Introduction
At present, there are >300 million individuals
with thyroid disease worldwide (1).
In particular, thyroid nodules, which are the second most common
endocrine system disease, have received increasing attention
(2,3).
Conventional ultrasonography was previously considered to be the
preferred diagnostic tool for patients with thyroid disease, and
has been reported as the most effective method for the clinical
assessment and treatment of this disorder (4). However, with the development of
high-frequency ultrasonography, the early detection of thyroid
lesions has improved (5). Thus,
high-frequency ultrasonography has become the preferred method for
the clinical evaluation and treatment of thyroid disease (6). Accordingly, thyroid nodules identified
on cervical ultrasonographic examination account for 50% of all
reported thyroid nodules (7).
Papillary thyroid microcarcinoma (TMC) is an early-stage subtype of
thyroid carcinoma with a maximum tumor diameter of ≤10 mm and a
frequency of 65–99% among all thyroid carcinomas (8). However, nodular thyroid lesions
measuring ≤1 cm in diameter may possess features that are
characteristic of different types of lesions, commonly resulting in
an uncertain diagnosis during pre-operative conventional
ultrasonography (8). Therefore, the
development of novel pre-operative diagnostic methods is
required.
In previous years, contrast-enhanced ultrasound
(CEUS) and ultrasonic elastography (UE) have become popular
research topics, and these modalities have been proposed for the
diagnosis and differential diagnosis of thyroid lesions, as well as
for enhancing the diagnostic confidence for such lesions (9–11).
However, due to differences in the patients investigated and the
evaluation indices and methods employed between studies, the
reported evaluation criteria are inconsistent (12). For example, Giuseppetti et al
(13) considered the enhanced
contrast patterns of thyroid nodules to be closely associated with
nodule size as opposed to pathological type, and proposed that
nodule size had a particular influence on the UE results. This
criterion may be appropriate as UE methodology is based on external
pressure applied to the inspected tissues, which may result in
compressive deformation, and therefore may objectively reflect
tissue stiffness. To date, the majority of studies investigating
thyroid nodule diagnosis using UE have focused on differentiating
between benign and malignant nodules (14–16).
Conversely, only a limited number of studies have been conducted on
the use of CEUS and UE to differentiate between papillary TMC and
other types of lesions. Thus, the present study proposes that these
2 novel technologies may have the potential to enhance the accuracy
of benign and malignant thyroid nodule diagnosis. Accordingly, the
aim of the current study was to explore the value of UE and CEUS in
the differential diagnosis of benign and malignant lesions of the
thyroid.
Subjects and methods
Subjects
Between May 2012 and September 2013, a retrospective
analysis was performed on 73 patients that possessed a total of 80
micro-thyroid space-occupying lesions. The patients all underwent
thyroid surgery in Shaanxi Provincial Cancer Hospital Affiliated to
Medical School (Xi'an, Shaanxi, China). The diagnoses of 50 TMC
tumors and 30 benign lesions were confirmed by pathological
examination. The patients consisted of 21 men and 52 women aged
19–61 years (mean age, 39.5±10.3 years). All patients underwent
pre-operative contrast examination with CEUS and UE based on
conventional ultrasonography. The present study was conducted in
accordance with the Declaration of Helsinki (17) and with approval from the Ethics
Committee of Xi'an Jiaotong University (Xi'an, China). Written
informed consent was obtained from all participants.
Instruments and reagents
The Philips iU22 xMATRIX ultrasonography diagnostic
apparatus and L9-3 probe (Koninklijke Philips N.V., Amsterdam,
Netherlands) were used to select the thyroid examination
conditions. The angiographic region of interest was defined as
thyroid nodules with surrounding healthy tissue. The inclusion
criterion was the presence of suspicious solid nodules without a
background of diffused thyroid lesions. The instrument was equipped
with UE and CEUS analysis software, with the mechanical index set
at 0.06–0.08. The contrast agent used was SonoVue, which consisted
of sulfur hexafluoride (SF6) packaged by lipid membrane (Bracco,
Milan, Italy). Prior to use, 59 mg dry lyophilized SonoVue powder
was added to 5 ml normal saline and repeatedly agitated to form the
white SF6 microbubble suspension.
Ultrasonography
The patients were placed in the supine position, and
conventional 2-dimensional and color Doppler ultrasonography of the
thyroid were performed for all patients. If suspicious nodules were
identified, the patient underwent qualitative evaluation of the
lesions in accordance with the benign and malignant lesion
differentiation standards proposed by Stacul et al (18).
Thyroid CEUS was conducted using the dual-contrast
mode and by adjusting the gain to only capture the thyroid tunica.
Additionally, the single focus was set at the lower level of the
lesion. Subsequent to noting the name, age and sex of each patient
and ensuring that the setting parameters were correct, the position
of the probe was fixed to prevent slippage. Next, the long axis
view was selected. The mode was switched to ultrasonography
contrast mode and a venous channel was created using a 20-ml trocar
to puncture the superficial elbow vein. Following agitation and
blending for the rapid bolus injection, 2.4 ml contrast agent was
intravenously injected prior to washing the pipe with 5.0 ml
saline. Simultaneously, and maintaining same observation view, the
timer was started. This allowed real-time observation of the
dynamic images to be performed exactly 3 min after administration
of the contrast agent. The recorded observations were stored on the
built-in hard disc for subsequent off-line analysis, with the
imaging data coded according to a random order. The ultrasonography
results included the size, location and shape of the radiographic
lesion. These data were confirmed by a surgeon and a pathologist to
ensure that the intraoperative lesion corresponded with the nodules
observed during contrast-enhanced ultrasonography and pathological
examination.
Following adjustment of the position and size of the
sampling frame to ensure that the lesions and surrounding healthy
tissue were contained, UE was conducted using the dual mode for
observation in the vertical section. The probe was vertically
vibrated using light pressure according to the indicator to ensure
that the pressure index remained constant between 3 and 4 for 3–4
s. Subsequently, the color change of the sampling frame was
observed and hardness scores were calculated for each lesion.
Different colors represent the elasticity and hardness of the
tissue. Red represents soft tissue, green represents moderate hard
tissue, and blue represents inelastic hard tissue; if >50% of
the lesion area was green, the lesion was considered predominantly
green, and vice versa for blue. All patients were examined by 2
senior clinicians specialized in contrast-enhanced
ultrasonography.
Result evaluation
A double blind method was used for the 2
examinations and evaluation methods. Prior to thorough discussion
of each case, the CEUS and UE findings were confirmed by 2
experienced ultrasonography specialists and diagnoses were
determined. The following 5-point method was used to analyze the UE
data: If the cystic lesions exhibited blue and green or
red-green-blue coloring, the score was 0 points; if the lesions and
the surrounding tissue demonstrated homogeneous green coloring, the
score was 1 point; if the lesion area was >90% green, the score
was 2 points; if the lesion exhibited no incidences of blue and
green, and predominantly blue (60–90%), the score was 3 points; and
if >90% of the lesion was blue, the score was 4 points. If the
lesion scored ≥3 points, the lesion was diagnosed as a malignant
tumor, whereas if the lesion scored ≤2 points, it was diagnosed as
a benign tumor (19). CEUS evaluation
was performed by initially comparing the enhancement degree of the
lesion and surrounding healthy tissue. Enhancement was categorized
into light and weak (low enhancement), equal and high enhancement,
according to the difference between nodules and surrounding
tissues, judged by eye. Next, the enhancement mode (homogeneous
degree) was divided into homogeneous and inhomogeneous enhancement.
The phenomenon of enhancement decreasing below surrounding tissues
quickly was defined as early regression. Inhomogeneous perfusion
and whole course low enhancement were considered to be the
diagnostic indices for malignant nodules (12,18).
Statistical analysis
The accuracy of the 2 diagnostic methods was
calculated using SPSS statistical software, version 13.0 (SPSS,
Inc., Chicago, IL, USA). Comparisons between the sensitivity,
specificity and accuracy rates were analyzed using the
χ2 test and P<0.05 was considered to indicate a
statistically significant difference.
Results
CEUS features
The CEUS features of the 80 thyroid micronodules are
indicated in Table I. With regards to
enhancement degree, 10 benign nodules and 31 malignant nodules
presented a low degree; 14 benign and 15 malignant nodules
presented an equal degree; and 6 benign and 4 malignant nodules
presented a high degree. A total of 22 benign and 37 malignant
nodules presented homogeneous enhancement, while 8 benign and 13
malignant nodules displayed no homogeneous enhancement. A total of
6 benign and 18 malignant nodules exhibited early regression, while
the remaining nodules did not. The CEUS indices exhibited no
significant differences between benign and malignant thyroid
nodules (diameter, ≤10 mm; P>0.05), indicating that CEUS has no
evident advantage in the diagnosis of TMC.
 | Table I.Comparison of contrast-enhanced
ultrasound enhancement modes between the benign and malignant
thyroid micronodules (diameter, ≤10 mm; n=80)a. |
Table I.
Comparison of contrast-enhanced
ultrasound enhancement modes between the benign and malignant
thyroid micronodules (diameter, ≤10 mm; n=80)a.
|
| Enhancement
degree | Homogeneous
enhancement | Early regression |
|---|
|
|
|
|
|
|---|
| Diagnosis | Low | Equal | High | Yes | No | Yes | No |
|---|
| Benign | 10 | 14 | 6 | 22 | 8 | 6 | 24 |
| Thyroid
microcarcinoma | 31 | 15 | 4 | 37 | 13 | 18 | 32 |
Comparison of CEUS and UE
The consistency and accuracy of CEUS and UE for the
diagnosis of thyroid micronodules are indicated in Table II. The results of the pathological
diagnosis were considered as the gold standard for diagnosis.
Accordingly, the correct diagnostic rate of CEUS was determined to
be 85% (68/80 thyroid micronodules), out of which 6 benign cases
were misdiagnosed as TMC and 6 cases of TMC were misdiagnosed as
benign nodules. The correct diagnostic rate of the UE 5-point
scoring method was 92.5% (74/80 cases), with 3 cases of TMC
misdiagnosed as benign nodules and 3 cases of benign nodules
misdiagnosed as TMC. An elasticity imaging score of ≥3 points for
the diagnosis of TMC was determined to have diagnostic sensitivity
of 94.0%, specificity of 90.0% and accuracy of 92.5%; whereas the
corresponding rates for CEUS were 88.0, 80.0 and 85.0%,
respectively. Thus, ultrasonographic elasticity imaging was
determined to demonstrate more statistically significant advantages
in the accurate diagnosis of TMC compared with CEUS
(P<0.05).
 | Table II.Results of CEUS and UE in the
examination of thyroid microlesions. |
Table II.
Results of CEUS and UE in the
examination of thyroid microlesions.
|
|
| Pathological
diagnosis |
|
|
|
|---|
|
|
|
|
|
|
|
|---|
| Examination
method | Imaging
diagnosis | TMC | Benign | Sensitivity, % | Specificity, % | Accuracy, % |
|---|
| CEUS | TMC | 44 | 6 | 88.0 | 80.0 | 85.0 |
|
| Benign | 6 | 24 |
|
|
|
| UE | TMC | 47 | 3 |
94.0a |
90.0a |
92.5a |
|
| Benign | 3 | 27 |
|
|
|
Ultrasound Contrast Imaging
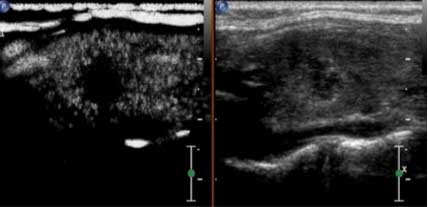
Four typical patients were selected for the
representative images. The first patient was female and 26 years
old, whose ultrasound contrast imaging of thyroid microcarcinoma
nodules indicated a lack of good blood supply, and therefore low
perfusion, and the ultrasonic appearance was consistent with the
pathological findings (Fig. 1.) The
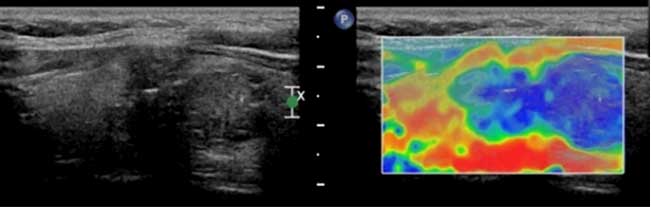
second patient was female and 38 years old, whose CEUS
cross-section demonstrated weak enhancement of the nodule. The
ultrasonic indication was that the nodular nature was to be
determined (Fig. 2A) Longitudinal UE
section demonstrating >90% blue coloring of the nodule (score, 4
points). The coloring indicates a malignant lesion, consistent with
the pathological findings (Fig. 2B).
The third patient was female and 61 years old whose
contrast-enhanced ultrasound of a nodular goiter demonstrated
uniform high enhancement (Fig. 3).
The fourth patient was female and 61 years old whose ultrasonic
elastography of a thyroid microcarcinoma, resulted in a score of 4
points, which was consistent with the pathological findings
(Fig. 4).
Discussion
Currently, there is significant controversy
regarding the application of CEUS in the diagnosis of thyroid
diseases, with different studies reporting contradictory results on
its value (12,20). Zhang et al (12) reported that thyroid nodule-enhancing
CEUS models may be divided into homogeneous, inhomogeneous
(heterogeneous), ring (ring-enhancing) and no enhancement nodules.
In addition, homogeneous enhancement was considered to be
characteristic of benign tumors, with the sensitivity and
specificity of heterogeneous low enhancement in the prediction of
malignant thyroid tumors determined as 88.2 and 92.5%,
respectively. Furthermore, Bartolotta et al (21) reported that nodules measuring <1 cm
in diameter were predominantly characterized by poorly-vascularized
features; nodules measuring 1–2 cm in diameter were characterized
by a low level of speckled enhancement; and nodules measuring >2
cm in diameter were characterized by a diffused enhanced
performance. In the present study, CEUS identified poor
vascularization, and a low (Fig. 1)
or faint/weak (Fig. 2) enhancement
degree in 31/50 TMC nodules (62%). By contrast, the remaining 19
nodules (38%) exhibited iso- or high enhancement, through high or
full coverage of the lesions by the contrast agent, in the lesions
and surrounding tissues when the contrast agent was injected. Among
these 19 nodules, 6 were diagnosed as benign. Among the 30
pathologically confirmed benign nodules, 20 nodules (66.66%)
exhibited iso- or high enhancement (Fig.
3), which pre-operatively confirmed the diagnosis of benign
nodules. However, the remaining 10 nodules (33.4%) exhibited low or
weak enhancement on CEUS. Among these, 6 nodules were misdiagnosed
as TMC. Fine-needle puncture biopsies were subsequently performed
to diagnose these nodules as 1 granulomatous nodule, 1 case of old
blood clot and 4 hyperplastic nodules with calcification. Thus, in
contrast to the findings of Bartolotta et al (21), the present study determined that
benign nodules measuring <1 cm in diameter and malignant thyroid
nodules exhibit numerous overlapping features on CEUS.
The results of the present study indicate that CEUS
has no significant advantage in the identification of TMCs. The
current authors hypothesize that the observed overlap in CEUS
perfusion was a result of the specific tissue biostructures of the
investigated nodules. Jebreel et al (22) reported that differences in the
microvessel density of benign lesions measuring <1 cm in
diameter and malignant nodules may not be evident when the tumors
are small. Furthermore, small malignant nodules may not exhibit
typical characteristics of malignant tumors, including a large
variation in vascular diameter and shape, a large number of
vascular branches, easy formation of arteriovenous fistulae, low
efficiency of new tumor vessels, or edema and fibrosis of the
lesion stroma (22). In addition, the
overlapping may be associated with the instrument sensitivity, and
the adjustments and parameters employed during use of the
instrument.
UE was originally reported by Ophir et al
(23) in 1991, and is based on the
interpretation of amplitude changes of echo signals prior to and
following compression of the tissue. The amplitude changes are
transferred into real-time color images, where the color elasticity
coding of various tissues reflects the tissue hardness (24). A long-term clinical study determined
that the hardness of lesions differs between malignant and benign
tissues, with increased hardness being associated with an increased
risk of malignancy (25).
Furthermore, a study conducted by Wang et al (26) identified that UE had high diagnostic
sensitivity and specificity for nodules measuring <1 mm in
diameter. Similarly, the sensitivity, specificity and accuracy of
UE diagnosis of the 80 TMC cases in the present study (Fig. 4) were 94.0, 90.0 and 92.5%,
respectively. These results were significantly increased compared
with the corresponding values obtained by CEUS (P<0.05).
The current authors propose that the advantages of
UE in diagnosing TMC compared with CEUS may be due to the close
association of the TMC nodule with the surrounding tissues. Thus,
the corresponding deformation required may have been small.
Furthermore, the nodules were commonly accompanied by psammoma
bodies, resulting in the lesions exhibiting a certain level
hardness and consequently a higher elasticity score, thus,
improving the diagnostic accuracy rate of UE for TMC (27).
However, the elastic coefficients of different
pathological tissues may overlap (28), indicating that UE is associated with
various influencing factors that may result in misdiagnosis.
Accordingly, 3/50 TMC nodules were misdiagnosed in the present
study due to elastic scores of ≤2 points, including 2 cases of
follicular carcinoma. We hypothesize that the reason for this
misdiagnosis may be that the investigated lesions were composed of
various degrees of differentiated follicular tissue. For example,
the stroma is rich in thin-walled vasculature and the blood flow is
more evident in these lesions compared with in papillary carcinoma
(29). If follicular differentiation
is good, the hardness does not markedly differ from that of healthy
thyroid tissue, resulting in a low elastic classification (29). In the present study, the other
misdiagnosed lesion was in a patient with Hashimoto's disease
accompanied by small papillary carcinoma. This lesion was likely
misdiagnosed due to the increasing hardness of the thyroid caused
by extensive damage to the thyroid parenchyma and tissue fibrosis
hyperplasia, resulting in hardness differences between the lesions
and a reduction in the size of peripheral tissues, thereby
resulting in a reduced nodular elastic classification. A total of 3
nodules in the benign group had an elastic score of 4 points, 1 of
which was diagnosed as a cystic adenoma. Due to the increase in
liquid tension in the cystic sac, the true hardness of internal
solid nodule was not accurately reflected, resulting in increased
hardness and a higher score, according to the elasticity imaging
5-point diagnostic method. Furthermore, the current cohort included
1 case of subacute thyroiditis and 1 case of Hashimoto's
thyroiditis, in which the nodules appeared hard due to repeated
stimulation of inflammation, fibrosis, hyalinization and other
pathological changes. Additionally, the hardness of the thyroid
gland itself may affect the thyroid nodules in elasticity imaging
(30).
According to the literature (31), ~20–50% of all TMC cases may be
associated with cervical lymph node metastasis. Lyshchik et
al (32) and Alam et al
(33) reported that UE may improve
the accuracy of the differential diagnosis of cervical lymph node
metastasis. The results of the present study require exploration in
future large-scale studies to clarify these findings.
In conclusion, it was determined that there was no
significant advantage of using CEUS in the diagnosis of TMC.
However, the novel technology of UE was demonstrated to be superior
to conventional ultrasonography in evaluating the tissue
biomechanics of TMC, and may provide relevant and useful data
regarding tissue hardness, expanding the use of ultrasonography for
diagnosis. Furthermore, the current results indicate a potential
role of UE and hardness testing in the diagnosis of small papillary
thyroid nodules.
Acknowledgements
The present study was supported by the Social
Development Research Plan Project of Shaanxi Provincial Department
of Science and Technology (grant nos. 2012K13-02-39 and
2011k15-06-14).
References
|
1
|
Müller O and Krawinkel M: Malnutrition and
health in developing countries. CMAJ. 173:279–286. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Golden SH, Robinson KA, Saldanha I, Anton
B and Ladenson PW: Clinical review: Prevalence and incidence of
endocrine and metabolic disorders in the United States: A
comprehensive review. J Clin Endocrinol Metab. 94:1853–1878. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Roti E, Degli Uberti EC, Bondanelli M and
Braverman LE: Thyroid papillary microcarcinoma: A descriptive and
meta-analysis study. Eur J Endocrinol. 159:659–673. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wong CK and Wheeler MH: Thyroid nodules:
rational management. World J Surg. 24:934–941. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Haber RS: Role of ultrasonography in the
diagnosis and management of thyroid cancer. Endocr Pract.
6:396–400. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Guth S, Theune U, Aberle J, Galach A and
Bamberger CM: Very high prevalence of thyroid nodules detected by
high frequency (13 MHz) ultrasound examination. Eur J Clin Invest.
39:699–706. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Isidro ML, Penín M and Cordido F:
Systematic ultrasound evaluation of thyroid nodules. Thyroid.
16:1324–1325. 2006.PubMed/NCBI
|
|
8
|
Roti E, Degli Uberti EC, Bondanelli M and
Braverman LE: Thyroid papillary microcarcinoma: a descriptive and
meta-analysis study. Eur J Endocrinol. 159:659–673. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Holtel MR: Emerging technology in head and
neck ultrasonography. Otolaryngol Clin North Am. 43:1267–1274.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yu D, Han Y and Chen T: Contrast-enhanced
ultrasound for differentiation of benign and malignant thyroid
lesions Meta-analysis. Otolaryngol Head Neck Surg. 151:909–915.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Carneiro-Pla D: Ultrasound elastography in
the evaluation of thyroid nodules for thyroid cancer. Curr Opin
Oncol. 25:1–5. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang B, Jiang YX, Liu JB, Yang M, Dai Q,
Zhu QL and Gao P: Utility of contrast-enhanced ultrasound for
evaluation of thyroid nodules. Thyroid. 20:51–57. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Giuseppetti GM, Martegani A, Di Cioccio B
and Baldassarre S: Elastosonography in the diagnosis of the nodular
breast lesions: preliminary report. Radiol Med. 110:69–76.
2005.PubMed/NCBI
|
|
14
|
Luo S, Kim EH, Dighe M and Kim Y: Thyroid
nodule classification using ultrasound elastography via linear
discriminant analysis. Ultrasonics. 51:425–431. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ding J, Cheng H, Ning C, Huang J and Zhang
Y: Quantitative measurement for Thyroid cancer characterization
based on elastography. J Ultrasound Med. 30:1259–1266.
2011.PubMed/NCBI
|
|
16
|
Bhatia KS, Rasalkar DP, Lee YP, Wong KT,
King AD, Yuen HY and Ahuja AT: Cystic change in change in thyroid
nodules: A confounding factor for real-time qualitative thyroid
ultrasound elastography. Clin Radiol. 66:799–807. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rickham PP: Human experimentation. Code of
ethics of the World Medical Association. Declaration of Helsinki.
Br Med J. 2:1771964.
|
|
18
|
Stacul F, Bertolotto M, De Gobbis F, et
al: US, colour-Doppler US and fine-needle aspiration biopsy in the
diagnosis of thyroid nodules. Radiol Med. 11:751–762. 2007.
View Article : Google Scholar
|
|
19
|
Rago T, Santini F, Scutari M, Pinchera A
and Vitti P: Elastography: new developments in ultrasound for
predicting malignancy in thyroid nodules. J Clin Endocrinol Metab.
92:2917–2922. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Friedrich-Rust M, Sperber A, Holzer K, et
al: Real-time elastography and contrast-enhanced ultrasound for the
assessment of thyroid nodules. Exp Clin Endocrinol Diabetes.
118:602–609. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bartolotta TV, Midiri M, Galia M, et al:
Qualitative and quantitative evaluation of solitary thyroid nodules
wih contrast-enhanced ultrasound: initial results. Eur Radiol.
16:2234–2241. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jebreel A, England J, Bedford K, Murphy J,
Karsai L and Atkin S: Vascular endothelial growth factor (VEGF),
VEGF receptors expression and microvascular density in benign and
malignant thyroid diseases. Int J Exp Pathol. 88:271–277. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ophir J, Céspedes I, Ponnekanti H, Yazdi Y
and Li X: Elastography: a quantitative method for imaging the
elasticity of biological tissues. Ultrason Imaging. 13:111–134.
1991. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Khalil AS, Chan RC, Chau AH, Bouma BE and
Mofrad MR: Tissue elasticity estimation with optical coherence
elastography: toward mechanical characterization of in vivo soft
tissue. Ann Biomed Eng. 33:1631–1639. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pacini F, Schlumberger M, Dralle H, Elisei
R, Smit JW and Wiersinga W: European Thyroid Cancer Taskforce:
European consensus for the management of patients with
differentiated thyroid carcinoma of the follicular epithelium. Eur
J Endocrinol. 154:787–803. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang Y, Dan HJ, Dan HY, Li T and Hu B:
Differential diagnosis of small single solid thyroid nodules using
real-time ultrasound elastography. Int Med Res. 38:466–472. 2010.
View Article : Google Scholar
|
|
27
|
Lyshchik A, Higashi T, Asato R, et al:
Thyroid gland tumor diagnosis at US elastography. Radiology.
237:202–211. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hiltawsky KM, Krüger M, Starke C, Heuser
L, Ermert H and Jensen A: Freehand ultrasound elastography of
breast lesions: clinical results. Ultrasound Med Biol.
27:1461–1469. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ren X, Zhan W and Zhou P: Comparative
study of real-time elastography and grey-scale ultrasonography in
the diagnosis of thyroid occupied lesions. Chin J Ultrasound Med.
25:128–132. 2009.
|
|
30
|
Shuzhen C: Comparison analysis between
conventional ultrasonography and ultrasound elastography of thyroid
nodules. Eur J Radiol. 81:1806–1811. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Costa S, Giugliano G, Santoro L, et al:
Role of prophylactic central neck dissection in cN0 papillary
thyroid cancer. Acta Otorhinolaryngol Ital. 29:61–69.
2009.PubMed/NCBI
|
|
32
|
Lyshchik A, Higashi T, Asato R, et al:
Cervical lymph node metastases: diagnosis at sonoelastography -
initial experience. Radiology. 243:258–267. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Alam F, Naito K, Horiguchi J, Fukuda H,
Tachikake T and Ito K: Accuracy of sonographic elastography in the
differential diagnosis of enlarged cervical lymph nodes: comparison
with conventional B-mode sonography. AJR Am J Roentgenol.
191:604–610. 2008. View Article : Google Scholar : PubMed/NCBI
|