermatologist may be helpful.
References
|
1
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Shepherd FA, Rodrigues Pereira J, Ciuleanu
T, et al National Cancer Institute of Canada Clinical Trials Group:
Erlotinib in previously treated non-small-cell lung cancer. N Engl
J Med. 353:123–132. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mok TS, Wu YL, Thongprasert S, et al:
Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N
Engl J Med. 361:947–957. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Braiteh F, Kurzrock R and Johnson FM:
Trichomegaly of the eyelashes after lung cancer treatment with the
epidermal growth factor receptor inhibitor erlotinib. J Clin Oncol.
26:3460–3462. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jeon SH, Ryu JS, Choi GS, et al: Erlotinib
induced trichomegaly of the eyelashes. Tuberc Respir Dis (Seoul).
74:37–40. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Khozin S, Blumenthal GM, Jiang X, et al:
U.S. Food and Drug Administration approval summary: Erlotinib for
the first-line treatment of metastatic non-small cell lung cancer
with epidermal growth factor receptor exon 19 deletions or exon 21
(L858R) substitution mutations. Oncologist. 19:774–779. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gray H: Trichomegaly or movie lashes.
Stanford Med Bull. 2:157–158. 1944.
|
|
8
|
Oliver GL and McFarlane DC: Congenital
trichomegaly: With associated pigmentary degeneration of the
retina, dwarfism, and mental retardation. Arch Ophthalmol.
74:169–171. 1965. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kline AD, Krantz ID, Sommer A, et al:
Cornelia de Lange syndrome: Clinical review, diagnostic and scoring
systems and anticipatory guidance. Am J Med Genet A.
143A:1287–1296. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ziakas NG, Jogiya A and Michaelides M: A
case of familial trichomegaly in association with oculocutaneous
albinism type 1. Eye (Lond). 18:863–864. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cohen PR, Escudier SM and Kurzrock R:
Cetuximab-associated elongation of the eyelashes: Case report and
review of eyelash trichomegaly secondary to epidermal growth factor
receptor inhibitors. Am J Clin Dermatol. 12:63–67. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pascual JC, Bañuls J, Belinchon I, Blanes
M and Massuti B: Trichomegaly following treatment with gefitinib
(ZD1839). Br J Dermatol. 151:1111–1112. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pucci N, Novembre E, Lombardi E, et al:
Long eyelashes in a case series of 93 children with vernal
keratoconjunctivitis. Pediatrics. 115:e86–e91. 2005.PubMed/NCBI
|
|
14
|
Alpsoy E: Hypotrichosis, long eyelashes
and atopic dermatitis: A new syndrome? J Eur Acad Dermatol
Venereol. 18:374–375. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Carser JE and Summers YJ: Trichomegaly of
the eyelashes after treatment with erlotinib in non-small cell lung
cancer. J Thorac Oncol. 1:1040–1041. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Iacovelli R, Palazzo A, Trenta P, et al:
Trichomegaly of the eyelashe induced by erlotinib therapy. Lung
Cancer. 64 (Suppl 1):S59–2009. View Article : Google Scholar
|
|
17
|
Lane K and Goldstein SM:
Erlotinib-associated trichomegaly. Ophthal Plast Reconstr Surg.
23:65–66. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Vergou T, Stratigos AJ, Karapanagiotou EM,
et al: Facial hypertrichosis and trichomegaly developing in
patients treated with the epidermal growth factor receptor
inhibitor erlotinib. J Am Acad Dermatol. 63:e56–e58. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yarden Y and Sliwkowski MX: Untangling the
ErbB signalling network. Nat Rev Mol Cell Biol. 2:127–137. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hirsch FR and Bunn PA Jr: EGFR testing in
lung cancer is ready for prime time. Lancet Oncol. 10:432–433.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Miller VA, Riely GJ, Zakowski MF, et al:
Molecular characteristics of bronchioloalveolar carcinoma and
adenocarcinoma, bronchioloalveolar carcinoma subtype, predict
response to erlotinib. J Clin Oncol. 26:1472–1478. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sequist LV, Martins RG, Spigel D, et al:
First-line gefitinib in patients with advanced non-small-cell lung
cancer harboring somatic EGFR mutations. J Clin Oncol.
26:2442–2449. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhou C, Wu YL, Chen G, et al: Erlotinib
versus chemotherapy as first-line treatment for patients with
advanced EGFR mutation-positive non-small-cell lung cancer
(OPTIMAL, CTONG-0802): A multicentre, open-label, randomised, phase
3 study. Lancet Oncol. 12:735–742. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Petrelli F, Borgonovo K, Cabiddu M, Lonati
V and Barni S: Relationship between skin rash and outcome in
non-small-cell lung cancer patients treated with anti-EGFR tyrosine
kinase inhibitors: A literature-based meta-analysis of 24 trials.
Lung Cancer. 78:8–15. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bouché O, Brixi-Benmansour H, Bertin A,
Perceau G and Lagarde S: Trichomegaly of the eyelashes following
treatment with cetuximab. Ann Oncol. 16:1711–1712. 2005. View Article : Google Scholar
|
|
26
|
Shah NT, Kris MG, Pao W, et al: Practical
management of patients with non-small-cell lung cancer treated with
gefitinib. J Clin Oncol. 23:165–174. 2005. View Article : Google Scholar : PubMed/NCBI
|