Introduction
Nestin, a 200-220-kDa intermediate filament protein,
is considered to be a neuronal stem cell marker. Nestin has been
shown to be expressed in newly formed blood vessels and tumour
cells in various neoplasms, including glioblastoma, and prostate,
pancreatic, lung, colorectal and breast cancer (1–6). The
expression of nestin indicates a poor prognosis in patients with
colorectal cancer and prostate cancer, where nestin expression is
found not in the cancer cells, but rather in the vascular
endothelial cells, suggesting one or more significant roles of
nestin in tumour neovascularization (2,5).
By contrast, the expression of nestin has been
confirmed in the tumour cytoplasm and vessel endothelial cells in
glioblastoma (7,8). Liu et al reported that nestin was
strongly expressed in two anaplastic thyroid cancer (ATC) tissues,
accompanied with areas of nestin-negative differentiated thyroid
cancer within the same samples. The study suggested that the
expression of nestin with a concomitant loss of E-cadherin was
associated with the stemness phenotype of ATC (9). To the best of our knowledge, the
prevalence and clinical significance of nestin expression in ATC
have not been reported.
ATC is characterized by its extremely rapid
progression and extremely poor prognosis. Curative surgery is rare,
as patients with ATC often demonstrate advanced-stage disease and
multiple distant metastases even at the initial presentation.
External beam radiotherapy (EBRT) may be able to temporarily
improve the local disease, but the majority of ATC patients
eventually suffer from rapid local recurrence (10). ATC treatment with chemotherapy is not
standardized and has shown only limited efficacy (11). It is thus of utmost importance to
identify the ATC patients for whom anticancer therapy can be
effective and the optimal treatment strategy.
Several clinical findings have been reported as
clinical indicators of prognosis in ATC patients, including acute
progression of local symptoms, leukocytosis, distant metastasis and
a tumour size >5 cm (12). A
biomarker that can indicate the prognosis of ATC has not been
reported. In the present study, clinically obtained samples were
used and the association between nestin expression and clinical
characteristics, including the prognosis in patients with ATC, were
evaluated in an attempt to reveal the significance of nestin
expression.
Materials and methods
Patients
A total of 23 patients (12 males and 11 females) who
were diagnosed with ATC histologically between 1997 and 2013 at
Osaka City University Hospital (Osaka, Japan) were analyzed. The
patient characteristics are shown in Table I. The median age of the patients was
73.0 years (range, 31–88 years). All but 1 patient succumbed due to
ATC. The median follow-up period was 139 days, with a range of
10–1,901 days.
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
| Characteristic | Value |
|---|
| Patients | 23 |
| Gender |
|
|
Female | 11 |
| Male | 12 |
| Age, years |
|
| Median
(range) | 73 (31–88) |
|
>70 | 14 |
| ≤70 | 9 |
| Maximal tumour
diameter, cm |
|
| Median
(range) | 6 (2.5–10) |
|
>5 | 16 |
| ≤5 | 7 |
| Lymph node
metastasis |
|
|
Positive | 21 |
|
Negative | 2 |
| Distant
metastasis |
|
|
Positive | 14 |
|
Negative | 9 |
| Acute symptoms |
|
|
Positive | 19 |
|
Negative | 4 |
| White blood cell
count |
|
|
>10,000 | 7 |
|
≤10,000 | 16 |
| Prognostic
indexa |
|
| 0,1 | 4 |
|
2,3,4 | 19 |
| Surgical
treatment | 15 |
| Complete
resection | 7 |
|
incomplete resection or
inoperative | 16 |
| Chemotherapy |
|
| No | 8 |
| Yes | 15 |
| EBRT |
|
| No | 10 |
| Yes | 13 |
In total, 13 patients underwent EBRT with at least
20 Gy; 2 underwent EBRT only, 2 underwent EBRT and surgery, 3
received chemoradiation and surgery and 6 as part of chemoradiation
therapy. Grossly complete resection (R0 or R1) was performed in 7
(47%) of the 15 patients who underwent surgery. In 15 patients,
chemotherapy was conducted with EP, a protocol using a combination
of etoposide and cisplatinum (6 patients) (13), and/or a taxane, either paclitaxel or
docetaxel (11 patients; including overlap in protocols). Written
informed consent was obtained from each patient, and the Osaka City
University Hospital Institutional Ethics Committee approved the
study protocol (#926).
Immunohistochemical staining
All tissues used in the present study were obtained
by needle biopsy, surgery or autopsy. Tissue specimens were fixed
in 10% neutral-buffered formalin immediately after resection and
embedded in paraffin. Sections of paraffin-embedded tissue (4-µm
thick) were prepared, and immunohistochemical staining for nestin
was performed using the avidin-biotin-peroxidase complex method as
previously described (14). Once the
specimens were deparaffinized and hydrated, they were heated for 15
min at 105°C in Target Retrieval Solution (Dako, Carpinteria, CA,
USA). The slides were incubated overnight at 4°C with anti-human
nestin mouse monoclonal antibody (clone 10c2; dilution 1:50; Santa
Cruz Biotechnology, Inc., Dallas, TX, USA). The immunoreaction was
visualized using the Histofine Simple Stain™ MAX PO (M) kit
(Nichirei Biosciences Inc., Tokyo, Japan).
The final evaluation of immunoreactivity was decided
by two authors without knowledge of the patients' clinical
characteristics. The staining intensity of newly formed blood
vessels within the samples was regarded as an internal positive
control. The cytoplasmic staining intensity of the cancer cells was
evaluated as a specific reaction, and positive staining was defined
when the specific immunoreaction was observed in ≥10% of the ATC
cells. The micrograph images were captured with an Olympus BX43F
microscope (Olympus, Tokyo, Japan).
Statistical analysis
All statistical analyses were performed using the
SPSS software program (SPSS 17.0; SPSS, Chicago, IL, USA). Fisher's
exact test was used to compare the prevalence or distribution of
two variables. Survival data were estimated by the Kaplan-Meier
method, and the log-rank test was used for the univariate survival
analysis. The Cox proportional hazards model was used for the
multivariate survival analysis. P<0.05 was considered to
indicate a statistically significant difference.
Results
Representative images of ATC tissues showing
positive or negative immunohistochemical staining for nestin are
shown in Fig. 1. Overall, 6 of the 23
cases (26.1%) were judged to show positive staining. In those
cases, cytoplasmic staining of nestin was observed in various
populations of tumour cells, and was distributed partially or
widely. Clear nestin expression was observed in the endothelial
cells of the newly formed blood vessels, and was used as an
internal positive control. The correlations between nestin
expression and the clinical factors of the patients were also
examined (Table II). Age, gender,
tumour size, prevalence of lymph node metastasis and distant
metastases were not found to be correlated with nestin expression
in the tumours.
 | Table II.Association between nestin expression
and clinical factors. |
Table II.
Association between nestin expression
and clinical factors.
|
| Nestin |
|
|---|
|
|
|
|
|---|
| Characteristic | Positive | Negative | P-value |
|---|
| Patients | 6 | 17 |
|
| Gender |
|
|
|
|
Female | 4 | 7 | 0.371 |
| Male | 2 | 10 |
|
| Age, years |
|
|
|
| Median
(range) | 66.5 (31–78) | 74 (57–88) |
|
|
>70 | 2 | 12 | 0.162 |
|
≤70 | 4 | 5 |
|
| Maximal tumour
diameter, cm |
|
|
|
| Median
(range) | 4.5 (3.4–10) | 6.4 (2.5–10) |
|
|
>5 | 3 | 13 | 0.319 |
| ≤5 | 3 | 4 |
|
| Lymph node
metastasis |
|
|
|
|
Positive | 6 | 15 | 1.000 |
|
Negative | 0 | 2 |
|
| Distant
metastasis |
|
|
|
|
Positive | 5 | 9 | 0.340 |
|
Negative | 1 | 8 |
|
| Acute symptoms |
|
|
|
|
Positive | 6 | 13 | 0.539 |
|
Negative | 0 | 4 |
|
| White blood cell
count |
|
|
|
|
≤10,000 | 5 | 11 | 0.621 |
| >10,000 | 1 | 6 |
|
| Prognostic
index |
|
|
|
|
0,1 | 0 | 4 | 0.539 |
| 2,3,4 | 6 | 13 |
|
| Surgical
treatment | 4 | 11 |
|
|
Complete resection | 1 | 6 | 0.621 |
|
Incomplete resection or
inoperative | 5 | 11 |
|
| Chemotherapy |
|
|
|
| No | 2 | 6 | 1.000 |
|
Yes | 4 | 11 |
|
| EBRT |
|
|
|
| No | 3 | 7 | 1.000 |
|
Yes | 3 | 10 |
|
For ATC patients, it has been reported that the
presence of acute symptoms, a large tumour (>5 cm in diameter),
distant metastasis and leukocytosis (>10,000/mm3) are
significant risk factors for poor prognosis, and that patients with
less than one of these four factors experience better survival
(12). In the present study, the
number of these four factors was defined as the prognostic index
(PI) according to the original report (12), and this was included as a clinical
indicator of the prognosis.
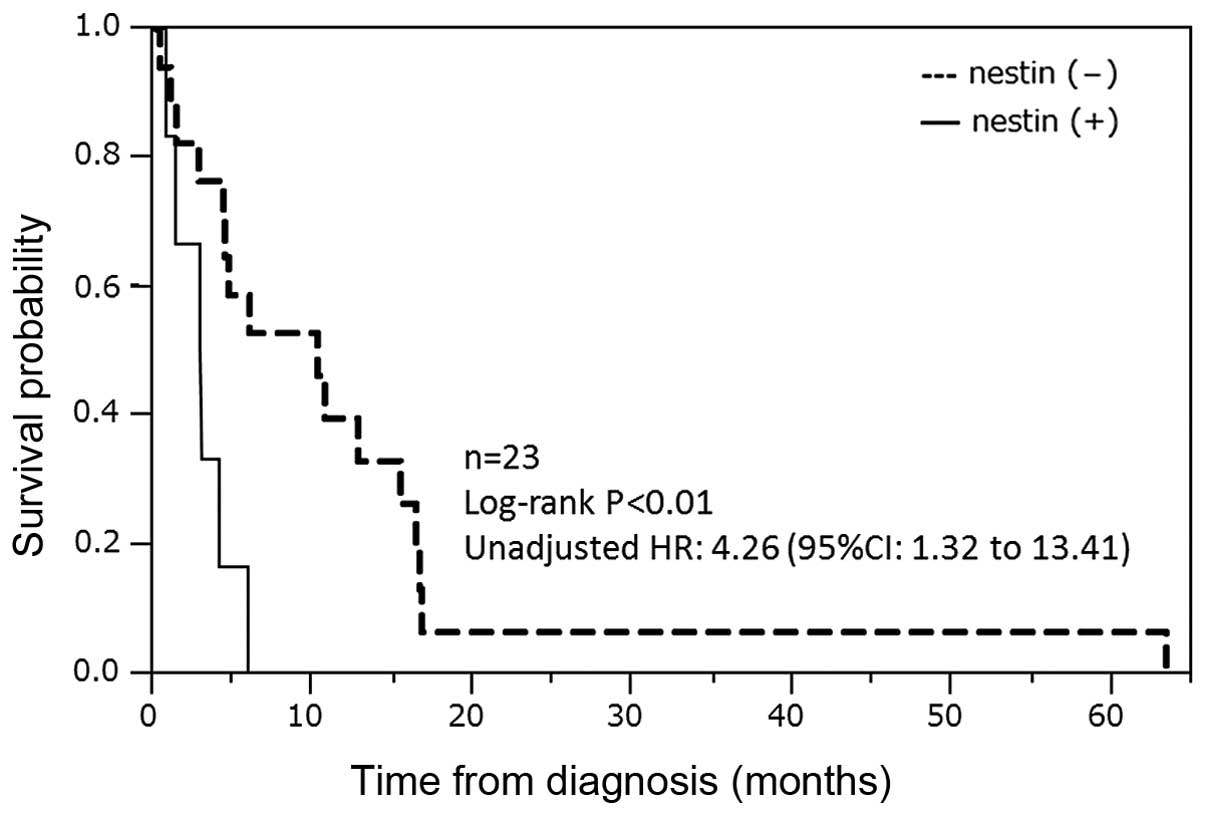
The Kaplan-Meier plot of the overall survival of the
ATC patients was determined according to the nestin expression
status in their tumours (Fig. 2). The
nestin-positive group (n=6) showed significantly poorer prognoses
compared with the nestin-negative group (n=17) [median survival
time (MST), 86.5 vs. 306 days; P<0.01].
Univariate and multivariate analyses demonstrated
that the patients with a nestin-expressing tumour or a high PI
score exhibited significantly poorer prognoses compared with the
patients without these factors (P=0.014; Table III). The multivariate analysis
demonstrated that the nestin expression status in the tumour
(P<0.01) and the PI of the patient (P<0.01) were mutually
independent prognostic factors, indicating the molecular and
clinical features of the ATC, respectively (Table IV).
 | Table III.Correlation between prognosis and
patient characteristics. |
Table III.
Correlation between prognosis and
patient characteristics.
| Factors | n | Median survival
(range), days | Survival ≥180 days,
n (%) | P-value |
|---|
| Patients | 23 | 139 (10–1901) | 9 (39.1) |
|
| Gender |
|
|
|
|
|
Female | 11 | 175 (39–502) | 4 (36.4) | 1.000 |
|
Male | 12 | 130 (10–1901) | 5 (41.7) |
|
| Age, years |
|
|
|
|
|
>70 | 14 | 180 (21–1901) | 7 (50.0) | 0.228 |
|
≤70 | 9 | 88 (10–491) | 2 (22.2) |
|
| Maximal tumour
diameter, cm |
|
|
|
|
|
>5 | 16 | 157 (30–498) | 6 (37.5) | 1.000 |
| ≤5 | 7 | 121 (10–1901) | 3 (42.9) |
|
| Lymph node
metastasis |
|
|
|
|
|
Positive | 21 | 132 (10–502) | 7 (33.3) | 0.142 |
|
Negative | 2 | 1042
(182–1901) | 2 (100.0) |
|
| Distant
metastasis |
|
|
|
|
|
Positive | 14 | 105 (10–1901) | 3 (21.4) | 0.077 |
|
Negative | 9 | 320 (83–502) | 6 (66.7) |
|
| Acute symptoms |
|
|
|
|
|
Positive | 19 | 129 (10–502) | 5 (26.3) | 0.014 |
|
Negative | 4 | 422 (182–1901) | 4 (100.0) |
|
| White blood cell
count |
|
|
|
|
|
>10,000 | 7 | 41 (10–1901) | 2 (28.6) | 0.657 |
|
≤10,000 | 16 | 177 (39–502) | 7 (43.8) |
|
| Prognostic
index |
|
|
|
|
|
0,1 | 4 | 422 (182–502) | 4 (100.0) | 0.014 |
|
2,3,4 | 19 | 129 (10–1901) | 5 (26.3) |
|
| Expression of
nestin |
|
|
|
|
|
Positive | 6 | 87 (10–175) | 0 (0.0) | 0.048 |
|
Negative | 17 | 306 (30–1901) | 9 (52.9) |
|
| Surgical
treatment |
|
|
|
|
|
Complete resection | 7 | 382 (175–1901) | 6 (85.7) | <0.010 |
|
Incomplete resection or
inoperative | 16 | 105 (10–498) | 3 (18.8) |
|
| Chemotherapy |
|
|
|
|
| No | 8 | 157 (10–502) | 2 (25.0) | 0.931 |
|
Yes | 15 | 132 (30–1901) | 7 (46.7) |
|
| EBRT |
|
|
|
|
| No | 13 | 62 (10–182) | 8 (61.5) | 0.029 |
|
Yes | 10 | 320 (85–1901) | 1 (10.0) |
|
 | Table IV.Univariate and multivariate analysis
of the factors affecting prognosis in anaplastic thyroid
cancer. |
Table IV.
Univariate and multivariate analysis
of the factors affecting prognosis in anaplastic thyroid
cancer.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Factor | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Nestin
expression | 4.26 |
1.32–13.41 | 0.017 | 5.59 | 1.63–19.50 | <0.01 |
| Prognostic
index | 1.78 | 1.10–3.02 | 0.019 | 2.13 | 1.21–4.07 | <0.01 |
While 4 patients were found to have a
nestin-negative tumours and a low PI score, with an MST of 422
days, by contrast, all 6 patients with nestin-positive tumours
exhibited high PI scores, with an MST of 86.5 days.
The present study also investigated the efficacy of
three different treatments for the ATC patients (Table V): Surgery, chemotherapy and EBRT. In
the patients with nestin-negative ATC, the 6-month survival rate
was significantly higher than that for the patients who underwent a
gross resection (R0 or R1) or radiation therapy (100 and 80%,
respectively), whereas no significant difference in survival rate
(63.6 vs. 33.3%) was found between the patients with and without
chemotherapy. By contrast, none of the three treatments (surgery,
chemotherapy or EBRT) improved the 6-month survival rate of the ATC
patients with tumours that positively expressed nestin.
 | Table V.A 6-month survival analysis of
anaplastic thyroid cancer patients. |
Table V.
A 6-month survival analysis of
anaplastic thyroid cancer patients.
|
|
Nestin-negative |
Nestin-positive |
|---|
|
|
|
|
|---|
| Treatment | Total | 6 months
survival | P-value | Total | 6 months
survival |
|---|
| Surgery (R0/1) |
|
|
|
|
|
|
Yes | 6 | 6
(100.0) | <0.010 | 1 | 0 (0.0) |
| No | 11 | 3 (27.3) |
| 5 | 0 (0.0) |
| Radiotherapy |
|
|
|
|
|
|
Yes | 10 | 8 (80.0) | 0.015 | 3 | 0 (0.0) |
| No | 7 | 1 (14.3) |
| 3 | 0 (0.0) |
| Chemotherapy |
|
|
|
|
|
|
Yes | 11 | 7 (63.6) | 0.335 | 4 | 0 (0.0) |
| No | 6 | 2 (33.3) |
| 2 | 0 (0.0) |
Discussion
ATC is a rare but extremely aggressive disease with
a extremely poor prognosis; the MST from the time of diagnosis is
3–5 months and the one-year survival rate is <20% (12,15).
Several clinical characteristics, including age, gender, tumour
size, leukocytosis, symptoms, distant metastasis and extrathyroidal
invasion have been reported as indicators of a poor prognosis
(16–18). Sugitani et al found that the
presence of acute symptoms, a large tumour (>5 cm in diameter),
distant metastasis and leukocytosis (>10,000/mm3)
were significant risk factors for a poor prognosis (12), and these findings were confirmed in a
prospective setting (19). In a
comparison of the clinical data of ATC patients in a nationwide
registry, another study by Sugitani et al found that
possession of less than one of the aforementioned four factors was
linked to better survival (20).
In the present series, the absence of nestin
expression, a low PI, a complete tumour resection and EBRT were
significantly associated with a longer survival time. The
univariate and multivariate analyses revealed that nestin
expression and the PI were significant indicators for prognosis
independently. In addition, no associations between nestin
expression and any clinicopathological factors were found in the
present series. These findings therefore clearly demonstrated that
nestin expression in the cytoplasm of the ATC cells is one of the
strong molecular factors indicating the prognosis of patients; a
factor that is not affected by clinicopathological features of the
tumour or the patient.
Nestin, a class VI intermediate protein, is known as
a marker for neural stem cells. In melanomas and prostate cancer,
nestin expression has been reported to be associate with the
migration, invasion and metastasis of the cancer cells (21,22).
Although the cytoplasmic expression of nestin protein in ATC cells,
along with the loss of E-cadherin expression, were reported in a
previous study (9), the study
described only two representative cases of ATC in association with
concomitant differentiated thyroid cancer. Therefore, nestin
expression in ATCs had not been fully investigated prior to the
present study, and associations between nestin expression in ATC
and the clinicopathological characteristics of the tumour or
prognosis of the patients were not established.
In the present study, the expression of nestin was
observed in the cytoplasm of the ATC cells in approximately
one-quarter of 23 patients with ATC, indicating that nestin
expression in ATC is not a universal phenomenon during anaplastic
changes of the tumour. Further studies are necessary to investigate
the involvement of nestin expression in the de-differentiation
steps of thyroid cancer, as no nestin-specific staining could be
detected in differentiated thyroid cancers (data not shown).
The present study also found that nestin expression
in the ATC patients exhibited significant prognostic meaning, based
on univariate and multivariate analyses. The acceleration of cancer
cell proliferation, invasive growth and migration have previously
been reported to correlate with nestin expression in several cancer
types (22–24). These features are cellular mechanisms
underlying cancer progression and metastasis, and they are also
common characteristics found in ATC cells. Recent basic research
demonstrated that nestin regulates actin and cell adhesion
molecules affecting migration, invasion and metastasis (25). In the present study, however, there
were no significant correlations between the expression of nestin
in the tumour and the clinicopathological characteristics of the
patients, indicating that nestin did not accelerate disease
progression in ATC.
ATC cancer cells are characterized by extremely
highly aggressive behavior compared with that of other organs
(9,10,12,15–18).
This unusually malignant nature of ATCs, even without nestin
expression, may have contributed to obscure the effect of
therapeutic efforts in patients with nestin-negative tumours. At
the same time, the resection of nestin-negative tumours may be
beneficial for better prognoses, suggesting that important
molecular mechanisms of nestin expression may exist in disease
progression.
Among the present 23 cases, 7 patients with
unresectable disease received EBRT (≥40 Gy or higher). A total of 3
out of the 4 ATC patients with nestin-negative tumours achieved
disease control with radiation therapy, while two-thirds of the
patients with nestin-expressing tumours could not obtain disease
control. In light of these data, nestin may have a role in
acquiring resistance to radiation therapy. Nestin has also been
reported to be expressed increasingly after radiation injury to the
brain (26), although the precise
mechanism of this finding was not identified. This observation may
indicate an important role of nestin in the response to cellular
injury by irradiation therapy. There is currently little evidence
regarding the association between nestin expression and the effect
of radiation therapy, and further investigation is required.
In conclusion, the present findings indicate that
the expression of cytoplasmic nestin is an independent indicator of
a poor prognosis for patients with ATC. In the present data, the
expression of nestin did not correlate with any clinicopathological
characteristic or therapeutic procedure. Nestin may therefore have
a role in acquiring resistance to radiation and surgical therapy.
Further investigations are desired to clarify the role of nestin in
the resistance to multimodal therapeutic approaches, using
authentic ATC cell lines (27). At
the same time, patients with a lower PI and nestin-expressing
tumours may be good candidates for receiving aggressive multimodal
treatment in the practical setting.
Acknowledgements
The authors would like to thank Dr Tamami Morisaki
(Department of Surgical Oncology, Osaka City University Graduate
School of Medicine) for providing advice. The present study was
supported in part by Grant-in-Aid for Scientific Research (C) (JSPS
KAKENHI; grant no. 25461992)
References
|
1
|
Maderna E, Salmaggi A, Calatozzolo C,
Limido L and Pollo B: Nestin, PDGFRbeta, CXCL12 and VEGF in glioma
patients: Different profiles of (pro-angiogenic) molecule
expression are related with tumor grade and may provide prognostic
information. Cancer Biol Ther. 6:1018–1024. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gravdal K, Halvorsen OJ, Haukaas SA and
Akslen LA: Proliferation of immature tumor vessels is a novel
marker of clinical progression in prostate cancer. Cancer Res.
69:4708–4715. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yamahatsu K, Matsuda Y, Ishiwata T, Uchida
E and Naito Z: Nestin as a novel therapeutic target for pancreatic
cancer via tumor angiogenesis. Int J Oncol. 40:1345–1357.
2012.PubMed/NCBI
|
|
4
|
Chen Z, Wang J, Cai L, Zhong B, Luo H, Hao
Y, Yu W, Wang B, Su C, Lei Y, Bella AE, Xiang AP and Wang T: Role
of the stem cell-associated intermediate filament nestin in
malignant proliferation of non-small cell lung cancer. PLoS One.
9:e855842014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Teranishi N, Naito Z, Ishiwata T, Tanaka
N, Furukawa K, Seya T, Shinji S and Tajiri T: Identification of
neovasculature using nestin in colorectal cancer. Int J Oncol.
30:593–603. 2007.PubMed/NCBI
|
|
6
|
Piras F, Ionta MT, Lai S, Perra MT, Atzori
F, Minerba L, Pusceddu V, Maxia C, Murtas D, Demurtas P, Massidda B
and Sirigu P: Nestin expression associates with poor prognosis and
triple negative phenotype in locally advanced (T4) breast cancer.
Eur J Histochem. 55:e392011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Strojnik T, Røsland GV, Sakariassen PO,
Kavalar R and Lah T: Neural stem cell markers, nestin and musashi
proteins, in the progression of human glioma: Correlation of nestin
with prognosis of patient survival. Surg Neurol. 68:133–144. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ishiwata T, Teduka K, Yamamoto T, Kawahara
K, Matsuda Y and Naito Z: Neuroepithelial stem cell marker nestin
regulates the migration, invasion and growth of human gliomas.
Oncol Rep. 26:91–99. 2011.PubMed/NCBI
|
|
9
|
Liu J and Brown RE: Immunohistochemical
detection of epithelialmesenchymal transition associated with
stemness phenotype in anaplastic thyroid carcinoma. Int J Clin Exp
Pathol. 3:755–762. 2010.PubMed/NCBI
|
|
10
|
Sherman SI: Thyroid carcinoma. Lancet.
361:501–511. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tennvall J, Lundell G, Wahlberg P,
Bergenfelz A, Grimelius L, Akerman M, Hjelm Skog AL and Wallin G:
Anaplastic thyroid carcinoma: three protocols combining
doxorubicin, hyperfractionated radiotherapy and surgery. Br J
Cancer. 86:1848–1853. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sugitani I, Kasai N, Fujimoto Y and
Yanagisawa A: Prognostic factors and therapeutic strategy for
anaplastic carcinoma of the thyroid. World J Surg. 25:617–622.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tsutsui K: Chemotherapy of the anaplastic
thyroid cancer. Naibunpitsu Geka. 12:133–139. 1995.(In
Japanese).
|
|
14
|
Kasiwagi S, Yashiro M, Takashima T,
Aomatsu N, Kawajiri H, Ogawa Y, Onoda N, Ishikawa T, Wakasa K and
Hirakawa K: c-Kit expression as a prognostic molecular marker in
patients with basal-like breast cancer. Br J Surg. 100:490–496.
2013. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Smallridge RC and Copland JA: Anaplastic
thyroid carcinoma: Pathogenesis and emerging therapies. Clin Oncol.
22:486–497. 2010. View Article : Google Scholar
|
|
16
|
Kebebew E, Greenspan FS, Clark OH, Woeber
KA and McMillan A: Anaplastic thyroid carcinoma. Treatment outcome
and prognostic factors. Cancer. 103:1330–1335. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yau T, Lo CY, Epstein RJ, Lam AK, Wan KY
and Lang BH: Treatment outcomes in anaplastic thyroid carcinoma:
Survival improvement in young patients with localized disease
treated by combination of surgery and radiotherapy. Ann Surg Oncol.
15:2500–2505. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Akaishi J, Sugino K, Kitagawa W, Nagahama
M, Kameyama K, Shimizu K and Ito K: Prognostic factors and
treatment outcomes of 100 cases of anaplastic thyroid carcinoma.
Thyroid. 21:1183–1189. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Orita Y, Sugitani I, Amemiya T and
Fujimoto Y: Prospective application of our novel prognostic index
in the treatment of anaplastic thyroid carcinoma. Surgery.
150:1212–1219. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sugitani I, Miyauchi A, Sugino K, Okamoto
T, Yoshida A and Suzuki S: Prognostic factors and treatment
outcomes for anaplastic thyroid carcinoma: ATC Research Consortium
of Japan cohort study of 677 patients. World J Surg. 36:1247–1254.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Brychtova S, Fiuraskova M, Hlobilková A,
Brychta T and Hirnak J: Nestin expression in cutaneous melanomas
and melanocytic nevi. J Cutan Pathol. 34:370–375. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kleeberger W, Bova GS, Nielsen ME, Herawi
M, Chuang AY, Epstein JI and Berman DM: Roles for the stem cell
associated intermediate filament Nestin in prostate cancer
migration and metastasis. Cancer Res. 67:9199–9206. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Matsuda Y, Hagio M and Ishiwata T: Nestin:
A novel angiogenesis marker and possible target for tumor
angiogenesis. World J Gastroenterol. 19:42–48. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Narita K, Matsuda Y, Seike M, Naito Z,
Gemma A and Ishiwata T: Nestin regulates proliferation, migration,
invasion and stemness of lung adenocarcinoma. Int J Oncol.
44:1118–1130. 2014.PubMed/NCBI
|
|
25
|
Matsuda Y, Naito Z, Kawahara K, Nakazawa
N, Korc M and Ishiwata T: Nestin is a novel target for suppressing
pancreatic cancer cell migration, invasion and metastasis. Cancer
Biol Ther. 11:512–523. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Suman S, Rodriguez OC, Winters TA, Fornace
AJ Jr, Albanese C and Datta K: Therapeutic and space radiation
exposure of mouse brain causes impaired DNA repair response and
premature senescence by chronic oxidant production. Aging (Albany
NY). 5:607–622. 2013.PubMed/NCBI
|
|
27
|
Onoda N, Nakamura M, Aomatsu N, Noda S,
Kashiwagi S and Hirakawa K: Establishment, characterization and
comparison of seven authentic anaplastic thyroid cancer cell lines
retaining clinical features of the original tumors. World J Surg.
38:688–695. 2014. View Article : Google Scholar : PubMed/NCBI
|