Introduction
As the ninth most common carcinoma diagnosis
worldwide, >330,000 new cases of bladder carcinoma are diagnosed
and >130,000 mortalities occur annually (1). Upon initial diagnosis of bladder cancer,
approximately two-thirds of cases are diagnosed as non-muscle
invasive bladder cancer (NMIBC) and almost one-third as
muscle-invasive bladder cancer (MIBC). Although radical cystectomy
is the gold standard treatment strategy for patients with MIBC,
~50% of patients diagnosed with MIBC have imperceptible metastases
at the time of treatment of the primary tumor. Furthermore, almost
one-quarter of patients that undergo radical cystectomy present
with lymph node involvement at the time of surgery. Therefore, this
gold standard treatment strategy only provides a five-year survival
rate of ~50% (2). To improve these
results, peri-operative chemotherapy was introduced in the 1980s.
Further studies have indicated that treatment with cisplatin-based
neoadjuvant chemotherapy may significantly enhance overall survival
(3). However, as only ~50% of
patients with MIBC respond to cisplatin-based chemotherapy
(3), the identification of novel
therapeutic strategies for the treatment of bladder carcinoma is
required.
Metformin is an anti-diabetic agent that is
typically prescribed to treat type 2 diabetes; however, it has
recently received attention as a potentially useful therapeutic
agent for the treatment of certain types of cancer (4–7). Previous
studies have demonstrated that the combination of metformin and
typical chemotherapeutic agents may inhibit the proliferation of
breast cancer cells (8). Furthermore,
in animal experiments, metformin in combination with the
chemotherapeutic agent doxorubicin exhibited significant efficacy
in the extermination of cancer stem cells, compared with that of
doxorubicin alone (9). However,
whether metformin is able to increase the sensitivity of bladder
cancer to the chemotherapeutic agent cisplatin, as well as its
exact mechanism remains to be elucidated.
Therefore, the current study aimed to analyze the
antitumor efficacy of metformin treatment in combination with
cisplatin on bladder cancer cell lines and explore the potential
mechanism underlying this effect.
Materials and methods
Cell lines, antibodies and
reagents
Two human bladder cancer cell lines, T24 and BIU-87,
were obtained from the Shanghai Cell Bank (Shanghai, China) and
cultured with 10% fetal bovine serum in a 37°C humidified
atmosphere containing 5% CO2. Metformin, cisplatin, MTT
and dimethyl sulfoxide (DMSO) were all obtained from Sigma-Aldrich
(St. Louis, MO, USA). Metformin and cisplatin were dissolved in
DMSO at a concentration of 25 mmol/l, aliquoted and stored at
−20°C. Rabbit anti-human polyclonal IgG antibodies against
adenosine monophosphate-activated protein kinase [AMPK; AMPKα1/2
(H-300); catalog no. sc-25792], phosphorylated (p)-AMPK [p-AMPKα1/2
(Thr 172); catalog no. sc-33524], mammalian target of rapamycin
(mTOR; H-266; catalog no. sc-8319), p-mTOR (Ser 2448; catalog no.
sc-101738), AKT [Akt1/2/3 (H-136); catalog no. sc-8312], p-AKT
[p-Akt1/2/3 (Ser 473); catalog no. sc-33437] and β-actin (N-21;
catalog no. sc-130656) were obtained from Santa Cruz Biotechnology,
Inc. (Dallas, TX, USA), and mouse anti-human monoclonal antibodies
against cluster of differentiation 34 (CD34; clone QBEnd 10;
catalog no. IR632/IS632) and Ki-67 (clone MIB-1; catalog no.
IR626/IS626) were purchased from Dako North America, Inc.
(Carpinteria, CA, USA). Horseradish peroxidase-conjugated goat
anti-mouse IgG (Affinipure; catalog no. SA00001-2) secondary
antibodies were obtained from Proteintech (Wuhan, China). In
addition, the SP immunohistochemistry kit was purchased from
Beijing Zhongshan Golden Bridge Biotechnology Co., Ltd. (Beijing,
China) and Matrigel was purchased from BD Biosciences (Mountain
View, CA, USA).
Treatment strategy
On day 1, a total of 5,000 cells/well were plated
and grown on 96-well plates prior undergoing the following four
treatment protocols: Untreated control, metformin alone, cisplatin
alone or metformin in combination with cisplatin. On day 2, new
medium containing DMSO, metformin alone, cisplatin alone or
metformin in combination with cisplatin was added following removal
of the culture medium. The cells were incubated at 37°C in an
atmosphere of 5% CO2. Subsequently, assays were
performed on day 4.
Cell viability assay
The effect of cisplatin and metformin on cell
viability was analyzed by performing an MTT assay. Briefly, T24 or
BIU-87 cells were subjected to the aforementioned treatment
protocol with metformin (0.5 µM) and/or cisplatin (2 µm) for 0, 24,
36 or 48 h. Following removal of the medium and the addition of MTT
solution, the cells were incubated for 1 h. Subsequently, the MTT
solution was replaced with 100 µl DMSO. Thereafter, the optical
density (OD) of the cells was measured using an xMark™ Microplate
Absorbance Spectrophotometer (Bio-Rad Laboratories, Inc., Hercules,
CA, USA; catalog no. 168–1150) at a wavelength of 560 nm. Cell
viability (%) was calculated using the following equation:
ODtreated wells / ODcontrol wells × 100%.
Cell cycle analysis
T24 and BIU-87 cells were treated as follows:
Metformin (0.5 µM), cisplatin (1 µM), metformin and cisplatin or
DMSO as a control for 48 h. Following treatment, cells were
collected by trypsin digestion, washed in phosphate-buffered saline
(PBS) and incubated for 24 h in 70% ethanol. Cells were resuspended
in PBS containing 10 µg/ml propidium iodide and incubated in the
dark for 30 min at 4°C. The DNA content of the cells was analyzed
by fluorescence-activated cell sorting (FACS) using a FACScan II
and CellQUEST software (Beckton Dickinson, Moutain View, CA, USA).
All remaining experimental steps for FACS analysis were performed
as previously described (10).
Western blot analysis
Cells were seeded in 96-well microtiter plates and
subjected to the aforementioned treatments, prior to the
preparation of cell lysates, followed by protein fractionation and
transfer, performed as previously described (11). The following human reactive antibodies
were used for western blot analysis: primary AMPK (1:100 dilution),
anti-p-AMPK (1:1,000 dilution), anti-mTOR (1:2,000 dilution) and
anti-p-mTOR (1:1,000 dilution) antibodies and corresponding
horseradish peroxidase-conjugated goat anti-mouse IgG secondary
antibodies, at dilutions of 1:2,000, 1:4,000, 1:2,000 and 1:5,000,
respectively. Protein expression was then visualized using Luminol
reagent (Santa Cruz Biotechnology, Inc.) and an Odyssey® scanner
(LI-COR Biosciences, Lincoln, NE, USA).
Establishment and treatment of mouse
xenograft models
In the present study, all animals were obtained
from, and experiments authorized by, the Experimental Animal Center
of Chongqing Medical University (Chongqing, China). A total of 32
four-week-old male athymic BALB/c nu/nu mice (mean weight, 24.2 g)
were kept in individually ventilated cage systems with sterilized
bedding at 20–25°C. The mice received sterlized food and acidified
water daily and were exposed to 12 h light/dark cycles. BIU-87
cells (2×107) in 100 µl RPMI-1640 and 200 µl Matrigel
were subcutaneously injected into the left hip of each mouse. Mice
were administered with metformin and cisplatin for four weeks and
tumor volumes were subsequently measured, as described previously
(12–14).
Immunohistochemical analysis of
xenograft tumors
Mice were sacrificed by dislocation of the cervical
vertebra, and tumors were surgically excised, fixed in 10%
formalin, embedded in paraffin and cut into 5-mm thick sections for
immunohistochemical staining. All staining steps were performed
according to the manufacturer's instructions. The primary
antibodies and incubation conditions were as follows: Mouse
monoclonal anti-Ki-67 at a 1:500 dilution in ready-to-use form at
room temperature for 30 min, rabbit polyclonal anti-AKT in a 1:500
dilution at room temperature for 2 h, rabbit polyclonal anti-p-AKT,
in a 1:1,000 dilution at room temperature for 2 h, and rabbit
polyclonal anti-CD34 in a 1:1,500 dilution at 4°C overnight. The
microvessel density (MVD) score was calculated as previously
described (15).
Statistical analysis
Prism software (version 5.0; GraphPad Software,
Inc., San Diego, CA, USA) was used to construct charts and perform
all statistical analyses. Data were analyzed using the Student's
t-test or one-way analysis of variance (Tukey's post test),
as indicated in the figure legends, and are expressed as the mean ±
standard deviation. P<0.05 was considered to indicate a
statistically significant difference.
Results
Metformin and cisplatin inhibit T24
and BIU-87 cell viability in vitro
The MTT assay results demonstrated that a metformin
concentration of >1 and >2 µM significantly inhibited T24 and
BIU-87 cell viability, respectively (Fig.
1A). By contrast, a significant change in the viability of T24
and BIU-87 cells was induced by cisplatin at concentrations of 3
and 4 µM, respectively (Fig. 1B).
Therefore, a concentration of 0.5 µM metformin and 2 µM cisplatin
was selected for use in the subsequent experiments.
Combined treatment with metformin in
combination with cisplatin inhibits bladder cancer cell
proliferation
To observe the efficacy of metformin alone or in
combination with cisplatin on the proliferation of T24 and BIU-87
cells, viable cells were treated with the indicated concentrations
of the therapeutic agents for 0–48 h (Fig. 1C and D). The MTT assay results
indicated that metformin in combination with cisplatin inhibited
T24 and BIU-87 cell proliferation in a time-dependent manner.
Furthermore, a significant decrease in the proliferation of T24 and
BIU-87 cells was observed in the combined treatment group compared
with that of the metformin and cisplatin alone treatment groups at
48 h (P<0.05).
Combined treatment with metformin and
cisplatin alters bladder cancer cell cycle distribution
Following the treatment of T24 and BIU-87 cells with
metformin alone, cisplatin alone or cisplatin in combination with
metformin for 48 h, PI staining and FACS were used to analyze
changes in the cell cycle in response to these agents. Cell cycle
analysis identified that co-treatment resulted in an enhanced
sub-G1 population when compared with monotherapy, for the two
investigated cells types (Fig. 2).
Thus, the current results indicated that the pro-apoptotic effect
of metformin combined with cisplatin is greater than that of
metformin and cisplatin treatment alone.
Cisplatin and metformin treatment
alter the expression of proteins associated with the AMPK and mTOR
signaling pathways in bladder cancer cell lines
Western blot analysis was performed to investigate
the mechanism underlying the effects of metformin and cisplatin
co-treatment on the cell cycle. In the T24 and BIU-87 cells,
cisplatin-induced downregulation of p-mTOR was reinforced by
co-treatment with metformin, while cisplatin-induced downregulation
of p-AMPK was not altered by co-treatment with metformin. However,
no marked changes in mTOR and AMPK expression were identified in
the two types of cell (Fig. 3).
Cisplatin and metformin co-treatment
decreases the size and weight of BIU-87 xenograft tumors
Considering that the aim of the present study was to
establish a novel therapeutic strategy for the treatment of
advanced bladder cancer cells, which are not sensitive to
cisplatin, the BIU-87 cell line was selected for the in vivo
investigation as its 50% inhibitory concentration following
treatment with cisplatin for 48 h was significantly higher than
that of T24 cells (data not shown). To assess the in vivo
antitumor efficacy of co-treatment with cisplatin and metformin,
nude mice bearing BIU-87 tumor xenografts were treated with
cisplatin, metformin or a combination of the two agents for four
weeks. The doses of cisplatin and metformin used were selected
based on the results of initial investigations aimed at identifying
the doses required to inhibit the growth of bladder cancer
xenografts (data not shown). The size and weight of the tumor
xenografts were significantly decreased following treatment with
cisplatin or metformin alone compared with those of the untreated
(control) xenografts (data not shown). Furthermore, the growth of
combined cisplatin and metformin-treated xenografts was
significantly inhibited when compared with monotherapy-treated
xenografts. However, no significant body weight loss was observed
during the four-week treatment process, indicating that the
treatment strategies were well-tolerated with no obvious toxicity.
Thus, the results of the present study indicate that the antitumor
efficacy of cisplatin combined with metformin is greater than that
of cisplatin and metformin alone.
Cisplatin in combination with
metformin decreases tumor growth and angiogenesis
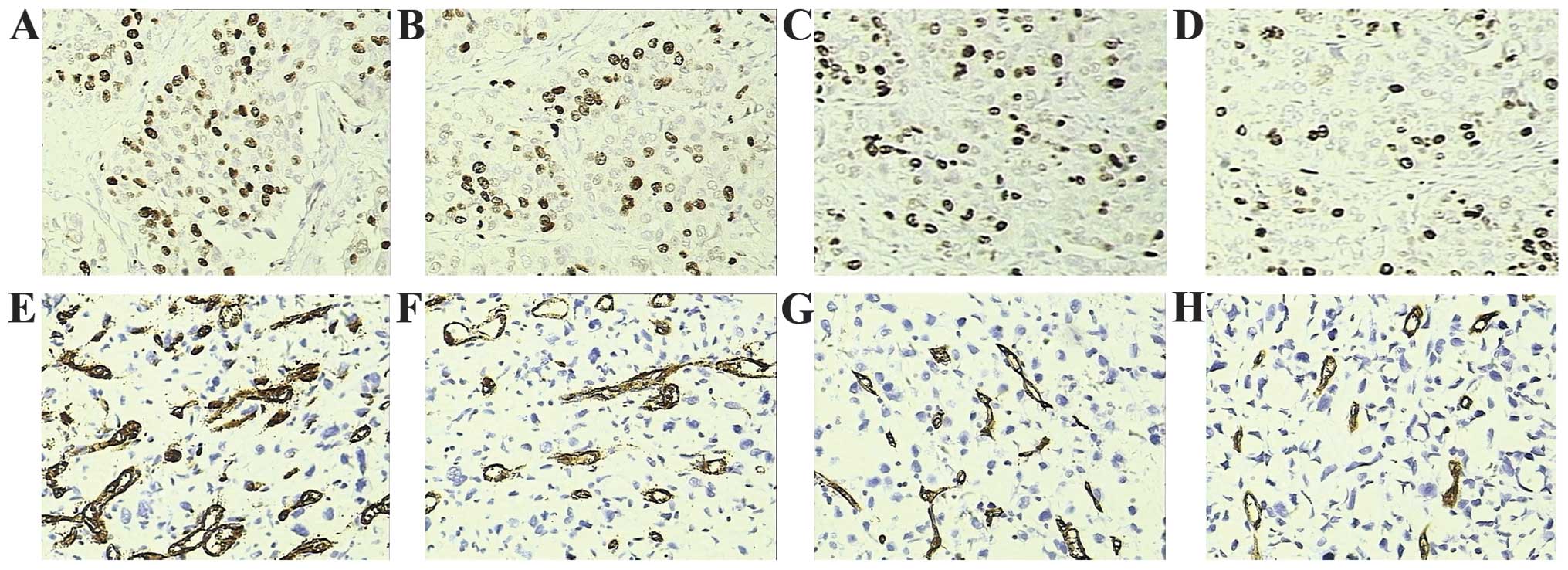
To investigate the tumor growth and angiogenesis
mechanisms of cisplatin and metformin, immunostainings of Ki-67 and
CD34 were performed, respectively (Fig.
4). The Ki-67 labeling index data revealed that tumors from
mice treated with cisplatin without metformin had decreased levels
of proliferation when compared with those of the control mice
(Fig. 4A and B). Furthermore,
combined treatment with metformin and cisplatin resulted in a
marked decrease in the Ki-67 labeling index compared with that of
cisplatin monotherapy (Fig. 4B and
D). In agreement with the aforementioned data, MVD analysis
revealed that mice treated with cisplatin or metformin alone
exhibited decreased MVD compared with that of the control group
(Fig. 4E–G). Similarly, a marked
decrease in MVD was observed in the metformin and cisplatin
co-treatment group compared with that of the cisplatin monotherapy
group (Fig. 4A and H).
Cisplatin and metformin co-treatment
decreases the expression on p-AKT in BIU-87 xenografts
Finally, the metformin-induced inhibition of tumor
growth and angiogenesis was analyzed by investigating the change in
AKT protein expression levels associated with the AKT/mTOR
signaling pathway. AKT expression was assessed by western blotting
using equal weights of BIU-87 xenografts from nude mice. Expression
of AKT protein was not significantly altered in the
metformin-treated cells compared with that of the control cells.
However, the expression levels of p-AKT protein were significantly
decreased in the co-treatment group compared with those the
monotherapy treatment groups (P<0.05) (data not shown).
Discussion
Human bladder cancer is one of the most fatal types
of cancer in the world and, in certain countries, the incidence
rate is increasing (1). In addition
to surgical treatment, systematic chemotherapy is a common
therapeutic strategy in the treatment of bladder cancer,
particularly for patients with advanced and metastatic bladder
cancer (16,17). However, despite rapid shrinkage of the
tumor mass following chemotherapy, the chemoresistance of carcinoma
cells typically results in subsequent recurrence and metastasis of
the cancer. For example, although a cisplatin-based combination
chemotherapeutic strategy is a realistic alternative to cystectomy
in advanced or metastatic bladder cancer, the development of
cisplatin resistance is common amongst patients with bladder cancer
(3). Recent studies have demonstrated
that metformin exhibits an antiproliferative effect on carcinoma
cells, directly as well as indirectly, by ameliorating insulin
sensitivity, and decreasing hyperinsulinemia, respectively
(4,18,19).
Therefore, the present study aimed to observe whether the
application of cisplatin in combination with metformin exhibited
greater efficacy compared with that of cisplatin monotherapy for
the treatment of human bladder cancer.
In the present study, only a marginal decrease in
the viability of cells treated with 2 µM cisplatin or 0.5 µM
metformin alone was identified. However, cell viability was
significantly reduced by co-treatment with cisplatin and metformin
at these concentrations for 48 h. These results indicated that
combined treatment with cisplatin and metformin may be more
effective against T24 and BIU-87 cell proliferation in vitro
than treatment with cisplatin and metformin alone. Subsequently,
the combined effect of cisplatin and metformin treatment was
compared with that of the effect of cisplatin treatment alone. Cell
cycle assays revealed an increased sub-G1 phase cell population in
the cisplatin and metformin treatment groups in all cell lines. In
particular, the ratio of sub-G1 cells was increased most potently
by co-treatment compared with that of cisplatin and metformin
monotherapy. The mechanism of this combined effect was then
examined by measuring the expression levels of proteins associated
with cellular AMPK and mTOR signaling. AMPK is an energy receptor
within cells. Thus, a change in intercellular pressure or the
consumption of glucose may increase the AMP/ adenosine triphosphate
(ATP) ratio, activating AMPK expression by phosphorylation, and
promoting the synthesis and utilization of glucose reabsorption.
Therefore, AMPK is a critical regulatory pathway under normal
physiological conditions (20).
However, the predominant function of AMPK in tumors is to inhibit
tumor cell proliferation and regulate apoptosis. It has been
reported that metformin may induce a loss of mitochondrial membrane
potential and inhibition of ATP production, which may consequently
activate expression of the AMPK protein in prostate cancer cells
(21,22). The results of the present study
demonstrated that treatment with cisplatin increased the expression
of p-AMPK; however, no significant change in the expression of
p-AMPK was detected following treatment with metformin. Therefore,
it appears that metformin does not inhibit the proliferation of
bladder cancer cells via the AMPK signaling pathway. mTOR is a
member of phosphatidyl inositol kinase-related enzyme family and is
key in mediating the cell proliferation process (23). A previous study revealed that mTOR
expression was increased in the majority cancer patients, and that
hyperthyroidism and the phosphorylation of mTOR may promote tumor
cell proliferation, differentiation, cell cycle regulation, growth
and angiogenesis. However, mTOR expression may also be associated
with the insensitivity to chemotherapy and targeted therapy of
malignant tumors (24). To explore
the mechanism by which metformin inhibits bladder cancer cells, the
present study investigated whether p-mTOR protein expression
levels, which represent the degree of activation of the mTOR
signaling pathway, were altered following treatment with cisplatin
alone or in combination with metformin by western blot analysis.
The present study verified the role of cisplatin in decreasing the
transcriptional activity of mTOR and determined that cisplatin in
combination with metformin exerted a more significant inhibitory
effect on the mTOR signaling pathway.
Subsequently, whether cisplatin and metformin
exhibited a combined effect on the xenograft tumor was examined. It
was identified that the volume and weight of the xenograft tumor
were significantly reduced by co-treatment with cisplatin and
metformin, compared with the control and monotherapy groups.
Furthermore, there was no apparent loss of body weight in the mice
co-treated with cisplatin and metformin. Considering these results,
it appears that combination therapy with cisplatin and metformin
may respresent a safe and effective strategy for the inhibition of
BIU-87 cell proliferation in vivo. To investigate the
mechanisms underlying this combination effect, the protein
expression levels of Ki-67 and CD34 were determined in isolated
xenografts using immunohistochemical analysis. Ki-67 protein is
typically considered to be a cell proliferation activity biomarker
and research tool, with decreased Ki-67 protein expression
indicating a general decline in cell proliferation (25). Thus, the present study assessed the
Ki-67 labeling index and MVD, and clarified that combination
therapy significantly inhibited tumor growth and angiogenesis
compared with monotherapy. AKT is a major signaling molecule
involved in regulating cell survival, proliferation, growth and
angiogenesis. Furthermore, mTOR is a key substrate of AKT, with AKT
able to activate mTOR and its downstream signaling pathways by
directly phosphorylating serine sites on mTOR (26). In the present study, expression of
p-AKT, the activated form of AKT, was significantly decreased in
xenograft tumors following treatment with combined therapy of
cisplatin and metformin, compared with that of the monotherapy
group. This data further indicated that metformin enhances the
cisplatin sensitivity of bladder cancer cells via the AKT/mTOR
signaling pathway, as opposed to the AMPK signaling pathway.
The present study was limited by the use of only two
types of bladder carcinoma cell and the generation of in
vivo data from a xenograft tumor of a single cell line
(BIU-87). Therefore, the evaluation of additional bladder cancer
cell lines is required to establish the clinical potential of
metformin and cisplatin co-administration for the treatment of
bladder cancer.
In conclusion, the present study demonstrated that
cisplatin combined with metformin had a synergistic anti-tumor
effect in the treatment of human bladder carcinoma cells. In
addition, the current study may encourage the future joint clinical
application of metformin and cisplatin in bladder cancer. However,
additional in vivo validation is required to determine
whether the clinical application of this treatment strategy is able
to achieve a satisfactory clinical outcome.
Acknowledgements
The authors would like to thank the Experimental
Animal Center of Chongqing Medical University (Chongqing, China)
for providing the animal research facility.
References
|
1
|
Ploeg M, Aben KK and Kiemeney LA: The
present and future burden of urinary bladder cancer in the world.
World J Urol. 27:289–293. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Stenzl A, Cowan NC, De Santis M, Kuczyk
MA, Merseburger AS, Ribal MJ, Sherif A and Witjes JAEuropean
Association of Urology: Treatment of muscle-invasive and metastatic
bladder cancer: Update of the EAU guidelines. Actas Urol Esp.
36:449–460. 2012.(In Spanish).
|
|
3
|
Herr HW, Dotan Z, Donat SM and Bajorin DF:
Defining optimal therapy for muscle invasive bladder cancer. J
Urol. 177:437–443. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bowker SL, Majumdar SR, Veugelers P and
Johnson JA: Increased cancer-related mortality for patients with
type 2 diabetes who use sulfonylureas or insulin: Response to
Farooki and Schneider. Diabetes Care. 29:1990–1991. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Evans JM, Donnelly LA, Emslie-Smith AM,
Alessi DR and Morris AD: Metformin and reduced risk of cancer in
diabetic patients. BMJ. 330:1304–1305. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ropelle ER, Pauli JR, Zecchin KG, Ueno M,
de Souza CT, Morari J, Faria MC, Velloso LA, Saad MJ and
Carvalheira JB: A central role for neuronal adenosine
5′-monophosphate-activated protein kinase in cancer-induced
anorexia. Endocrinology. 148:5220–5229. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Franciosi M, Lucisano G, Lapice E,
Strippoli GF, Pellegrini F and Nicolucci A: Metformin therapy and
risk of cancer in patients with type 2 diabetes: Systematic review.
PLoS One. 8:e715832013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu B, Fan Z, Edgerton SM, Deng XS,
Alimova IN, Lind SE and Thor AD: Metformin induces unique
biological and molecular responses in triple negative breast cancer
cells. Cell Cycle. 8:2031–2040. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hirsch HA, Iliopoulos D, Tsichlis PN and
Struhl K: Metformin selectively targets cancer stem cells, and acts
together with chemotherapy to block tumor growth and prolong
remission. Cancer Res. 69:7507–7511. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Abdelnour-Berchtold E, Cerantola Y, Roulin
D, Dormond-Meuwly A, Demartines N and Dormond O: Rapamycin-mediated
FOXO1 inactivation reduces the anticancer efficacy of rapamycin.
Anticancer Res. 30:799–804. 2010.PubMed/NCBI
|
|
11
|
Iwata H, Sato H, Suzuki R, Yamada R,
Ichinomiya S, Yanagihara M, Okabe H, Sekine Y, Yano T and Ueno K: A
demethylating agent enhances chemosensitivity to vinblastine in a
xenograft model of renal cell carcinoma. Int J Oncol. 38:1653–1661.
2011.PubMed/NCBI
|
|
12
|
Jensen MM, Jørgensen JT, Binderup T and
Kjaer A: Tumor volume in subcutaneous mouse xenografts measured by
microCT is more accurate and reproducible than determined by
18F-FDG-microPET or external caliper. BMC Med Imaging. 8:162008.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu LZ, Zhou XD, Qian G, Shi X, Fang J and
Jiang BH: AKT1 amplification regulates cisplatin resistance in
human lung cancer cells through the mammalian target of
rapamycin/p70S6K1 pathway. Cancer Res. 67:6325–6332. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rocha GZ, Dias MM, Ropelle ER, et al:
Metformin amplifies chemotherapy-induced AMPK activation and
antitumoral growth. Clin Cancer Res. 17:3993–4005. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Miyake M, Anai S, Fujimoto K, et al:
5-fluorouracil enhances the antitumor effect of sorafenib and
sunitinib in a xenograft model of human renal cell carcinoma. Oncol
Lett. 3:1195–1202. 2012.PubMed/NCBI
|
|
16
|
Juffs HG, Moore MJ and Tannock IF: The
role of systemic chemotherapy in the management of muscle-invasive
bladder cancer. Lancet Oncol. 3:738–747. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gupta S and Mahipal A: Role of systemic
chemotherapy in urothelial urinary bladder cancer. Cancer Control.
20:200–210. 2013.PubMed/NCBI
|
|
18
|
Fonseca EA, de Oliveira MA, Lobato NS,
Akamine EH, Colquhoun A, de Carvalho MH, Zyngier SB and Fortes ZB:
Metformin reduces the stimulatory effect of obesity on in vivo
Walker-256 tumor development and increases the area of tumor
necrosis. Life Sci. 88:846–852. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pollack MN: Insulin, insulin-like growth
factors, insulin resistance, and neoplasia. Am J Clin Nutr.
86:s820–s822. 2007.PubMed/NCBI
|
|
20
|
Jalving M, Gietema JA, Lefrandt JD, de
Jong S, Reyners AK, Gans RO and de Vries EG: Metformin: Taking away
the candy for cancer? Eur J Cancer. 46:2369–2380. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kaaks R, Lukanova A and Kurzer MS:
Obesity, endogenous hormones, and endometrial cancer risk: A
synthetic review. Cancer Epidemiol Biomarkers Prev. 11:1531–1543.
2002.PubMed/NCBI
|
|
22
|
Ben Sahra I, Laurent K, Giuliano S,
Larbret F, Ponzio G, Gounon P, Le Marchand-Brustel Y,
Giorgetti-Peraldi S, Cormont M, Bertolotto C, et al: Targeting
cancer cell metabolism: The combination of metformin and
2-deoxyglucose induces p53-dependent apoptosis in prostate cancer
cells. Cancer Res. 70:2465–2475. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Harris TE and Lawrence JC Jr: TOR
signaling. Sci STKE. 2003:re152003.PubMed/NCBI
|
|
24
|
Zakikhani M, Blouin MJ, Piura E and Pollak
MN: Metformin and rapamycin have distinct effects on the AKT
pathway and proliferation in breast cancer cells. Breast Cancer Res
Treat. 123:271–279. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jiang XL, Zhang Y, Luo CL and Wu XH:
Targeting renal cell carcinoma with gambogic acid in combination
with sunitinib in vitro and in vivo. Asian Pac J Cancer Prev.
13:6463–6468. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Brazil DP and Hemmings BA: Ten years of
protein kinase B signalling: A hard Akt to follow. Trends Biochem
Sci. 26:657–664. 2001. View Article : Google Scholar : PubMed/NCBI
|