Introduction
Merkel cell carcinoma (MCC) was initially described
by Toker in 1972 as trabecular carcinoma of the skin in a report of
five patients with unusual skin tumors (1). In 1875, the German anatomist and
histopathologist Merkel termed these cells, which are localized at
the basal layer of the skin and mucosa, as ‘touch cells’, prior to
their subsequent renaming to Merkel cells (2,3). In 1978,
Tang and Toker (4) identified dense
core granules, a morphological hallmark of Merkel cells, in three
of the original five tumors investigated by Toker using electron
microscopy. Thus, it was assumed that this type of trabecular
carcinoma arises from Merkel cells. However, the exact origin of
MCC remains a controversial topic, with the two predominant
theories stating that MCC descends from stem cells of neural crest
origin or from epithelial cells of the epidermis. To date, the
first hypothesis appears to be the most widely accepted (3,5).
MCC is a rare condition with an estimated annual
incidence of 0.23 cases per 100,000 individuals throughout the
Caucasian population (6). However,
according to age-adapted incidence rates, the occurrence of MCC
increased between 1986 and 2001, exhibiting a statistically
significant annual increase of 8% (7). Possible reasons for this increase
include longer life expectancy, greater sun exposure and a growing
number of immunocompromised individuals (6). Furthermore, there is a higher incidence
in men and the elderly (6).
The etiology of MCC is unknown. However, the
pioneering identification of a novel Merkel cell polyomavirus
(MCPyV), which was associated with the development of MCC by Feng
et al (8), led to numerous
attempts to investigate viral oncogenesis. Feng et al
(8) detected MCPyV in 8 out of 10 MCC
patients. Furthermore, in a study conducted by Fukutomo et
al (9), frequent detection of
MCPyV DNA in human papillomavirus-1 (HIV-1)-positive patients was
reported, indicating that MCPyV viremia is associated with host
immunity. Notably, it was also reported that patients with acquired
immunodeficiency syndrome (AIDS) demonstrate a 13.4-fold increase
in the risk of developing MCC compared with healthy individuals
(10).
Additionally, evidence regarding the origin of MCC
has been provided by a number of strongly associated risk factors
and frequent clinical features, presented below. MCC can be
challenging to diagnose and may be overlooked in the early stages,
as the development tends to be asymptomatic, without pathognomonic
clinical features. Typically, MCC presents as a small, firm, red to
purple painless papule or nodule that grows progressively in size.
MCC is predominantly observed in the head and neck region; however,
alternative locations include the upper (19%) and the lower limbs
(16%), which are associated with the best prognosis, and the trunk
(11%), a location more often associated with distant metastasis at
the time of diagnosis (11). In a
study conducted by Heath et al (12), the most common clinical features in
195 patients were described using the acronym AEIOU, indicating the
following: Asymptomatic, which occurs in 88% of patients; expanding
rapidly, which occurs in 63% of patients; immune-suppressed,
including patients with AIDS or chronic lymphocytic leukemia, or
those that have undergone transplantation, comprising 7.8% of
patients; >50 and >65 years old, which accounts for 90 and
75% of patients, respectively; and UV-exposed and fair-skinned,
which accounts for 81 and 98% of patients, respectively.
A definitive diagnosis of MCC is determined by a
histopathological examination performed subsequent to biopsy, and
is predominantly based on the expression of cytokeratin 20 (CK20),
a protein that is highly specific for MCC (13). In a review by Jaeger et al
(14), it was demonstrated that, as
well as CK20, the expression of neuron-specific enolase (NSE) and
neurofilament protein are specific for MCC. Other neuroendocrine
markers commonly used for MCC diagnosis are chromogranin and
synaptophysin, and negative markers include thyroid transcription
factor-1 (TTF-1), CK7, diagnostic markers for small cell lung
cancer and leukocyte common antigen for lymphoma (3). Furthermore, the differential diagnosis
between MCC and malignant melanoma is based on the presence of CK20
expression, and the absence of human melanoma black 45, NKI/C3 and
S-100 expression (3). Data regarding
the regional and metastatic disease is obtained using various
imaging techniques, including ultrasound (US), computed tomography
(CT) and magnetic resonance imaging (MRI), as well as nuclear
medicine modalities. Therefore, these techniques facilitate the
staging, management and follow-up of patients with MCC (3,7).
Surgery is considered to be the primary treatment
strategy for patients with MCC. Specifically, the National
Comprehensive Cancer Network guidelines (www.nccn.org/professionals/physician_gls/f_guidelines.asp)
recommend a margin of 1–2 cm for wide local excision or,
alternatively, treatment with Mohs surgery. In patients treated
with wide local excision, sentinel lymph node biopsy (SLNB) is
performed intraoperatively, as in the present case. By contrast,
SLNB should be performed prior to undergoing Mohs surgery as the
surgical technique may alter the lymphatic drainage, leading to
changes in sentinel node scintigraphy (5). MCC is a radiosensitive malignancy;
however, the use of radiotherapy (RT) as monotherapy is typically
reserved for patients that are not eligible for surgery (5). Mojica et al (15) reported a median survival time of 63
months for patients who were treated with RT as an adjuvant to
surgery, compared with 45 months for those who underwent surgery
alone. The treatment of MCC with chemotherapy remains under
evaluation. Although MCC is considered to be a chemosensitive
malignancy, a standard chemotherapeutic treatment scheme does not
yet exist. Due to MCC exhibiting similar biological behavior to
small cell lung cancer, chemotherapeutic regimens, such as
etoposide/carboplatin or cyclophosphamide/doxorubicin/vincristine,
have been used (5). Peptide receptor
radionuclide therapy (PRRT) is an alternative treatment option in
appropriate cases (16).
The typically poor prognosis of patients with MCC
appears to be associated with the degree of expansion at the time
of presentation. The aggressive course of this rare neoplasm is
reflected by the following five-year survival rates: Local disease,
64%; regional nodal involvement, 39%; and distant metastatic
disease, 18% (17). Furthermore, MCC
appears to have a high three-year mortality rate of 33%. This rate
is ~15% higher than that of the less aggressive skin cancer
melanoma (12). Even following
treatment, close monitoring of patients is required due to the
following high recurrence rates: Local recurrence within 12 months,
30–40%; regional recurrence within two years, 50%; and distant
metastasis, 36–49% (18).
Case report
In 2012, a 43-year-old Caucasian man presented to
St. Savvas Anticancer-Oncology Hospital (Athens, Greece) with a
painless skin lesion on the posterior side of the left thigh that
was reported to be growing in size. The patient was HIV-positive
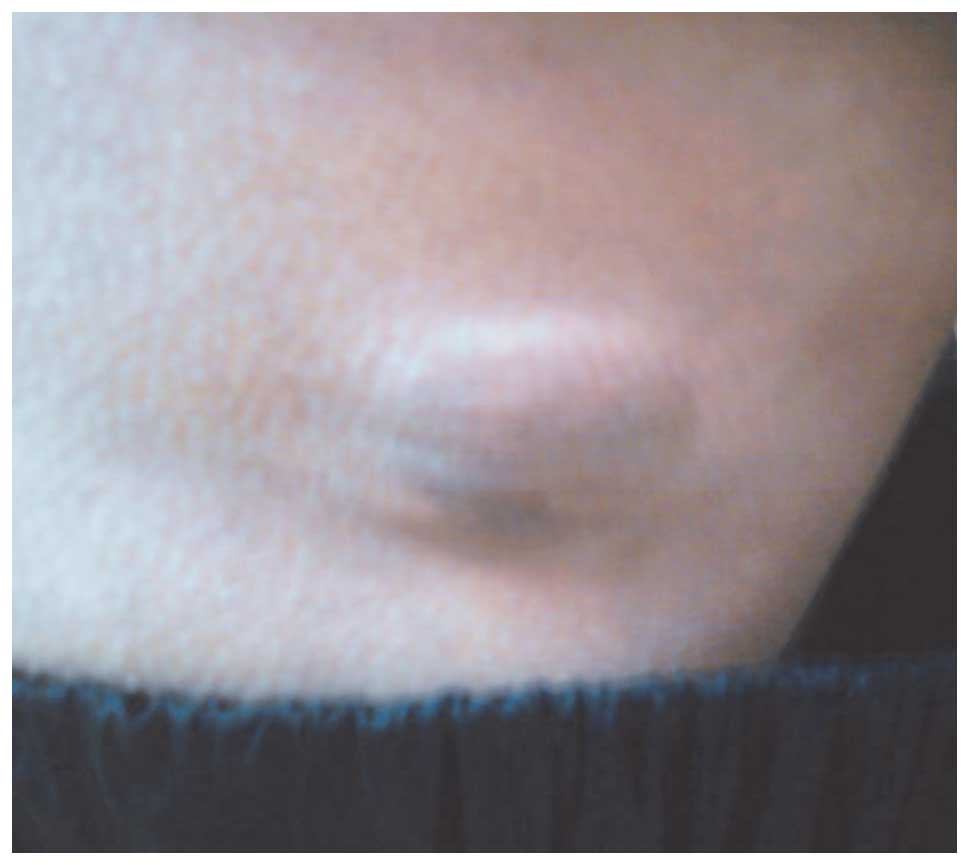
and undergoing treatment. Upon physical examination, a skin-colored
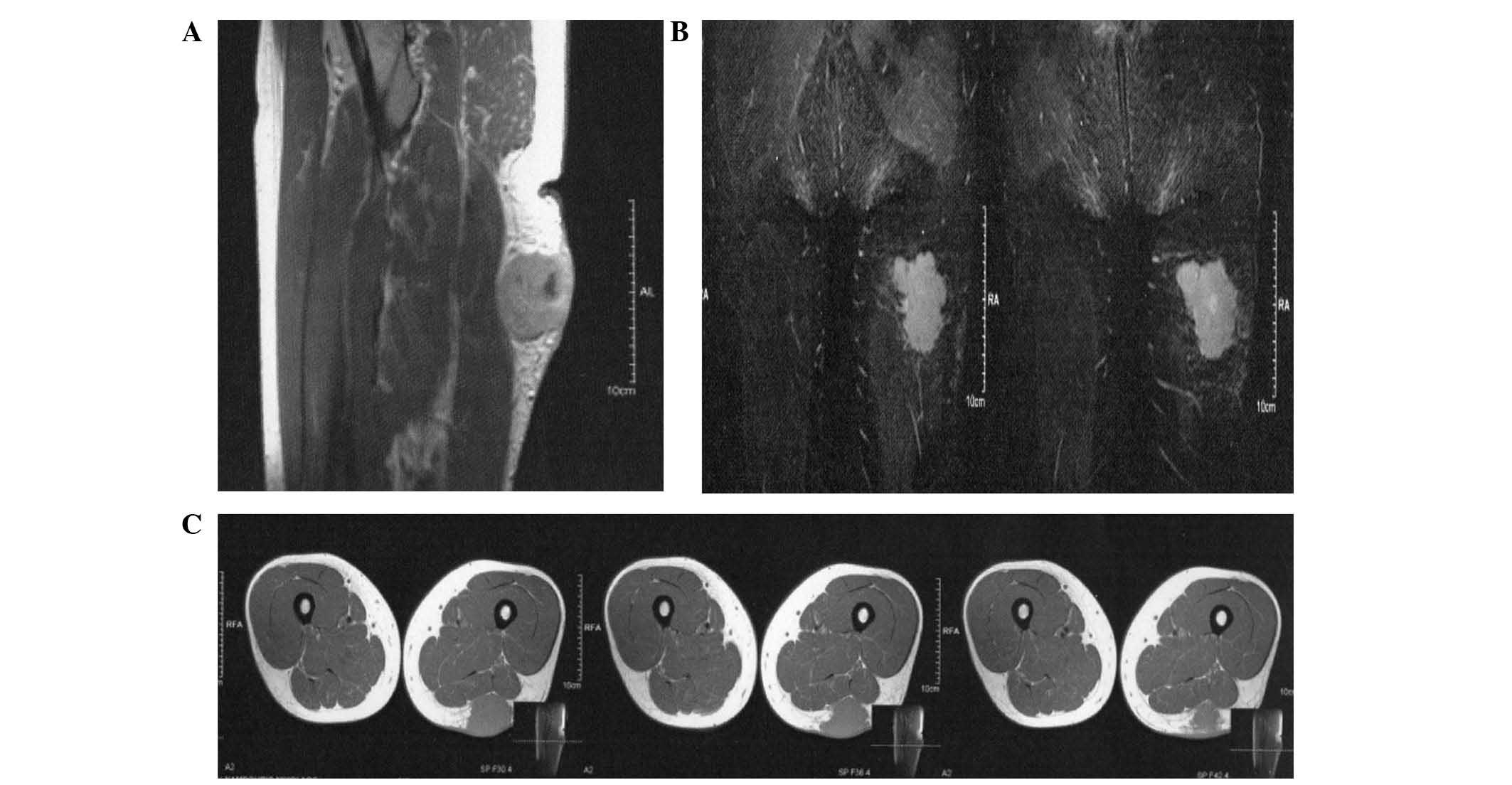
lesion of 4-cm diameter was observed (Fig. 1) and MRI revealed a suspicious mitotic
space-occupying lesion (Fig. 2).
Thus, a cytological and histological examination with fine needle
aspiration and biopsy was immediately performed under CT. The
cytological profile of the lesion was consistent with MCC; however,
the histological findings indicated a diagnosis of neuroendocrine
carcinoma of the skin. Immunohistochemistry identified that the
lesion was CK20(+), chromogranin(+), synaptophysin(+), NSE(+) and
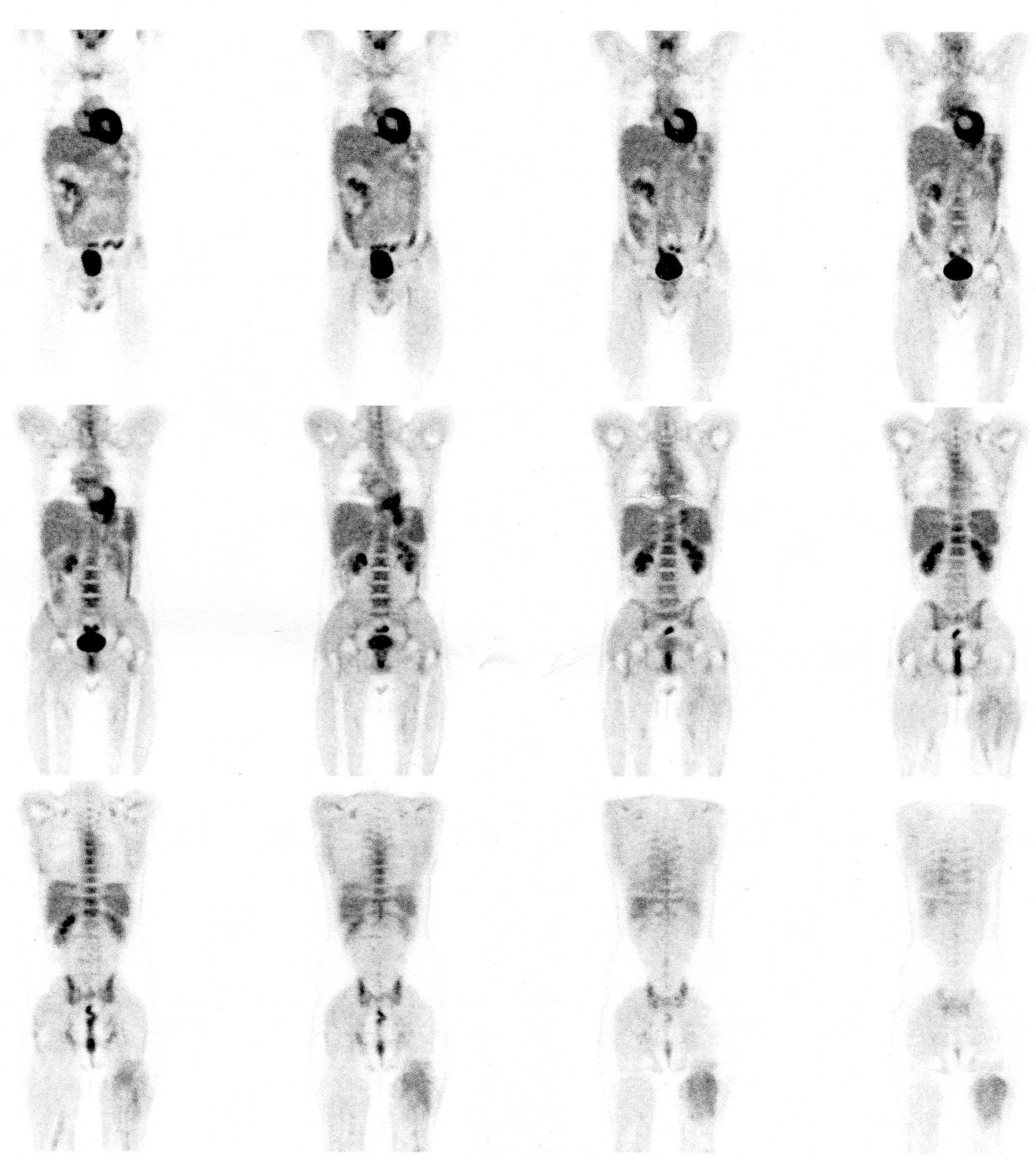
CK8/18(+). The patient subsequently underwent somatostatin receptor
scintigraphy. High uptake of the injected radiopharmaceutical was
observed in the anatomical location of the lesion, indicating
overexpression of somatostatin receptor subtypes 1 and 5 (SSTR-2
and SSTR-5, respectively; Fig. 3).
Considering the scintigraphy results, the patient was examined for
metastatic disease using CT. No indications of distant metastasis,
such as enlarged lymph nodes or lesion elsewhere on the body, were
identified. Thus, wide local excision of the lesion with an
intraoperative biopsy of the sentinel lymph node, identified by
scintigraphy, was performed. Subsequent histological analysis
determined that the node was free from malignant infiltration while
the whole tumor indicated the following immunohistochemical
expression pattern: CK AE1/AE3(+); CK20(+); synaptophysin(+);
chromogranin(+); epithelial membrane antigen(+); cluster of
differentiation (CD) 56(+); S100(−); CD117(−); CD99(−); CD20(−);
CD2(−); CD3(−); and CD43(−). Considering the aforementioned
findings, a diagnosis of tumor-node-metastasis (TNM) stage IIA
(T2pN0M0) MCC was determined. The patient received 8 21-day cycles
of chemotherapy with adjuvant RT. The chemotherapeutic regimen
consisted of epirubicin (35 mg) on days 1–3, cyclophosphamide (400
mg) on days 1–3 and vincristine (2 mg) on day 1, and was
well-tolerated by the patient.
At follow-up, which was conducted 6 months
subsequent to completion of treatment, a full body examination with
18F-fluorodeoxyglucose (FDG) positron emission
tomography (PET)/CT was performed. A dose of 10 mCi (370 Mbq)
18F-FDG was intravenously injected and imaging was
performed 60 min later. Analysis of the captured images
demonstrated low 18F-FDG uptake in the posterior surface
of the left thigh [maximum standardized uptake value
(SUVmax), 1.2], predominantly in the area of the excised
lesion, which was possibly due to the recent radiation treatment
(Figs. 4 and 5A). By contrast, increased
18F-FDG uptake was observed in the internal jugular
lymph nodes bilaterally and in lymph nodes of the left posterior
cervical triangle (SUVmax=2.6). This increase in uptake
may be attributed to inflammation, acute or as a result of the
patient's chronic HIV infection (Figs.
4 and 5B). According to the FDG
findings, it was determined that the patient's response to
treatment was complete, the possibility of recurrence was low and
the disease was in remission. However, due to the aforementioned
high recurrence rates, the patient continues to be monitored with
regular follow-up examinations. Furthermore, in consideration of
the current data, the patient is recommended to undergo
18F-FDG PET/CT 12 months subsequent to treatment.
Written informed consent was obtained from the patient.
Discussion
MCC is a rare and aggressive clinical entity that
has exhibited increasing frequency in recent years (18). Among the various imaging methods
available, nuclear medicine techniques appear to be of significant
value, providing crucial information for the staging, management
and follow-up of the patient (18).
Considering that regional lymph node metastases
occur frequently and early in the course of the disease, sentinel
lymph node scintigraphy may be used to identify lymph nodes that
could accommodate micrometastases (19). The sentinel lymph node is defined as
the first lymph node in a regional lymphatic basin to receive lymph
flow of tumor cells from a tumor site (19). The sentinel node is the target for
this imaging technique. The patient is subcutaneously injected with
a 99mTc-labelled colloid around the lesion. Within 2 h,
the sentinel node is pre-operatively imaged and marked on the body
of the patient, or intraoperatively identified with the use of a γ
probe detector, and removed for biopsy (18).
Absence of metastasis in this sentinel lymph node
has a high negative predictive value, as metastases that bypass the
sentinel lymph node are rare (<2%). Therefore, if a sentinel
lymph node is not infiltrated with tumor cells, it is unlikely that
other nodes in the regional lymph node basin will. In the majority
of cases, the pattern of lymphatic drainage is predictable when the
lesion is located in the extremities. However, in the head, neck
and trunk this pattern is much less predictable. In particular,
unexpected nodal drainage patterns are observed in 37–84% of cases
and are often missed without the use of scintigraphic guidance
(20). It can take ≤8 months for
nodal metastases to become clinically apparent; therefore, patients
with negative lymph nodes upon clinical examination alone may have
occult microscopic metastatic disease (19). In 2010, the first consensus staging
system for MCC was adopted by the American Joint Committee on
Cancer and the International Union Against Cancer. This system was
based on a study of 5823 cases conducted by Lemos et al
(17) and, in contrast to previous
staging systems, takes into consideration whether examination of
regional lymph nodes occurred clinically or pathologically. A worse
prognosis was presented for those with undetectable lymph nodes on
clinical examination alone compared with those who had
pathologically proven negative lymph nodes. The former, according
to the new staging system, are classified as stage IB or IIB (cN0)
disease, while the latter are categorized as stage IA or IIA (pN0).
Considering the aforementioned studies and that regional nodal
disease is a predictor of outcome, rather than crucial to the
outcome, all patients with MCC should undergo sentinel lymph node
scintigraphy and biopsy prior to surgery.
Nuclear medicine with PET/CT has recently gained
ground in the diagnostic imaging of patients with MCC. The
rationale for the application of PET in oncology is that cancer
cells typically have a high metabolic activity compared with
healthy tissue and use more glucose. Within tumor cells, glucose is
phosphorylated by hexokinase and undergoes additional metabolism.
18F-FDG is a glucose analogue that is transported into
the cell where it is phosphorylated through the same mechanism as
glucose, by hexokinase. However, in contrast to glucose,
18F-FDG undergoes no subsequent metabolism, is unable to
diffuse extracellularly and remains trapped in the cell.
Following intravenous injection, 18F-FDG
is rapidly distributed throughout the body and the patient
undergoes imaging 40–60 min later. Clearance of the radiotracer
occurs in the kidneys, and excretion through the bowel also occurs.
18F-FDG is important for the imaging of tumors with high
proliferative activity. 18F-FDG PET is typically more
sensitive in the detection of poorly differentiated high-grade
neuroendocrine tumors (NETs), with a Ki-67 index of >20%,
compared with highly differentiated tumors, providing valuable
prognostic information that may influence the therapeutic plan. In
addition, a negative 18F-FDG scan should generally be
considered as a true negative result, since a negative result
indicates a highly differentiated tumor and, therefore, a better
prognosis. Furthermore, it has been demonstrated that the positive
prognostic value of 18F-FDG PET for patient outcome is
better than that of traditional markers, such as Ki-67,
chromogranin A and liver metastasis (21).
MCC, which presents as a rapidly growing malignancy
in the majority of cases, can be imaged using 18F-FDG
PET/CT, allowing for differentiation between healthy and malignant
tissue. Yao et al (22)
reported two cases in which 18F-FDG PET detected
metastatic disease in subcentimeter nodes that were not detected
using CT. As CT relies on tumor size and architectural change,
nodes are often inaccurately characterized as benign due to a lack
of enlargement. Lin et al (23) reported the increased sensitivity of
18F-FDG PET for detecting MCC recurrence compared with
CT. Furthermore, in a study by Belhocine et al (24), 18F-FDG PET/CT was compared
with CT, MRI and bone scan alone in 11 patients. A sensitivity of
92% and specificity of 100% were reported, indicating that whole
body 18F-FDG PET may be useful in the management of MCC
patients during pretreatment staging, as well as in post-treatment
follow-up. However, it was highlighted that a normal
18F-FDG distribution cannot exclude the possibility of
an MCC with low proliferative activity, as previously discussed. In
the same study, it was reported that 18F-FDG PET was
able to detect a second neoplasm in 4/11 patients with MCC.
MCC is associated with secondary neoplasms, and
therefore the performance of whole body 18F-FDG PET may
provide useful information for the restaging of patients. Concannon
et al (25) conducted a
retrospective review of 18 patients with MCC that underwent
18F-FDG PET/CT, and reported that the 18F-FDG
PET/CT findings resulted in changes in the management of nine
patients. Similarly, in a study conducted by Shintani et al
(26), it was reported that
18F-FDG PET/CT findings caused two out of five patients
with MCC to have their post-surgical treatment strategy altered. In
a retrospective study, Peloschek et al (27) set the sensitivity of
18F-FDG PET at 85.7% and the specificity at 96.2%, in
comparison to values of 95.5% and 89.1%, respectively, that are
used in conventional imaging modalities. Furthermore, in a review
by Enzenhofer et al (20),
18F-FDG PET/CT was recommended as first-line imaging
technique for patients with MCC.
The rationale for the implementation of somatostatin
receptor scintigraphy (SRS) in patients with MCC is based on the
neuroendocrine characteristics of the malignancy (28). Somatostatin is a 14 amino acid peptide
produced in the hypothalamus, pituitary gland, brainstem,
gastrointestinal tract and pancreas. In the central nervous system,
somatostatin acts as a neurotransmitter and, external to the brain,
acts as a hormone that inhibits the release of growth hormone,
insulin, glucagon, gastrin, serotonin and calcitonin (28). Somatostatin also acts as a tumor
growth inhibitor and an angiogenesis inhibitor. Somatostatin
receptors are glycoproteins of the cell membrane and are expressed
in various healthy cell types, as well as in tumors of
neuroendocrine origin. Five different subtypes of somatostatin
receptors have been recognized at present (SSTR1-5) (28).
The radiopharmaceutical used in SRS is
111In-diethylene triamine pentaacetic acid
(DTPA)-octreotide at a dose of 111–222 MBq (3–6 mCi). Octreotide
predominantly binds to the SSTR2 and SSTR5 somatostatin receptor
subtypes, less commonly to SSTR3, and never binds SSTR1 and SSTR4.
Imaging is typically performed 24 and 48 h after the intravenous
injection. Furthermore, clearance of the radiotracer primarily
occurs through the kidneys and partially through the hepatobiliary
pathway. Due to the latter clearance method, the use of laxatives
and 48-h imaging is occasionally required (28).
The applications of 111In-DTPA-octreotide
imaging for patients with MCC and other NETs include staging and
restaging to detect primary tumors and metastatic sites, follow-up
to detect relapse or progression, assessment of prognosis to
predict the response to therapy, monitoring of the effects of
treatment, and selection of patients to undergo PPRT (29).
An important advantage of SRS imaging compared with
other conventional techniques, such as CT, MRI and US, is that this
technique allows whole body imaging of the patient (18,28).
Considering that the majority of NETs are non-functional and appear
late with tumor mass effects, distant metastasis or both, more than
one modality is often required in order to gather sufficient
information. In 1992, Kwekkeboom et al (30) demonstrated that SRS was associated
with equivalent or greater sensitivity for imaging of MCC compared
with CT. Furthermore, in a study conducted by Guitera-Rovel et
al (31), 20 patients with stages
I, II and III MCC exhibited SRS sensitivity of ~78% and specificity
of 96%. In comparison with conventional modalities, SRS identified
4 out of 5 primary tumor sites, 6 out of 8 lymph node sites, no
skin metastases, 2 out of 3 thoracic metastases and none of the 2
hepatic metastases. Thus, it was concluded that, in addition to
conventional imaging techniques, full body SRS pre- and
post-therapeutic monitoring may provide useful information for the
detection of metastases and recurrence.
Limitations of SRS include non-targeted uptake in
various organs, such as the liver, adrenal glands, pancreas,
thyroid gland and spleen. Non-targeted uptake results in a low
tumor to background ratio, making difficult to detect metastases.
Additional limitations consist of the inability to detect small
lesions due to suboptimal spatial resolution, relatively high cost
compared with PET imaging and longer image acquisition protocol
(32). In addition, SRS is limited by
the diagnostic dilemma of determining whether a negative
18F-FDG PET scan represents the absence of a tumor or a
well-differentiated tumor that has a high possibility of expressing
somatostatin receptors. Due to the aforementioned limitations of
SRS, a novel imaging technique using positron-emitting somatostatin
analogues, such as
68Ga-1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic
acid-(Tyr3)-octreotate (68Ga-DOTATATE), has
emerged.
Following the application of SRS in the diagnosis of
NETs, the next step for patients with inoperable or metastatic
disease is to shrink the tumor using PPRT with
111Indium-, 90Ytrium- or
177Lutetium-labeled somatostatin analogues. Proof of
somatostatin receptor overexpression is required for a patient to
be a candidate for such a treatment regime. Few studies have been
conducted concerning this theranostic approach in patients with
MCC. Schmidt et al (33)
reported the cases of two patients exhibiting MCC with extensive
lymph node metastases. The extent of the disease was diagnosed
using 68Ga-DOTATATE PET/CT and after four cycles of
chemotherapy, the patients underwent PRRT with
90Y-DOTATATE or 177Lu-DOTATATE in combination
with capecitabine. A temporary partial response was achieved in the
two patients; however, progression of the disease with fatal
outcome occurred 10 and 14 months after the first symptoms
occurred, respectively. For typical NETs, symptomatic control can
be achieved using all the available radiolabelled somatostatin
analogues. However, 90Y-DOTATOC and
177Lu-DOTATATE are the most promising, providing
long-lasting responses and good survival rates (16). In addition, the use of cold
somatostatin analogues is notable. In a case report by Fakiha et
al (34), an 87-year-old patient
diagnosed with inoperable MCC was intramuscularly injected with 15
mg lanreotide every two weeks. Follow-up with octreoscan after 17
months revealed that the patient experienced a good quality of
life, with no recurrence or side-effects.
In conclusion, MCC is a highly aggressive type of
skin cancer with a high rate of metastasis and mortality.
Considering that the median time to recurrence is nine months and
that 90% of recurrences occur within the first two years of
diagnosis, more frequent imaging during this period is advocated
(5). Peloschek et al (27) recommended repetition of
18F-FDG PET three months and one year after treatment.
Considering that survival of patients with MCC is highly associated
with the extent of disease at presentation, identifying patients
earlier and performing adequate staging is desirable. Furthermore,
nuclear medicine in combination with various evaluation methods,
such as sentinel lymph node scintigraphy, SRS and PET/CT, appears
to provide additional functional information regarding MCC. This
additional information may have significant impact on the selection
of the treatment strategy and therefore result in an optimal
outcome. At present, there continues to be no imaging algorithm for
MCC and the preferred modality has yet to be established;
therefore, additional studies are required. Due to the rarity of
the malignancy and despite good understanding of a large extent of
the biology of this neoplasm, there continue to be numerous
unanswered questions regarding MCC. The current study proposes that
greater understanding of this disease entity can be achieved by
thoroughly evaluating each individual case. The purpose of the
present study was to establish the role of nuclear medicine
techniques, as applied in the current case, and to contribute to an
evidence-based imaging approach to MCC. Specifically, sentinel
lymph node biopsy guided by the scintigraphy, along with the
preoperative somatostatin receptor scintigraphy contributed to the
accurate staging of the patient, and thus influenced the
therapeutic decision. Additionally, 18F-FDG PET was used
for the whole body post-therapeutic monitoring of metastases and
recurrence.
References
|
1
|
Toker C: Trabecular carcinoma of the skin.
Arch Dermatol. 105:107–110. 1972. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Merkel F: Tastzellen und Tastkörperchen
bei den Hausthieren und beim Menschen. Archiv für Mikroskopische
Anatomie. 11:636–652. 1875. View Article : Google Scholar
|
|
3
|
Erovic I and Erovic BM: Merkel cell
carcinoma: The past, the present and the future. J Skin Cancer.
2013:9293642013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tang CK and Toker C: Trabecular carcinoma
of the skin: An ultrastructural study. Cancer. 42:2311–2321. 1978.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Han SY, North JP, Canavan T, et al: Merkel
cell carcinoma. Hematol Oncol Clin North Am. 26:1351–1374. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Duprat JP, Landman G, Salvajoli JV and
Brechtbühl ER: A review of the epidemiology and treatment of Merkel
cell carcinoma. Clinics (Sao Paulo). 66:1817–1823. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Becker JC: Merkel cell carcinoma. Ann
Oncol. 21:(Sul 7). vii81–vii85. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Feng H, Shuda M, Chang Y and Moore PS:
Clonal integration of a polyomavirus in human Merkel cell
carcinoma. Science. 319:1096–1100. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fukumoto H, Sato Y, Hasegawa H and Katano
H: Frequent detection of Merkel cell polyomavirus DNA in sera of
HIV-1-positive patients. Virol J. 10:842013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Engels EA, Frisch M, Goedert JJ, et al:
Merkel cell carcinoma and HIV infection. Lancet. 359:497–498. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Agelli M and Clegg LX: Epidemiology of
primary Merkel cell carcinoma in the United States. J Am Acad
Dermatol. 49:832–841. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Heath M, Jaimes N, Lemos B, et al:
Clinical characteristics of Merkel cell carcinoma at diagnosis in
195 patients: The AEIOU features. J Am Acad Dermatol. 58:375–381.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Moll R, Löwe A, Laufer J and Franke WW:
Cytokeratin 20 in human carcinomas. A new histodiagnostic marker
detected by monoclonal antibodies. Am J Pathol. 140:427–447.
1992.PubMed/NCBI
|
|
14
|
Jaeger T, Ring J and Andres C:
Histological, immunohistological and clinical features of merkel
cell carcinoma in correlation to merkel cell polyomavirus status. J
Skin Cancer. 2012:9834212012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mojica P, Smith D and Ellenhorn JD:
Adjuvant radiation therapy is associated with improved survival in
Merkel cell carcinoma of the skin. J Clin Oncol. 25:1043–1047.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Teunissen JJ, Kwekkeboom DJ, Valkema R and
Krenning EP: Nuclear medicine techniques for the imaging and
treatment of neuroendocrine tumours. Endocr Relat Cancer. 18:(Sul
1). S27–S51. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lemos BD, Storer BE, Iyer JG, et al:
Pathologic nodal evaluation improves prognostic accuracy in Merkel
cell carcinoma: Analysis of 5823 cases as the basis of the first
consensus staging system. J Am Acad Dermatol. 63:751–761. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nguyen BD and McCullough AE: Imaging of
Merkel cell carcinoma. Radiographics. 22:367–376. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Arruda EP and Higgins KM: Role of sentinel
lymph node biopsy in the management of merkel cell carcinoma. J
Skin Cancer. 2012:1761732012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Enzenhofer E, Ubl P, Czerny C and Erovic
BM: Imaging in patients with merkel cell carcinoma. J Skin Cancer.
2013:9731232013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Panagiotidis E and Bomanji J: Role of
18 F-fluorodeoxyglucose PET in the study of
neuroendocrine tumors. PET Clin. 9:43–55. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yao M, Smith RB, Hoffman HT, et al: Merkel
cell carcinoma: Two case reports focusing on the role of
fluorodeoxyglucose positron emission tomography imaging in staging
and surveillance. Am J Clin Oncol. 28:205–210. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lin O, Thomas A, Singh A and Greenspan B:
Complementary role of positron emission tomography in merkel cell
carcinoma. South Med J. 97:1110–1112. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Belhocine T, Pierard GE, Frühling J, et
al: Clinical added-value of 18 FDG PET in
neuroendocrine-merkel cell carcinoma. Oncol Rep. 16:347–352.
2006.PubMed/NCBI
|
|
25
|
Concannon R, Larcos GS and Veness M: The
impact of (18)F-FDG PET-CT scanning for staging and management of
Merkel cell carcinoma: Results from Westmead Hospital, Sydney,
Australia. J Am Acad Dermatol. 62:76–84. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shintani SA, Foote RL, Lowe VJ, et al:
Utility of PET/CT imaging performed early after surgical resection
in the adjuvant treatment planning for head and neck cancer. Int J
Radiat Oncol Biol Phys. 70:322–329. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Peloschek P, Novotny C, Mueller-Mang C, et
al: Diagnostic imaging in Merkel cell carcinoma: Lessons to learn
from 16 cases with correlation of sonography, CT, MRI and PET. Eur
J Radiol. 73:317–323. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ziessman H, O'Malley JP and Thrall JH:
Nuclear Medicine: The Requisites. 3rd. Mosby; Maryland Heights, MO,
USA: pp. 279–281. 2006
|
|
29
|
Pepe G, Bombardieri E, Lorenzoni A and
Chiti A: Single-photon emission computed tomography tracers in the
diagnostics of neuroendocrine tumors. PET Clin. 9:11–26. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kwekkeboom DJ, Hoff AM, Lamberts SW, et
al: Somatostatin analogue scintigraphy: A simple and sensitive
method for the in vivo visualization of Merkel cell tumors and
their metastases. Arch Dermatol. 128:818–821. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Guitera-Rovel P, Lumbroso J,
Gautier-Gougis MS, et al: Indium-111 octreotide scintigraphy of
Merkel cell carcinomas and their metastases. Ann Oncol. 12:807–811.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ambrosini V and Fanti S:
68Ga-DOTA-peptides in the diagnosis of NET. PET Clin. 9:37–42.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Schmidt MC, Uhrhan K, Markiefka B, et al:
(68)Ga-DotaTATE PET-CT followed by Peptide Receptor Radiotherapy in
combination with capecitabine in two patients with Merkel Cell
Carcinoma. Int J Clin Exp Med. 5:363–366. 2012.PubMed/NCBI
|
|
34
|
Fakiha M, Letertre P, Vuillez JP and
Lebeau J: Remission of Merkel cell tumor after somatostatin analog
treatment. J Cancer Res Ther. 6:382–384. 2010. View Article : Google Scholar : PubMed/NCBI
|