Introduction
Survivin was first identified in 1997 as a member of
the inhibitor of apoptosis protein (IAP) family, which contain a
family-specific baculovirus IAP repeat (BIR) (1). This BIR domain exists at the N-terminal
and is associated with the inhibition of apoptosis (2). The human survivin gene is located on
chromosome 17 (17q25) (3). Survivin
is expressed in the nucleus and/or cytoplasm of various malignant
tumor cells (4), and is also
expressed in fetal and certain proliferating adult tissues,
although remains undetectable in differentiated tissues (5). In the cytoplasm, survivin functions as
an inhibitor of apoptosis, while in the nucleus, survivin regulates
cell proliferation (6).
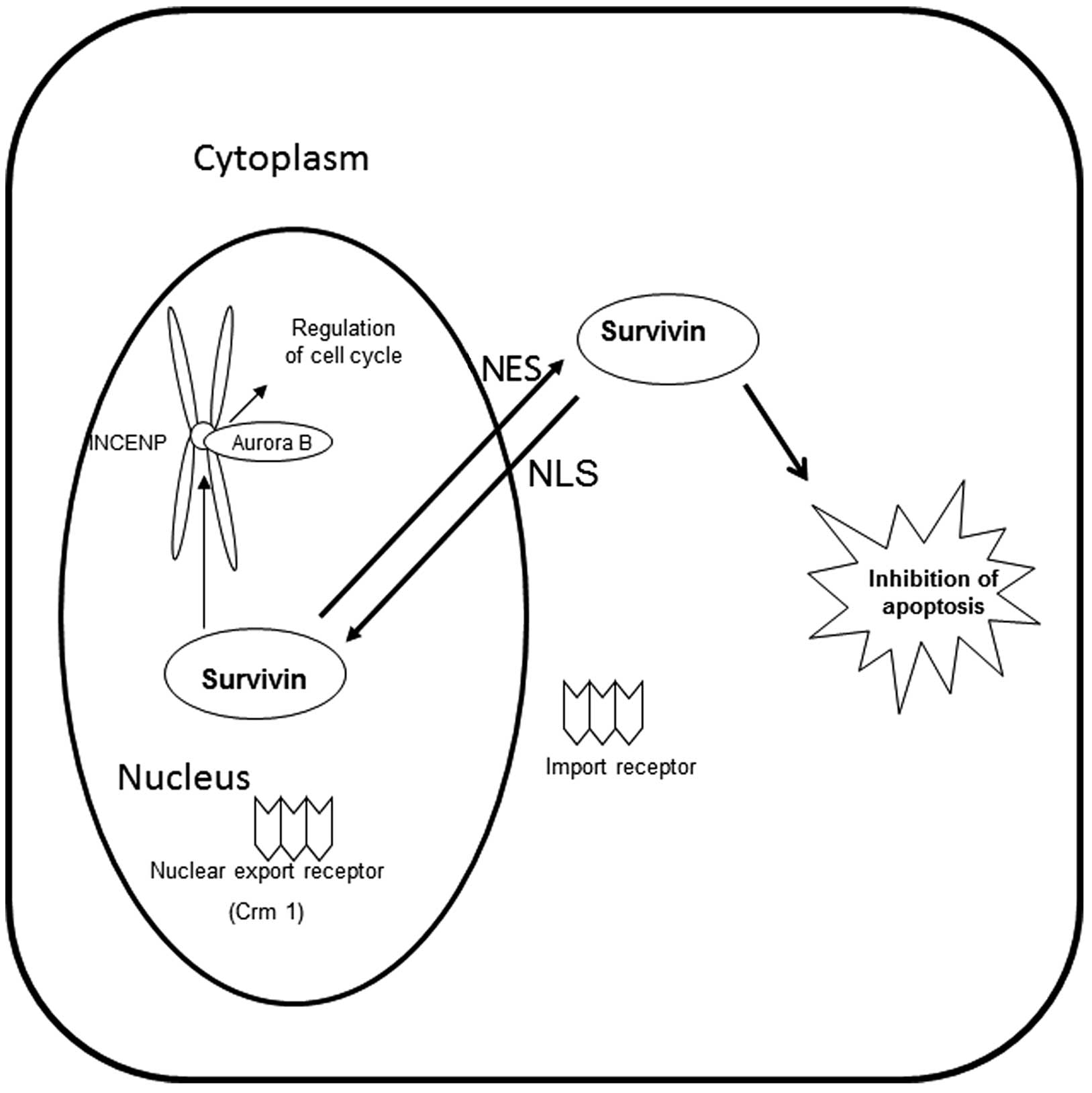
Survivin possesses a nuclear export signal (NES),
which allows it to bind to its export receptor, chromosome region
maintenance protein 1 (Crm1). In the nucleus, survivin forms a
complex with aurora kinase B, the inner centromere protein (INCENP)
and borealin to complete mitosis. Crm1 is critical in tethering
this complex to the centromere (Fig.
1) (7). At the end of mitosis,
Crm1 is released from this complex and exports survivin from the
nucleus to the cytoplasm, where it functions as an inhibitor of
apoptosis.
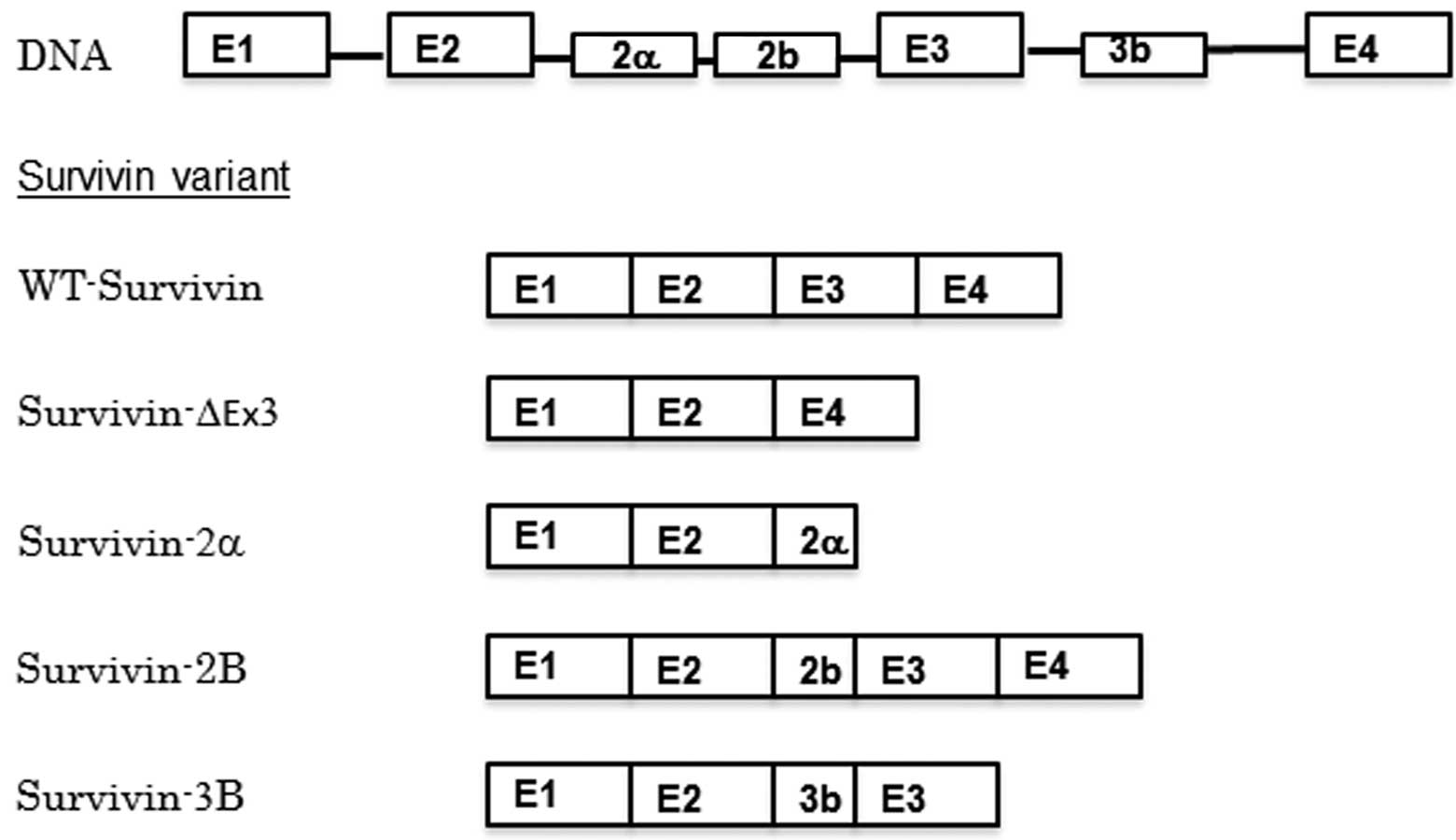
Human survivin has multiple splice variants, which
have various functions and localizations; wild type survivin
(WT-survivin), survivin-2a, survivin-2B, survivin-ΔEx3 and
survivin-3B have been identified to date (Fig. 2) (8).
Survivin-ΔEx3 and survivin-2a lack a nuclear localization signal
(NLS), and remain in the cytoplasm, while WT-survivin, survivin-2B
and survivin-3B possess an NLS and are able to function in the
completion of mitosis in the nucleus (9).
Various types of cancer express survivin. As
survivin has a dual role as an apoptosis inhibitor and a mitotic
effector, the expression of survivin in cancer cells may protect
them from therapeutic drug-induced apoptosis and promote their
proliferation. The association between the aggressiveness of cancer
cells and the expression of survivin has been investigated in
numerous types of cancer (10–12).
However, various studies have reported inconsistent findings with
regard to the significance of survivin as a prognostic factor for
cancer patients. Nuclear survivin expression has been reported to
be correlated with poor prognosis in patients with various types of
cancer, including hepatocellular carcinoma, esophageal squamous
cell carcinoma, as well as urinary bladder and ovarian cancer
(12–15). However, it has been reported to be a
valuable prognostic factor in other types of cancer, for example
gastric cancer and invasive breast cancer (10,16). With
regard to lung cancer, nuclear survivin expression has been
reported to be a useful prognostic factor for patients with
advanced non-small cell cancers (stages III and IV), and a poor
prognostic factor for patients with non-small cell carcinomas at
earlier stages (I and II). Although a number of studies have
investigated survivin expression in non-small cell lung carcinomas
(11,17,18),
little data is available with regard to survivin expression in lung
cancers of various histological types at various stages, or the
association between survivin expression and smoking history. The
paraffin-embedded blocks of lung tumor tissue removed during
surgeries performed in Toneyama National Hospital (Toyonaka, Osaka,
Japan) between 2002 and 2011 included sufficient numbers to allow
statistical analysis of adenocarcinomas from smokers and
non-smokers at p-stages I, II and III, as well as squamous cell
carcinomas (SqCCs), large cell neuroendocrine carcinomas (LCNECs)
and small cell carcinomas (SmCCs) from smokers at p-stage I. Among
the proliferative markers of tumors, the Ki-67 labeling index
(Ki67-LI) is widely accepted as one of the most reliable markers
for estimating the malignancy grade and the prognosis of various
types of tumor (19). The Ki-67 LI of
non-small cell lung carcinoma has prognostic value (20). The present study investigated the
expression of survivin and Ki-67 in these tumors.
Materials and methods
Patients and specimens
Paraffin-embedded tissue blocks of adenocarcinomas
of p-stages I, II and III, as well as SqCCs, LCNECs and SmCCs at
p-stage I, were selected. All tissues were obtained during
surgeries performed at Toneyama National Hospital (Osaka, Japan)
from January 2002 to December 2011. None of the patients whose
tissues were used in the present study had received chemotherapy or
radiotherapy prior to surgery. The numbers of samples obtained are
presented in Table I. The Brinkman
index (BI), which is described in Table
I represents the number of pieces of tobacco smoked/day,
multiplied by the number of years smoking history. The mortality of
lung cancer patients with a BI ≥400 is ~4.9 times higher compared
with that of non-smoking patients (21). In the present study, the majority of
the patients with SqCC, LCNEC and SmCC had a high BI, and the poor
prognosis of those histological types is possibly due to an
increased BI. The adenocarcinoma samples were almost evenly
distributed between p-stages I, II and III, and were identified in
non-smokers and smokers (including current smokers and ex-smokers)
(Table I). By contrast, the majority
of SqCCs, LCNECs and SmCCs were of p-stage I and almost all
patients who had undergone surgery for these types of tumor were
smokers (Table I).
 | Table I.Clinical features of patients with
various subtypes of lung cancer. |
Table I.
Clinical features of patients with
various subtypes of lung cancer.
| A, Adenocarcinoma
(n=87) |
|
|
|
|---|
|
|---|
| Characteristic | Stage I | Stage II | Stage III |
|---|
| Patients, n | 34 | 22 | 31 |
| Age, years |
|
|
|
| Mean ±
SE | 66.4±1.5 | 66.6±1.8 | 65.8±1.2 |
|
Range | 50–80 | 53–80 | 47–81 |
| Gender, n |
|
|
|
| Male | 20 | 15 | 18 |
|
Female | 14 | 7 | 13 |
| Smoking status,
n |
|
|
|
|
Non-smoker | 13 | 4 | 8 |
|
Smoker | 21 | 18 | 23 |
| BI of smoker, mean ±
SE | 921.2±57.5 | 829.2±108.0 | 1137.6±137.9 |
|
| B, Squamous cell
carcinoma (n=44) |
|
|
|
|
| Characteristic | Stage I | Stage II | Stage III |
|
| Patients, n | 33 | 2 | 9 |
| Age, years |
|
|
|
| Mean ±
SE | 68.4±1.5 | 66.5±7.6 | 70.9±3.4 |
|
Range | 55–79 | 59–74 | 48–82 |
| Gender, n |
|
|
|
| Male | 30 | 2 | 7 |
|
Female | 3 | 1 | 2 |
| Smoking status,
n |
|
|
|
|
Non-smoker | 1 | 0 | 1 |
|
Smoker | 32 | 2 | 8 |
| BI of smoker, mean ±
SE | 1179.4±83.7 |
| 935.6±231.1 |
|
| C, Large cell
neuroendocrine carcinoma (n=13) |
|
|
|
|
| Characteristic | Stage I | Stage II | Stage III |
|
| Patients, n | 13 | 0 | 0 |
| Age, years |
|
|
|
| Mean ±
SE | 69.3±2.8 |
|
|
|
Range | 58–79 |
|
|
| Gender, n |
|
|
|
|
Male | 12 |
|
|
|
Female | 1 |
|
|
| Smoking status,
n |
|
|
|
|
Non-smoker | 1 |
|
|
|
Smoker | 12 |
|
|
| BI of smoker, mean
± SE | 1012.9±118.0 |
|
|
|
| D, Small cell
carcinoma (n=13) |
|
|
|
|
| Characteristic | Stage I | Stage II | Stage III |
|
| Patients, n | 10 | 2 | 1 |
| Age, years |
|
|
|
| Mean ±
SE | 69.1±2.9 |
|
|
|
Range | 56–79 | 62–73 | 80 |
| Gender, n |
|
|
|
|
Male | 9 | 2 | 0 |
|
Female | 1 | 1 | 1 |
| Smoking status,
n |
|
|
|
|
Non-smoker | 0 | 0 | 0 |
|
Smoker | 10 | 2 | 1 |
| BI of smoker, mean
± SEM | 948±127.4 |
|
|
The paraffin-embedded tissue blocks used for this
study were produced following the fixation of tumor tissues in 0.01
M phosphate-buffered 10% formalin (pH 7.4). Clinical data,
including follow-up findings, were available for all cases. Written
informed consent from each patient, allowing the arbitrary use of
tumor tissues for pathological studies, was obtained prior to
surgery. The present study was approved by the ethics committee of
Toneyama National Hospital (approval number 1334).
Immunohistochemistry
For immunohistochemical examinations of survivin,
one representative tissue block from each tumor was used, with 5-mm
sections prepared. Immunohistochemical staining was performed using
an avidin-streptavidin immunoperoxidase method with a rabbit
polyclonal anti-human survivin antibody (Novus Biologicals,
Littleton, CO, USA) at a 1:500 dilution, or with a prediluted
anti-human Ki-67 mouse monoclonal antibody (Dako, Glostrup,
Denmark). The primary antibodies were detected using the iView DAB
universal kit (Ventana Medical Systems Inc., Tucson, AZ, USA),
which is a detection kit including from a mixture of anti-mouse Ig
and anti-rabbit Ig biotinylated secondary antibodies using DAB/H2O2
as substrates. Antigen retrieval was conducted by incubation of
deparaffinized sections in cell condition 1 solution from the
aforementioned kit for 64 min at 100°C and immunohistochemical
staining was conducted using an automated Benchmark system (Ventana
Medical System, Tuscon, AZ, USA), according to the manufacturer's
instructions. To estimate the labeling index (LI) of nuclear
survivin or Ki-67 in each tumor, ~1,000 nuclei stained positively
or negatively were counted automatically, using Win Roof software
(Mitani Co, Tokyo, Japan). To determine positive staining of
cytoplasmic survivin, tumors were classified into two groups based
on the percentage of positively stained cells: >10%, positive
staining; and <10%, negative staining.
Statistical analysis
Statistical analyses were performed using the Excel
Statistics 2012 software package for Windows (SSRI, Tokyo, Japan).
P<0.05 was considered to indicate a statistically significant
difference. Categorical data was analyzed using a χ2
test. Data comprising multiple values are presented as the mean ±
standard error of the mean and these data were analyzed using
Bonferroni's multiple comparison test. The survival curves were
analyzed by the Kaplan-Meier method, followed by the Log rank
test.
Results
Survivin expression in adenocarcinomas
of non-smokers and smokers
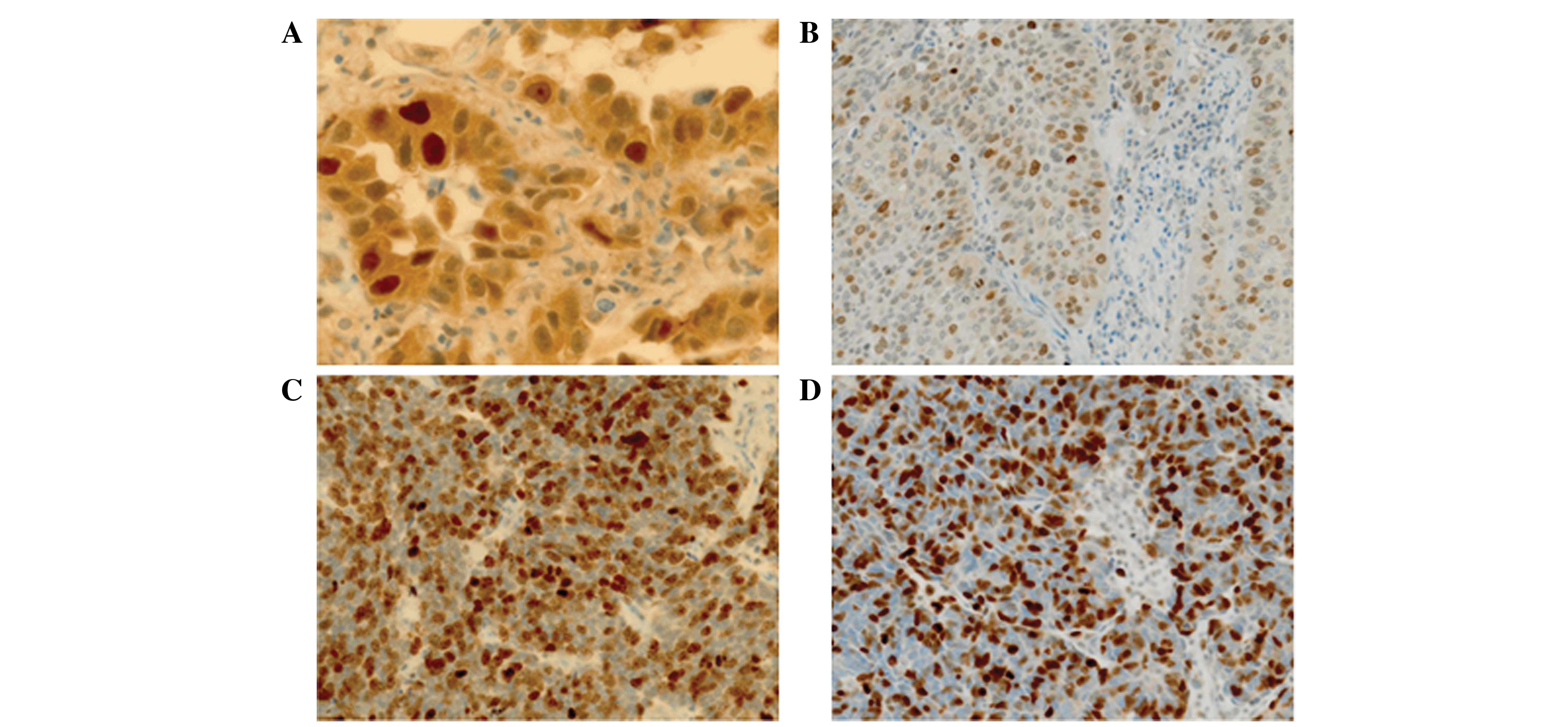
In adenocarcinomas at p-stage I, the nuclear
survivin LIs were significantly higher for smokers than for
non-smokers (P<0.05), while there was no significant difference
in the cytoplasmic survivin expression between adenocarcinomas from
smokers and non-smokers (Table II;
Fig. 3A). The Ki-67 LIs of the
adenocarcinomas of smokers were also significantly higher compared
with those of the adenocarcinomas of non-smokers. In
adenocarcinomas at p-stage II and III, smoking history was not
significantly associated with the nuclear survivin LI, cytoplasmic
survivin expression or Ki-67 LI. The nuclear survivin LIs and Ki-67
LIs in adenocarcinomas at p-stage II and III were similar to those
in adenocarcinomas of smokers at p-stage I.
 | Table II.Survivin expression and Ki-67
labeling indices in adenocarcinomas of non-smokers and smokers at
p-stage I, II or III. |
Table II.
Survivin expression and Ki-67
labeling indices in adenocarcinomas of non-smokers and smokers at
p-stage I, II or III.
| Stage | Non-smokers | Smokers | Difference |
|---|
| Stage I, n | 13 | 21 |
|
|
Survivin nuclear labeling
index, % |
2.9±1.0 |
9.7±2.4 | P<0.05 |
|
Survivin cytoplasm expression
>10%, n (%) | 12 (92.3) | 16 (76.2) | NS |
| Ki-67
labeling index, % | 12.0±1.6 | 20.5±2.8 | P<0.05 |
| Stage II, n | 4 | 18 |
|
|
Survivin nuclear labeling
index, % |
9.0±2.7 | 12.3±2.1 | NS |
|
Survivin cytoplasm expression
>10%, n (%) | 3
(75.0) | 9
(50.0) | NS |
| Ki-67
labeling index, % | 17.8±4.5 | 28.1±3.3 | NS |
| Stage III, n | 8 | 23 |
|
|
Survivin nuclear labeling
index, % |
7.1±1.8 | 13.2±2.8 | NS |
|
Survivin cytoplasm expression
>10%, n (%) | 7
(87.5) | 12 (52.2) | NS |
| Ki-67
labeling index, % | 15.1±2.6 | 21.9±2.1 | NS |
Survivin expression in lung cancers of
various histological types
Survivin expression and Ki-67 LIs were compared
between adenocarcinomas and other histological types of lung
carcinoma, including SqCC, LCNEC and SmCC, from smokers at p-stage
I, as almost all patients who had undergone surgery for the
treatment of SqCC, LCNEC and SmCC were smokers (Table II). The nuclear survivin and Ki-67
LIs in the adenocarcinoma samples were significantly lower compared
with those of the SqCC, LCNEC and SmCC samples (P<0.05;Table III; Fig.
3B, C and D). By contrast, the cytoplasmic survivin expression
in SqCC samples was similar to that of adenocarcinoma samples,
whilst it was decreased in LCNEC and SmCC compared with
adenocarcinoma.
 | Table III.Survivin expression and Ki-67
labeling indices in various histological types of lung cancer in
smokers. |
Table III.
Survivin expression and Ki-67
labeling indices in various histological types of lung cancer in
smokers.
| Stage | Adenocarcinoma | SqCC | LCNEC | SmCC |
|---|
| Stage I, n | 21 | 32 | 12 | 10 |
|
Survivin nuclear labeling
index, % |
9.7±2.4b,c,d |
29.1±2.9a,c |
41.5±2.3a,b |
33.3±3.2a |
|
Survivin cytoplasm expression
>10%, n (%) | 16
(76.2)c,d | 29
(90.6)c,d | 3
(25.0)a,b | 3
(30.0)a,b |
| Ki-67
labeling index, % |
20.5±2.8b,c,d |
45.5±2.7a |
46.9±7.4a |
54.2±4.0a |
| Stage III, n | 23 | 8 |
|
|
|
Survivin nuclear labeling
index, % | 13.2±2.8 | 19.4±4.2 |
|
|
|
Survivin cytoplasm expression
>10%, n (%) | 12 (52.2) | 7
(87.5) |
|
|
| Ki-67
labeling index, % |
21.9±2.1b |
32.2±4.5a |
|
|
Survival rate of patients with
different histological types of lung carcinoma
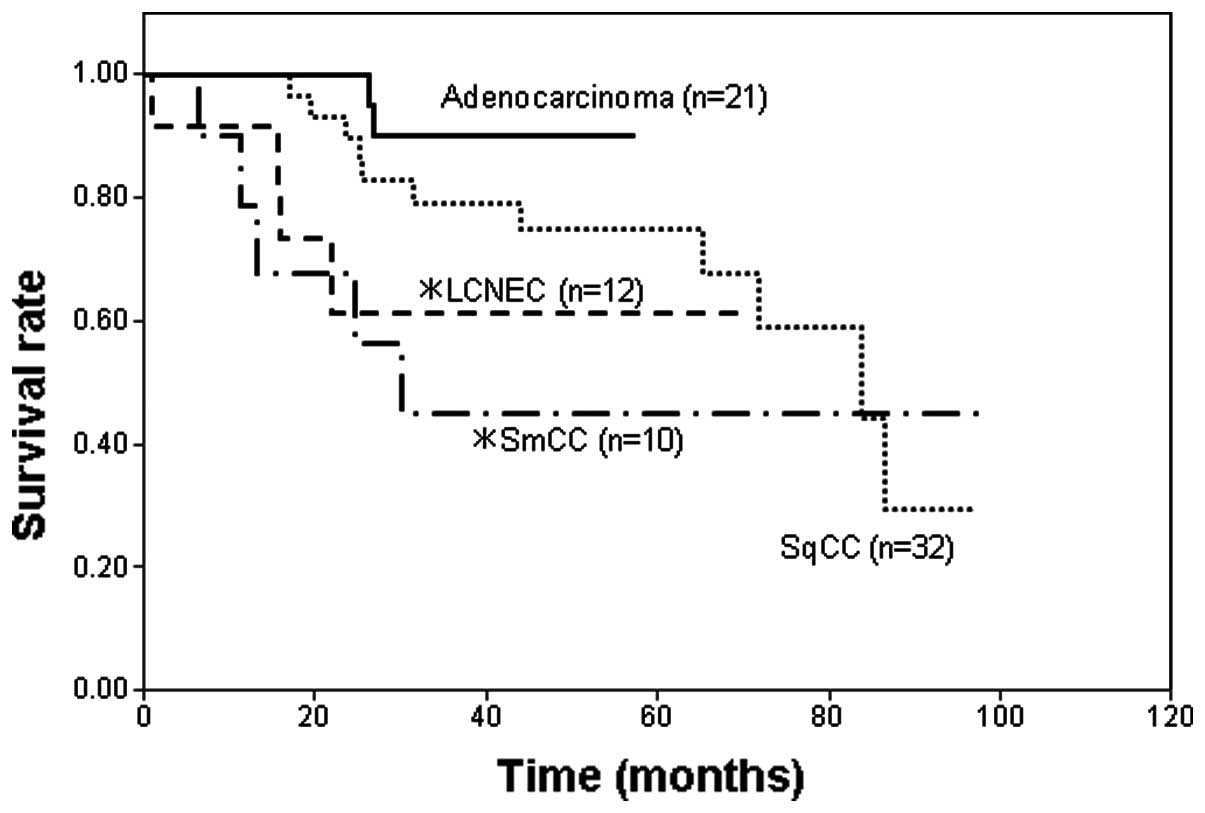
When the survival rates were compared among smokers
with adenocarcinoma, SqCC, LCNEC and SmCC at p-stage I, the
survival rates of smokers with LCNEC and SmCC were significantly
poorer compared with that of smokers with adenocarcinoma (Fig. 4). The survival rate of SqCC patients
was also poorer than that of adenocarcinoma patients, however, this
difference was not statistically significant. The duration of the
follow-up for the p-stage I adenocarcinoma patients was shorter
compared with the other type of lung carcinoma patients, since
p-stage I adenocarcinoma has a more favorable prognosis; therefore,
a 5-year follow-up was considered to be sufficient.
In conclusion, the present study indicates that
smoking is associated with the histogenesis of lung adenocarcinoma
but not with the development of lung adenocarcinoma, based on the
nuclear expression levels of Ki-67 and survivin in lung cancer
tissue samples.
Discussion
In adenocarcinomas at p-stage I, the LIs of nuclear
survivin and Ki-67 were significantly higher in the adenocarcinomas
of smokers than in adenocarcinomas of non-smokers. These results
are consistent with the results of a previous study by our group,
in which an association was identified between smoking and nuclear
survivin expression and Ki-67 LI in p-stage IA adenocarcinomas
(18). Dasgupta et al
(22) have reported that the
cigarette component nicotine upregulates survivin expression in
human non-small cell carcinoma cell lines. Therefore, it is
conceivable that smoking induces survivin expression at an early
developmental stage of lung adenocarcinoma. The increased nuclear
survivin expression in the adenocarcinomas of smokers compared with
that of non-smokers may be associated with the higher Ki-67 LIs in
these adenocarcinomas. Maeda et al (23) reported that smoking was associated
with poor outcomes in patients with clinical stage IA
adenocarcinomas. The poor prognoses of these patients may be
associated with the increased nuclear survivin expression.
Notably, nuclear survivin and Ki-67 LIs, in
adenocarcinomas at p-stages II and III of non-smokers and smokers
were similar to those in p-stage I adenocarcinomas of smokers.
These results indicate that nuclear survivin expression and
proliferative activity may be increased during the progression of
disease following the early developmental stages of
adenocarcinoma.
Nuclear survivin and Ki-67 LIs in adenocarcinomas of
smokers at p-stage I were significantly lower compared with those
of SqCCs, LCNECs and SmCCs of smokers at the same stage. These
results suggested that survivin expression may depend on the
histological type of lung cancer, at least at p-stage I. The higher
Ki-67 LIs in SqCCs, LCNECs and SmCCs may be associated with higher
nuclear survivin expression in these histological types of lung
cancer. Inconsistent with the higher Ki-67 LIs in SqCCs, LCNECs and
SmCCs compared with that of adenocarcinomas of smokers at p-stage
I, the survival rates of smokers with SqCCs, LCNECs and SmCCs were
poorer than those of smokers with adenocarcinoma.
In contrast to the nuclear expression of survivin,
cytoplasmic survivin expression was not increased in
non-adenocarcinoma lung cancer types compared with that in
adenocarcinoma, and the poorer survival rates of patients with
these types of cancer were not associated with its increase.
Therefore, cytoplasmic survivin expression may have little effect
on the proliferation or prognosis of lung cancer.
In conclusion, the present results suggest that a
history of smoking is accompanied by an increase in nuclear
survivin and Ki-67 expression in lung adenocarcinomas at p-stage I,
but not at p-stage II and III; and that nuclear survivin and Ki-67
expression in adenocarcinomas of smokers at p-stage I is lower than
that in other types of lung cancer of smokers at p-stage I, which
is associated with an enhanced survival rate.
Acknowledgements
The authors would like to thank Mr. Akira Kimura and
Mr. Hiroshi Yamada (Laboratory Medicine, Toneyama National
Hospital) for their technical assistance, and Ms. Yuko Ito
(Laboratory Medicine, Toneyama National Hospital) for providing
secretarial assistance.
Glossary
Abbreviations
Abbreviations:
|
IAP
|
inhibitor of apoptosis protein
|
|
BIR
|
baculovirus IAP repeat
|
|
NES
|
nuclear export signal
|
|
NLS
|
nuclear localization signal
|
|
Crm1
|
chromosome region maintenance protein
1
|
|
INCENP
|
inner centromere protein
|
|
SqCC
|
squamous cell carcinoma
|
|
LCNEC
|
large cell neuroendocrine
carcinoma
|
|
SmCC
|
small cell carcinoma
|
References
|
1
|
Ambrosini G, Adida C and Altieri DC: A
novel anti-apoptosis gene, survivin, expressed in cancer and
lymphoma. Nat Med. 3:917–921. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Srinivasula SM and Ashwell JD: IAPs:
What's in a name? Mol Cell. 30:123–135. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Storlazzi CT, Brekke HR, Mandahl N, et al:
Identification of a novel amplicon at distal 17q containing the
BIRC5/SURVIVIN gene in malignant peripheral nerve sheath tumours. J
Pathol. 209:492–500. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Connell CM, Colnaghi R and Wheatley SP:
Nuclear survivin has reduced stability and is not cytoprotective. J
Biol Chem. 283:3289–3296. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Salvesen GS and Duckett CS: IAP proteins:
Blocking the road to death's door. Nat Rev Mol Cell Biol.
3:401–410. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Stauber RH, Mann W and Knauer SK: Nuclear
and cytoplasmic survivin: Molecular mechanism, prognostic, and
therapeutic potential. Cancer Res. 67:5999–6002. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Knauer SK, Mann W and Stauber RH:
Survivin's dual role: An export's view. Cell Cycle. 6:518–521.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mull AN, Klar A and Navara CS:
Differential localization and high expression of SURVIVIN splice
variants in human embryonic stem cells but not in differentiated
cells implicate a role for SURVIVIN in pluripotency. Stem Cell Res
(Amst). 12:539–549. 2014. View Article : Google Scholar
|
|
9
|
Li F and Ling X: Survivin study: An update
of ‘What is the next wave’? J Cell Physiol. 208:476–486. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hernandez JM, Farma JM, Coppola D, et al:
Expression of the antiapoptotic protein survivin in colon cancer.
Clin Colorectal Cancer. 10:188–193. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xie YL, An L, Jiang H and Wang J: Nuclear
survivin expression is associated with a poor prognosis in
Caucasian non-small cell lung cancer patients. Clin Chim Acta.
414:41–43. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Vivas-Mejia PE, Rodriguez-Aguayo C, Han
HD, et al: Silencing survivin splice variant 2B leads to antitumor
activity in taxane-resistant ovarian cancer. Clin Cancer Res.
17:3716–3726. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Morinaga S, Nakamura Y, Ishiwa N, et al:
Expression of survivin mRNA associates with apoptosis,
proliferation and histologically aggressive features in
hepatocellular carcinoma. Oncol Rep. 12:1189–1194. 2004.PubMed/NCBI
|
|
14
|
Zhu H, Wang Q, Hu C, et al: High
expression of survivin predicts poor prognosis in esophageal
squamous cell carcinoma following radiotherapy. Tumour Biol.
32:1147–1153. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sun YW, Xuan Q, Shu QA, et al: Correlation
of tumor relapse and elevated expression of survivin and vascular
endothelial growth factor in superficial bladder transitional cell
carcinoma. Genet Mol Res. 12:1045–1053. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Vallböhmer D, Drebber U, Schneider PM, et
al: Survivin expression in gastric cancer: Association with
histomorphological response to neoadjuvant therapy and prognosis. J
Surg Oncol. 99:409–413. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ichiki Y, Hanagiri T, Takenoyama M, et al:
Tumor specific expression of survivin-2B in lung cancer as a novel
target of immunotherapy. Lung Cancer. 48:281–289. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hirano H, Maeda H, Takeuchi Y, et al:
Association of cigarette smoking with the expression of nuclear
survivin in pathological Stage IA lung adenocarcinomas. Med Mol
Morphol. 47:196–200. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gerdes J, Schwab U, Lemke H and Stein H:
Production of a mouse monoclonal antibody reactive with a nuclear
antigen associated with cell proliferation. Int J Cancer. 31:13–20.
1983. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nguyen VN, Mirejovský P, Mirejovský T, et
al: Expression of cyclin D1, Ki-67 and PCNA in non-small cell lung
cancer: Prognostic significance and comparison with p53 and bcl-2.
Acta Histochem. 102:323–338. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Maeshima AM, Tochigi N, Tsuta K, et al:
Histological evaluation of the effect of smoking on peripheral
small adenocarcinomas of the lung. J Thorac Oncol. 3:698–703. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dasgupta P, Kinkade R, Joshi B, et al:
Nicotine inhibits apoptosis induced by chemotherapeutic drugs by
up-regulating XIAP and survivin. Proc Natl Acad Sci USA.
103:6332–6337. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Maeda R, Yoshida J, Ishii G, et al:
Influence of cigarette smoking on survival and tumor invasiveness
in clinical stage IA lung adenocarcinoma. Ann Thorac Surg.
93:1626–1632. 2012. View Article : Google Scholar : PubMed/NCBI
|