Introduction
Ulcerative colitis (UC) is a non-specific intestinal
inflammatory disease and the pathogenesis of the disease has not
been fully elucidated at present. Previous studies have revealed
that individuals suffering from UC are at an increased risk of
developing colitis-associated cancer (CAC), which accounts for 15%
of inflammatory bowel disease (IBD)-associated mortalities
(1). Meanwhile, patients with Crohn's
disease (CD), a subtype of IBD, have a lower risk for colorectal
cancer compared with UC patients, and the pathogenesis is largely
unknown. The main strategy for CAC prevention in patients with
chronic UC is currently based on the identification of neoplasia by
surveillance colonoscopy. However, there is considerable interest
in the possibility of primary chemoprevention (2).
Peroxisome proliferator-activated receptors (PPARs)
are ligand-activated transcription factors that belong to a
superfamily of nuclear hormone receptors and comprise a subfamily
with three different isoforms, PPAR-α, PPAR-γ and PPAR-β/δ.
Previous studies (3–5) have reported that PPAR-γ plays an
important role in maintaining the intestinal immune balance, and
may exert anti-inflammatory and antineoplastic effects.
A standard drug used for the maintenance of the
remission of UC is 5-aminosalicylic acid (5-ASA). Epidemiological
data has suggested that 5-ASA prevents colorectal cancer in
patients receiving the drug (6),
although the mechanism of action has yet to be fully elucidated. As
5-ASA is a ligand for PPAR-γ (7), the
dependence of the anti-proliferative effects of 5-ASA on PPAR-γ has
been investigated, but the results have been inconsistent. Schwab
et al (5) revealed that 5-ASA
arrested the cell cycle of Caco2, HT29 and HCT116 cells in the
G0/G1 phase and induced apoptosis in a manner
that partly depended on PPAR-γ. However, Koelink et al
(8) found that GW9662 (a selective
PPAR-γ antagonist) was not able to block the anti-proliferative
effects of 5-ASA, while the agent was able to block the
anti-proliferative effect of troglitazone. Kohno et al
(9) reported that the administration
of the PPAR ligand troglitazone significantly inhibited the
incidence and multiplicity of the colonic adenocarcinoma induced by
azoxymethane (AOM)/dextran sodium sulfate (DSS) in mice. The
administration of troglitazone also decreased the proliferating
cell nuclear antigen (PCNA)-labeling index and the expression of
β-catenin, COX-2, inducible nitric oxide synthase (iNOS) and
nitrotyrosine (9). The treatments
increased the apoptosis index in the colonic adenocarcinoma
tissues. Clearly, systematic studies are required to confirm the
role of PPAR-γ in IBD and to confirm the anti-proliferative effects
mediated by 5-ASA.
To understand the pathogenesis of IBD and CAC,
several animal models have been established. One widely used and
thoroughly described CAC model is the AOM/DSS model. In this model,
AOM is administered to promote tumor formation and DSS dissolved in
the drinking water of rodents induces intestinal inflammation, as
well as crypt damage and crypt loss. When DSS is administered for a
number of days, followed by a period of normal drinking water, the
inflammatory period is followed by a period of healing and repair
that resembles the exacerbation and remission stages in human UC
(10,11). DSS-induced colitis in the AOM mouse
model has been demonstrated to accelerate the development of
colorectal tumors without altering the pathological characteristics
of the tumors and to be useful as a colitis-associated intestinal
cancer model (12,13).
The present study aimed to evaluate the PPAR-γ
levels in IBD and the association between PPAR-γ and the clinical
features of patients with IBD. In addition, an AOM/DSS mouse model
of colitis-associated neoplasia was established to investigate the
protective effect of 5-ASA and to explore the changes in the
expression of PPAR-γ during this process.
Materials and methods
Sample collection
In total, 66 IBD biopsy samples, consisting of 38 UC
and 28 Crohn's disease (CD) tissue samples, and 30 healthy
colorectal mucosa specimens were obtained from the Endoscopy Center
of Shanghai First People's Hospital (Shanghai, China). The tissue
specimens were collected between March 2010 and March 2011. The UC
patients consisted of 20 males and 18 females, with ages that
ranged between 17 and 62 years and a median age of 39 years. The CD
patients consisted of 18 males and 10 females, with ages that
ranged between 16 and 59 years and a median age of 36.9 years. Out
of the patients with UC and CD, 25 UD and 20 CD patients were in an
active stage of disease. Of the 30 healthy controls, consisting of
11 males and 19 females, the ages ranged between 25 and 57 years,
with a median age of 43 years. Ethical approval was obtained from
the Research Ethics Committee of the First People's Hospital of
Shanghai Jiaotong University. All tissue samples were anonymized
according to ethical and legal standards [Standard for quality
management of clinical research (SFDA-GCP), 2003; http://www.sda.gov.cn/WS01/CL0053/24473.html] and
informed consent was obtained from each patient or their
family.
Animals and treatment
In total, 36 female BALB/c mice were obtained from
the Shanghai Experimental Animal Facility of the Chinese Academy of
Sciences (Shanghai, China). The mice were purchased at six weeks of
age. The mice were housed in an environment with controlled
temperature (20–25°C), humidity (40–70%) and day-night cycles (12 h
of light and 12 h of darkness), with free access to standard
laboratory feed and water. The investigations were performed in
accordance with the guidelines set by the Ministry of Health of the
People's Republic of China regarding the care and use of animals
for experimental procedures (http://www.gov.cn/gongbao/content/2011/content_1860757.htm).
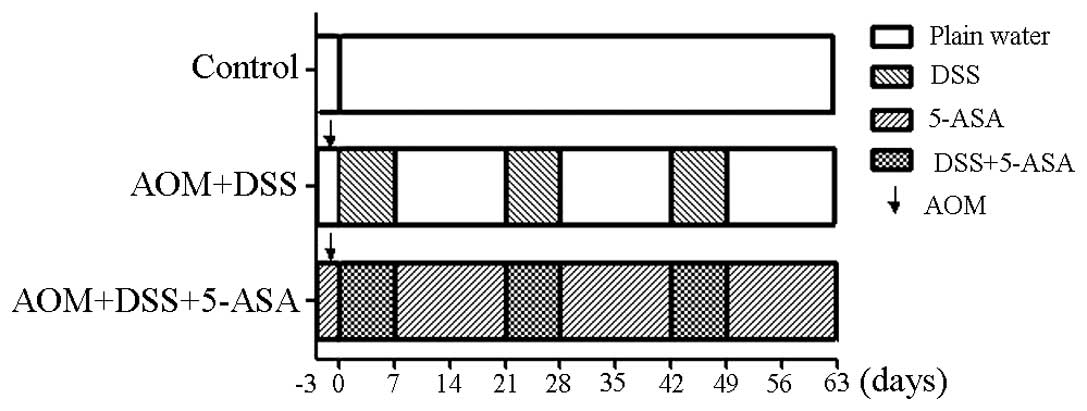
The AOM/DSS model of colitis-associated colorectal carcinogenesis
was employed in the present study according to the experimental
treatment protocol outlined in Fig.
1. Following an acclimation period, the mice were randomly and
equally divided into three groups, with 12 mice per group. For the
model group, 10 mg/kg AOM (Sigma-Aldrich, St. Louis, MO, USA) was
administered to the mice through an injection when they reached
seven weeks of age. Treatment with DSS (molecular mass, 30–50 kDa;
MP Biomedicals LLC, Santa Ana, CA, USA) began three days subsequent
to the administration of 5-ASA and continued for three cycles, with
each cycle consisting of treatment with drinking solution
containing 4% DSS for seven days, followed by 14 days of untreated
water. The treatment group received 150 mg/kg 5-ASA (Tokyo Chemical
Industry, Co., Ltd, Tokyo, Japan) through a diet containing 0.15%
5-ASA, which was manufactured by the Shanghai Experiment Animal
Facility of Chinese Academy of Sciences and stored in light-free
conditions. The 5-ASA dose was calculated based on the estimation
that a mouse weighing 20 g consumes a daily diet of 5 g, consuming
2 g and wasting 3 g per day. The remaining group acted as a control
and received untreated drinking water at all times.
Assessment of inflammation
At the end of first week, the clinical disease
activity index (DAI) was measured using the previously described
protocol (14). The DAI ranged
between 0 and 4 and was calculated from the sum of scores provided
for percentage loss of body weight, stool consistency and presence
or absence of fecal blood. Body weight loss was scored as follows:
0, none; 1, 1–5%; 2, 5–10%; 3, 10–20%; and 4, >20%. Stool
consistency was scored as: 0, well-formed pellets; 2, loose stools;
and 4, diarrhea. The presence or absence of fecal blood was scored
as: 0, negative hemoccult test; 2, positive hemoccult test; and 4,
gross bleeding. Three mice in each group were randomly selected and
sacrificed at the end of the first week for the analysis of colonic
inflammation and ulceration. Hematoxylin and eosin (HE) staining was
performed using a previously described protocol (15) to assess the inflammation and integrity
of the intestinal mucosa.
General observation and gross
anatomy
The body weight of the mice was recorded at the time
of AOM injection, and at the beginning and end of each cycle of DSS
administration. During the period of DSS consumption and for the
first week of untreated water consumption, data regarding the
mental state, gloss of body hair, appearance of perianal region,
presence of thin or blood-containing stool, and mortality of the
mice were also recorded. Particular attention was paid to the
perianal tumor at day 42, during the third cycle of DSS. The
remaining animals were sacrificed subsequent to three cycles of
treatment. The entire colon and rectum were excised, sliced
longitudinally, and rinsed in saline. The length of the colon and
the number of tumors present were also determined.
Immunohistochemistry and
assessment
A total of 66 biopsy specimens of colorectal tissue
obtained from patients with IBD and 30 normal controls were
immunohistochemically stained for the expression of PPAR-γ.
Paraffin-embedded 4-µm thick sections of the tissues were
deparaffinized in 100% xylene and rehydrated in descending ethanol
dilutions, according to standard protocols (16). Heat-induced antigen retrieval was
performed in CBS buffer (pH 6.0) for 10 min at 100°C. Endogenous
peroxidase activity and non-specific antigens were blocked with
peroxidase blocking reagent containing 3% hydrogen peroxide and
serum, followed by incubation with rabbit polyclonal antibody
against human PPAR-γ (dilution, 1:50; Abcam, Cambridge, UK)
overnight at 4°C. Subsequent to washing, the sections were
incubated with HRP-labeled secondary antibody for 40 min at 37°C.
The peroxidase reaction was developed using 3,3′-diaminobenzidine
(DAB) chromogen solution in DAB buffer substrate (ChemMate and
EnVision Detection kit; Gene Tech (Shanghai) Co., Ltd., Shanghai,
China). Sections were visualized using DAB counterstained with
hematoxylin, mounted in neutral gum and analyzed using a bright
field microscope (Olympus Corporation, Tokyo, Japan).
The stained samples were scored by two independent
observers for the proportion of epithelial cells that stained for
PPAR-γ expression, resulting in scores of 1 for <25% of cells
being stained, 2 for 25–50% and 3 for >50%. The samples were
also scored for the intensity of epithelial staining relative to
that observed in adjacent endothelial cells, resulting in scores of
0–3 for absent, weak, moderate and strong staining, respectively.
The adjacent endothelial cells acted as an internal positive
control. PPAR-γ expression was quantified subjectively by summating
the scores obtained for the intensity and proportion of stained
cells, as follows: Absent expression, 0–1; weakly positive (+), 2;
moderately positive (++), 3–4; and strongly positive (+++), 5–6.
For statistical analysis, patients with staining between + and +++
were grouped together as the PPAR-γ-positive group. Associations
were assessed between the proportion and intensity of PPAR-γ
immunoreactivity and the clinical characteristics of the
patients.
Reverse transcription quantitative
polymerase chain reaction (PCR)
Total RNA was extracted from frozen tissues using
the TRIzol reagent method. Briefly, 1 ml of TRIzol (Life
Technologies, Grand Island, NY, USA) was added to 50–100 mg of
tissue and the tissue was homogenized in an RNase-free environment.
Chloroform was then added at a concentration of 200 µl chloroform
per 1 ml TRIzol, and the samples were centrifuged at 12,000 × g for
15 min at 4°C. The aqueous layer was then transferred into a fresh
tube and RNA was precipitated with isopropanol followed by one wash
using 70% ethanol. The RNA precipitate was then dissolved into
10–15 µl of RNase-free water and analyzed for quantity and quality
using a spectrophotometer. A two-step reverse transcription-PCR
procedure was performed. Total RNA was reverse transcribed using
the GeneAmp kit (Takara Bio, Inc., Otsu, Shiga, Japan).
Quantitative PCR was performed on an MJ Opticon 2 real-time PCR
system (Opticon, Hoofddorp, Netherlands) according to the
manufacturer's instructions. Subsequent to setting the
amplification conditions, the experiments were repeated twice. The
primers used were as follows: PPAR-γ forward,
5′-GCCTCCCTGATGAATAAAGATG-3′ and reverse,
5′-AGGCTCCATAAAGTCACCAAAG-3′; GAPDH forward,
5′-GGTGAAGGTCGGTGTGAACG-3′ and reverse, 5′-CTCGCTCCTGGAAGATGGTG-3′.
The PCR cycling program was changed to 95°C for 1 min, and then 40
cycles were performed at 94°C for 30 sec, 60°C for 30 sec and 72°C
for 30 sec. Data analysis was performed using the Opticon Monitor 3
software. The results were expressed as fold-change in relative
mRNA expression level, calculated using the 2−ΔΔCt
method, with GAPDH acting as the reference gene and the normal
tissue providing a baseline.
Statistical analyses
Data are presented as the mean ± standard deviation.
Statistical analysis was performed using SPSS statistical software,
version 13.0, for Windows (SPSS, Inc., Chicago, IL, USA). For
comparisons between groups, analysis of variance and Student's
t-test were used. P<0.05 was considered to indicate a
statistically significant difference.
Results
Expression of PPAR-γ is attenuated in
patients with active UC and in the AOM/DSS mouse model
Immunohistochemical analysis revealed that PPAR-γ
was expressed in the cytoplasm and nuclei of cells in IBD tissues
and normal intestinal epithelium. As shown in Fig. 2 and Table
I, compared with healthy control individuals and CD patients,
the expression of PPAR-γ in the intestinal tissue of patients with
UC was significantly decreased (P=0.027 and 0.046, respectively).
The expression of PPAR-γ was significantly associated with the
activity of UC (P=0.028). However, PPAR-γ expression was not
associated with the severity of disease, site of lesions or
clinical characteristics of patients with CD (P>0.05; Table II). Downregulation of PPAR-γ mRNA in
the intestinal tissue of the AOM/DSS model compared with that of
the AOM/DSS/5-ASA and control group was also identified (Fig. 3), and this downregulation was
statistically significant (P<0.001).
 | Table I.Expression of PPAR-γ in patients with
inflammatory bowel disease (n=96). |
Table I.
Expression of PPAR-γ in patients with
inflammatory bowel disease (n=96).
|
|
| PPAR-γ
expression |
|
|---|
|
|
|
|
|
|---|
| Group | n | – | + | ++ | +++ | Positive rate, n
(%) |
|---|
| CD | 28 | 11 | 6 | 9 | 2 | 17
(60.71)a |
| UC | 38 | 25 | 7 | 4 | 2 | 13 (34.21) |
| Control | 30 | 11 | 3 | 13 | 3 | 19
(63.33)a |
 | Table II.Characteristics of the patients with
regard to peroxisome proliferator-activated receptor-γ
expression. |
Table II.
Characteristics of the patients with
regard to peroxisome proliferator-activated receptor-γ
expression.
| A, Patients with
ulcerative colitis (n=38) |
|
|
|---|
|
|---|
|
Characteristics | n | Positive rate, n
(%) |
|---|
| Activity of
diseasea |
|
|
|
Remission | 13 | 8
(61.54) |
|
Active | 25 | 5
(20.00) |
|
Severityb |
|
|
|
Mild | 9 | 4
(44.44) |
|
Moderate | 13 | 1 (7.69) |
|
Severe | 3 | 0 (0.00) |
| Lesion site |
|
|
| Whole
colon | 11 | 3
(27.27) |
| Left
colon | 16 | 7
(43.75) |
| Rectum
and sigmoid | 6 | 2
(33.33) |
|
Rectum | 5 | 1
(20.00) |
|
| B, Patients with
Crohn's disease (n=28) |
|
|
|
|
Characteristics | n | Positive rate, n
(%) |
|
| Activity of
disease |
|
|
|
Remission | 8 | 6
(75.00) |
|
Active | 20 | 11 (55.00) |
| Severity |
|
|
|
Mild | 7 | 4
(57.14) |
|
Moderate | 11 | 6
(54.55) |
|
Severe | 2 | 1
(50.00) |
| Lesion site |
|
|
|
Intestinal | 16 | 10 (62.50) |
|
Colon | 7 | 5
(71.43) |
|
Ileocolon | 5 | 2
(40.00) |
Colitis-associated colorectal
dysplasias are inhibited and the expression of PPAR-γ is promoted
in the intestinal tract by 5-ASA
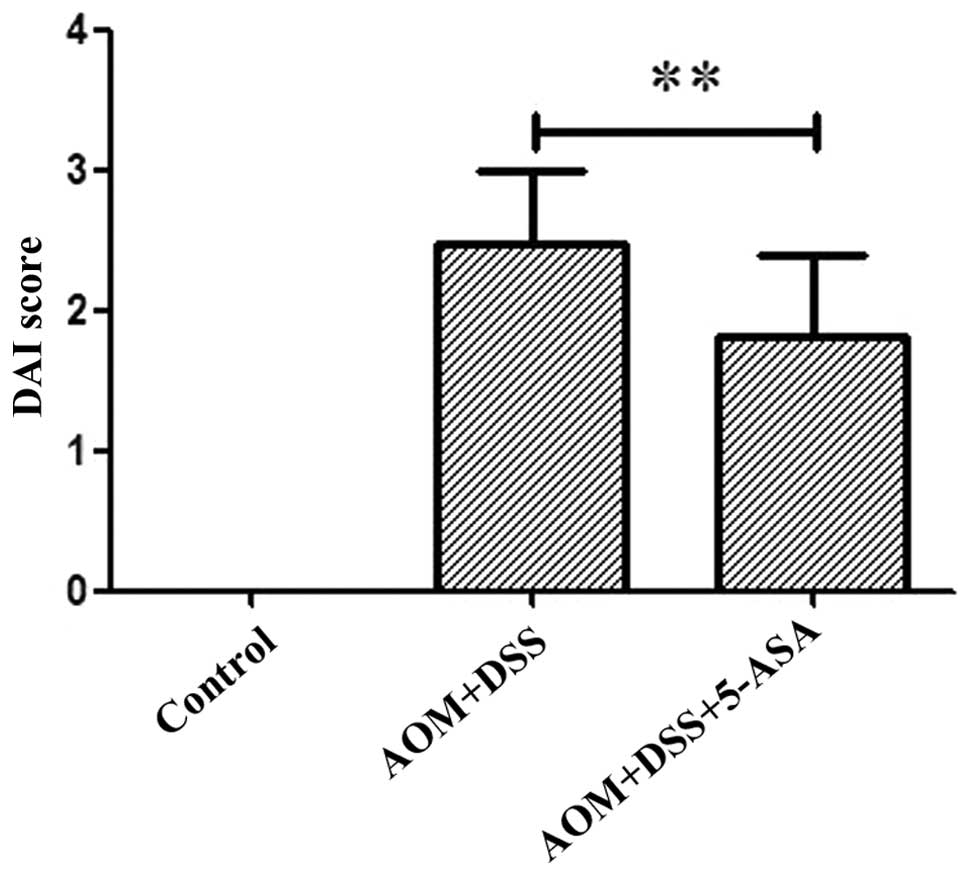
Colorectal inflammation was utilized to assess the
symptoms associated with colitis, and the DAI was recorded at the
end of the first week. The AOM/DSS group demonstrated an increase
in the DAI compared with the control group (Fig. 4), and this parameter was clearly
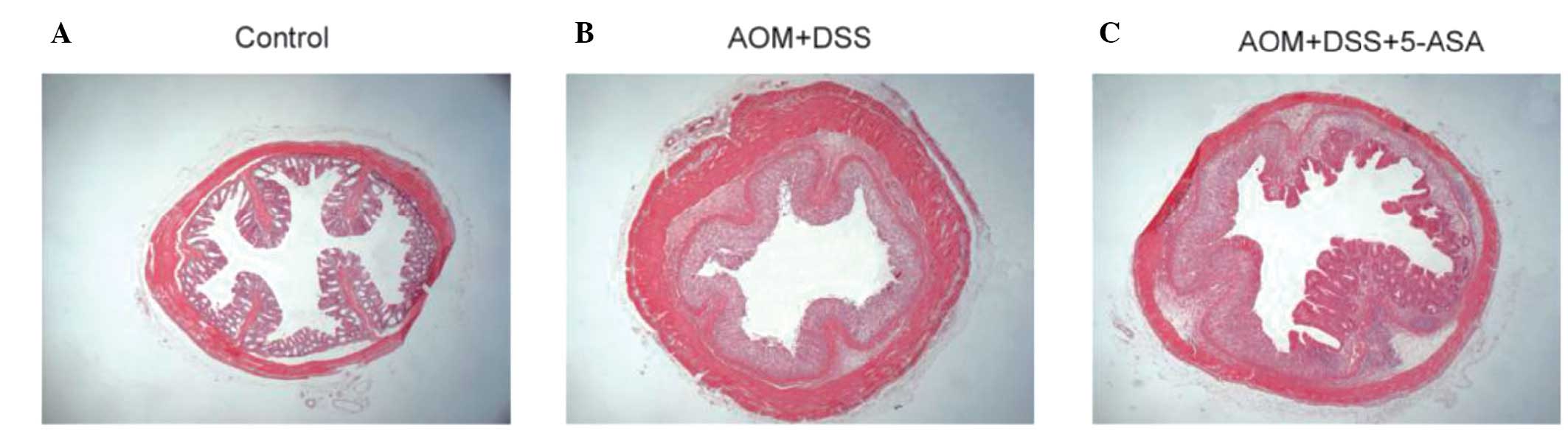
reduced in the 5-ASA group (P=0.009). HE staining revealed
neutrophil infiltration of the lamina propria and mucosa and
destruction of the intestinal glands in the AOM/DSS group. However,
the 5-ASA group demonstrated attenuated inflammation and renewal of
the crypts (Fig. 5).
In total, two mortalities occurred when the animals
were challenged with DSS in cycles 1 and 3, respectively. Diarrhea
and blood in the stool initially appeared 3–4 days subsequent to
the initiation of DSS administration in the AOM/DSS group, and
ceased at 7–9 days after the removal of DSS from the water supply,
during which the mice exhibited messy body hair, malaise and a lack
of exercise. The average appearance of diarrhea and bloody stools
in the 5-ASA treatment group commenced 1–2 days subsequent to the
initial administration of DSS, with mild symptoms and a faster
recovery. Three mice were observed to have developed perianal
tumors in the AOM/DSS group, one of which comprised ulcers and
bleeding. During the observation period, the AOM/DSS and 5-ASA
groups each demonstrated an inconsistent weight loss and recovery
curve (Fig. 6A). The difference
between the body weight of mice in the two groups in week 9 was
statistically significant (P=0.002; Table III).
 | Table III.General observations and gross
anatomy of the mice in the treatment and control groups. |
Table III.
General observations and gross
anatomy of the mice in the treatment and control groups.
| Group | Survival rate, n
(%) | Body weight, g | Length of colon,
cm | Tumor number |
|---|
| Control | 9
(100.0) | 23.31±0.47 | 8.59±0.63 | 0 |
| AOM+DSS | 7 (77.8) | 19.90±1.37 | 7.26±0.60 | 9.71±2.29 |
| 5-ASA
treatment | 9
(100.0) |
21.80±0.56b |
8.22±0.67a |
4.11±1.05b |
In visual observation, the AOM/DSS group possessed
intestinal edema, intestinal deformation, brittle intestines and
bled easily. The administration of 5-ASA significantly prevented
the shortening of the colorectum in the treatment group (P=0.01),
and the average number of tumors in the group administered with
5-ASA was significantly reduced (P<0.001), which demonstrated
the protective properties of 5-ASA (Fig.
6B and C; Table III). Flat
dysplasia and dysplasia-associated lesions or masses were each
revealed in the AOM/DSS group by HE staining, with the presence of
a high degree of atypical hyperplasia and carcinoma in situ.
However, atypical hyperplasia was more common in the group treated
with 5-ASA (data not shown).
PPAR-γ was considered to play a role in
5-ASA-mediated protection against AOM/DSS-induced
colitis-associated neoplasia. Colon homogenates from the treatment
group revealed that 5-ASA increased the transcriptional expression
of PPAR-γ (P<0.001; Fig. 3).
Discussion
PPAR-γ is a ligand-activated transcription factor
that was originally identified as a receptor expressed in adipose
tissue, where it played a role in adipocyte differentiation and in
the regulation of insulin responses. The human PPAR-γ gene is
located on chromosome 3p25, and three mRNA transcript variants that
encode two isoforms of PPAR-γ, PPAR-γ1 and PPAR-γ2, have been
found. The colon has been identified as one of the tissues
expressing the highest levels of PPAR-γ, next to adipose tissue in
epithelial cells and, to a lesser degree, macrophages and
lymphocytes (17,18). In the cellular system, PPAR exists as
a heterodimer formed with retinoid X receptor (RXR). Upon
activation by endogenous or synthetic ligands, PPAR binds to
specific PPAR response elements (PPREs) in the target genes and
acts as a transcriptional regulator (19). In addition, PPAR-γ is also able to
interfere with other transcriptional factors through a non-DNA
binding mode. This interference is mediated by physical association
between the heterodimer of PPAR and RXR and other activated
transcription factors, such as Janus kinase/signal transducer and
activator of transcription (20),
nuclear factor-κB (21) and nuclear
factor of activated T-cells (22),
thereby blocking the functions of the factors. In addition,
excessive binding of the shared co-activators with the PPAR-RXR
dimer renders these co-activators unavailable for other
transcription factors and therefore prevents the activation of the
transcription factors (23).
Macrophage-specific PPAR-γ deletion significantly impaired the
splenic and mesenteric lymph node regulatory T cell component. In
addition, macrophage-specific PPAR-γ deletion increased the
proportion of lamina propria CD8+ T cells expressing
CD40, Ly6C and TLR-4 on the cell surface. The expression of colonic
IFN-γ, CXCL9, CXCL10, IL-22, IL1RL1, CCR1, suppressor of cytokine
signaling 3 and MHC class II was also upregulated in mice with IBD
(24).
In the present study, it was found that PPAR-γ was
downregulated in active UC and the expression of PPAR-γ was
significantly associated with disease activity. These findings
demonstrated that deregulation of PPAR-γ may play an important role
in the pathogenic process of UC. The present data were also
consistent with the results obtained by Yamamoto-Furusho et
al (25), which indicated that
the mRNA expression of PPAR-γ was decreased in rectal mucosa from
patients with active UC compared with UC patients in remission, and
PPAR-γ gene expression was negatively correlated with endoscopic
activity, as determined by Spearman correlation test. The present
study did not identify a correlation between the expression of
PPAR-γ and the site of UC lesions, which indicates that PPAR-γ was
involved in an alternative component of the UC intestinal
inflammation.
UC is associated with an increased risk of
colorectal cancer. It is estimated that only 20–50% of colonic
neoplasms are detected during routine colonoscopy (26). The chemopreventive effect of 5-ASA is
therefore of considerable interest, and several studies (27,28) have
examined the ability of 5-ASA to inhibit colorectal cancer in
murine models, but the mechanism of the anti-neoplastic effects of
5-ASA remain ambiguous. The pathogenesis of CAC is widely
considered to involve a step-wise progression from inflamed and
hyperplastic epithelia to flat dysplasia and finally to
adenocarcinoma (29). CAC is probably
promoted by chronic inflammation, although the mechanism remains
unclear. In the present study, preventive oral administration of
5-ASA attenuated acute colitis as observed by a significant
reduction of the disease activity index (DAI),
histological/microscopic damage score in colonic tissue. The
anti-neoplastic effect may be partly attributable to the inhibition
of inflammation.
However, previous studies have reported that the
antineoplastic effects of 5-ASA did not completely rely on the
anti-inflammation effect. Clapper et al (30) reported that an inverse association was
observed between the dose of 5-ASA administered and the
multiplicity of colonic dysplasias. Inflammation was least severe
in the group administered with 75 mg/kg 5-ASA, which exhibited the
fewest number of colorectal tumors. This contradictory result
suggested that 5-ASA may utilize an alternative mechanism for
suppressing carcinoma. Rousseaux et al (7) reported that 5-ASA exerted its effects in
the colon through direct PPAR activation, as it was of interest to
elucidate the dependence of the 5-ASA anti-proliferative effects on
PPAR-γ. Due to the association between decreased PPAR-γ expression
and colonic carcinoma and inflammation in the present study, it was
hypothesized that the chemopreventive effects of 5-ASA appear to be
mediated, at least in part, by PPAR-γ activation.
The wnt/β-catenin-TCF signaling pathway has been
reported to play a critical role in the process of colorectal
cancer (31). β-catenin is an
important element of the wnt/β-catenin-TCF signaling pathway and is
also a key regulator of the cadherin-mediated cell-cell adhesion
system. The increased expression of β-catenin that results from
mutation of the APC gene has been considered as the primary
mediator of colon carcinogenesis (23). In sporadic colon cancer, PPAR-γ was
found to interfere with transcription in the wnt signaling pathway
through competition with β-catenin for co-activating factors;
mutations of the tumor suppressor gene APC that result in APC
inhibition and activation of glycogen synthase kinase 3β (32) accelerated the phosphorylation of
β-catenin. A previous study demonstrated that 5-ASA treatment did
not alter the expression of β-catenin (28). These data suggest that the protective
effect exerted by PPAR-γ may not involve the wnt/β-catenin-TCF
signaling pathway. The etiology underlying impaired PPAR-γ
expression in colonic epithelial cells in UC and CAC has yet to be
elucidated. One common polymorphism in the PPAR-γ gene is a proline
to alanine substitution (Pro12Ala) that results in a missense
substitution from CCA to GCA in codon 12 of exon 2 of the PPAR-γ
gene. Previous studies (33,34) have aimed to explore the association
between the Pro12Ala polymorphism in the PPAR-γ gene and UC disease
activity. The results indicated that the frequency of Pro/Alo
heterozygotes for the PPAR-γ gene was not significantly different
between the UC and control groups, which suggested that the
dysfunction of PPAR-γ may not be regulated at the gene level, but
at the post-transcription level. Previous studies have found that
during the process of LPS-stimulated downregulation of PPAR-γ, the
half-life of the PPAR-γ mRNA was shortened, and small non-coding
RNA, termed microRNA, may be involved in this process (35). To clarify the downregulation mechanism
of PPAR-γ under inflammatory status may become the focus of further
study.
In conclusion, the present study demonstrated that
PPAR-γ plays an important role in the pathogenic process of UC and
CAC. Based on the present findings, the chemopreventive effect was
considered to possibly be explained in part by the upregulation of
PPAR-γ. Therefore, targeting PPAR-γ may provide a novel and
promising approach for the treatment of UC and the prevention of
CAC.
Acknowledgements
The authors would like to thank Yueqin Tang and
Chengmin Qiu for their technical assistance.
References
|
1
|
Munkholm P: Review article: The incidence
and prevalence of colorectal cancer in inflammatory bowel disease.
Aliment Pharmacol Ther. 18:(Suppl 2). 1–5. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cheng Y and Desreumaux P: 5-aminosalicylic
acid is an attractive candidate agent for chemoprevention of colon
cancer in patients with inflammatory bowel disease. World J
Gastroenterol. 11:309–314. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Debril MB, Renaud JP, Fajas L and Auwerx
J: The pleiotropic functions of peroxisome proliferator-activated
receptor gamma. J Mol Med Berl. 79:30–47. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hontecillas R and Bassaganya-Riera J:
Peroxisome proliferator-activated receptor gamma is required for
regulatory CD4+ T cell-mediated protection against colitis. J
Immunol. 178:2940–2949. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Schwab M, Reynders V, Loitsch S, Shastri
YM, Steinhilber D, Schröder O and Stein J: PPARgamma is involved in
mesalazine-mediated induction of apoptosis and inhibition of cell
growth in colon cancer cells. Carcinogenesis. 29:1407–1414. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
van Staa TP, Card T, Logan RF and Leufkens
HG: 5-Aminosalicylate use and colorectal cancer risk in
inflammatory bowel disease: A large epidemiological study. Gut.
54:1573–1578. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rousseaux C, Lefebvre B, Dubuquoy L,
Lefebvre P, Romano O, Auwerx J, Metzger D, Wahli W, Desvergne B,
Naccari GC, et al: Intestinal antiinflammatory effect of
5-aminosalicylic acid is dependent on peroxisome
proliferator-activated receptor-gamma. J Exp Med. 201:1205–1215.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Koelink PJ, Mieremet-Ooms MA, Corver WE,
Wolanin K, Hommes DW, Lamers CB and Verspaget HW: 5-aminosalicylic
acid interferes in the cell cycle of colorectal cancer cells and
induces cell death modes. Inflamm Bowel Dis. 16:379–389. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kohno H, Suzuki R, Sugie S and Tanaka T:
Suppression of colitis-related mouse colon carcinogenesis by a
COX-2 inhibitor and PPAR ligands. BMC Cancer. 5:462005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cooper HS, Murthy SN, Shah RS and
Sedergran DJ: Clinicopathologic study of dextran sulfate sodium
experimental murine colitis. Lab Invest. 69:238–249.
1993.PubMed/NCBI
|
|
11
|
Ni J, Chen SF and Hollander D: Effects of
dextran sulphate sodium on intestinal epithelial cells and
intestinal lymphocytes. Gut. 39:234–241. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cooper HS, Everley L, Chang WC, Pfeiffer
G, Lee B, Murthy S and Clapper ML: The role of mutant Apc in the
development of dysplasia and cancer in the mouse model of dextran
sulfate sodium-induced colitis. Gastroenterology. 121:1407–1416.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tanaka T, Kohno H, Suzuki R, Hata K, Sugie
S, Niho N, Sakano K, Takahashi M and Wakabayashi K: Dextran sodium
sulfate strongly promotes colorectal carcinogenesis in Apc(Min/+)
mice: Inflammatory stimuli by dextran sodium sulfate results in
development of multiple colonic neoplasms. Int J Cancer. 118:25–34.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Murano M, Maemura K, Hirata I, Toshina K,
Nishikawa T, Hamamoto N, Sasaki S, Saitoh O and Katsu K:
Therapeutic effect of intracolonically administered nuclear factor
kappa B (p65) antisense oligonucleotide on mouse dextran sulphate
sodium (DSS)-induced colitis. Clin Exp Immunol. 120:51–58. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Onderdonk AB and Bartlett JG:
Bacteriological studies of experimental ulcerative colitis. Am J
Clin Nutr. 32:258–265. 1979.PubMed/NCBI
|
|
16
|
Jin Z, Jiang W, Jiao F, Hu H, Guo Z and
Wang L and Wang L: Decreased expression of histone deacetylase 10
predicts poor prognosis of gastric cancer patients. Int J Clin Exp
Pathol. 7:5872–5879. 2014.PubMed/NCBI
|
|
17
|
Dubuquoy L, Dharancy S, Nutten S,
Pettersson S, Auwerx J and Desreumaux P: Role of peroxisome
proliferator-activated receptor gamma and retinoid X receptor
heterodimer in hepatogastroenterological diseases. Lancet.
360:1410–1418. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dubuquoy L, Rousseaux C, Thuru X,
Peyrin-Biroulet L, Romano O, Chavatte P, Chamaillard M and
Desreumaux P: PPARgamma as a new therapeutic target in inflammatory
bowel diseases. Gut. 55:1341–1349. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Grommes C, Landreth GE and Heneka MT:
Antineoplastic effects of peroxisome proliferator-activated
receptor gamma agonists. Lancet Oncol. 5:419–429. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen CW, Chang YH, Tsi CJ and Lin WW:
Inhibition of IFN-gamma-mediated inducible nitric oxide synthase
induction by the peroxisome proliferator-activated receptor gamma
agonist, 15-deoxy-delta 12,14-prostaglandin J2, involves inhibition
of the upstream Janus kinase/STAT1 signaling pathway. J Immunol.
171:979–988. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sánchez-Hidalgo M, Martín AR, Villegas I
and Alarcón De La Lastra C: Rosiglitazone, an agonist of peroxisome
proliferator-activated receptor gamma, reduces chronic colonic
inflammation in rats. Biochem Pharmacol. 69:1733–1744. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yang XY, Wang LH, Chen T, Hodge DR, Resau
JH, DaSilva L and Farrar WL: Activation of human T lymphocytes is
inhibited by peroxisome proliferator-activated receptor gamma
(PPARgamma) agonists. PPARgamma co-association with transcription
factor NFAT. J Biol Chem. 275:4541–4544. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Krishnan A, Nair SA and Pillai MR: Biology
of PPAR gamma in cancer: A critical review on existing lacunae.
Curr Mol Med. 7:532–540. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hontecillas R, Horne WT, Climent M, Guri
AJ, Evans C, Zhang Y, Sobral BW and Bassaganya-Riera J:
Immunoregulatory mechanisms of macrophage PPAR-γ in mice with
experimental inflammatory bowel disease. Mucosal Immunol.
4:304–313. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yamamoto-Furusho JK, Peñaloza-Coronel A,
Sánchez-Muñoz F, Barreto-Zuñiga R and Dominguez-Lopez A: Peroxisome
proliferator-activated receptor-gamma (PPAR-γ) expression is
downregulated in patients with active ulcerative colitis. Inflamm
Bowel Dis. 17:680–681. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Farrell RJ and Peppercorn MA: Ulcerative
colitis. Lancet. 359:331–340. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ikeda I, Tomimoto A, Wada K, Fujisawa T,
Fujita K, Yonemitsu K, Nozaki Y, Endo H, Takahashi H, Yoneda M, et
al: 5-aminosalicylic acid given in the remission stage of colitis
suppresses colitis-associated cancer in a mouse colitis model. Clin
Cancer Res. 13:6527–6531. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Koelink PJ, Robanus-Maandag EC, Devilee P,
Hommes DW, Lamers CB and Verspaget HW: 5-Aminosalicylic acid
inhibits colitis-associated but not sporadic colorectal neoplasia
in a novel conditional Apc mouse model. Carcinogenesis.
30:1217–1224. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Riddell RH, Goldman H, Ransohoff DF,
Appelman HD, Fenoglio CM, Haggitt RC, Ahren C, Correa P, Hamilton
SR, Morson BC, et al: Dysplasia in inflammatory bowel disease:
Standardized classification with provisional clinical applications.
Hum Pathol. 14:931–968. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Clapper ML, Gary MA, Coudry RA, Litwin S,
Chang WC, Devarajan K, Lubet RA and Cooper HS: 5-aminosalicylic
acid inhibits colitis-associated colorectal dysplasias in the mouse
model of azoxymethane/dextran sulfate sodium-induced colitis.
Inflamm Bowel Dis. 14:1341–1347. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Saif MW and Chu E: Biology of colorectal
cancer. Cancer J. 16:196–201. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liu J and Farmer SR: Regulating the
balance between peroxisome proliferator-activated receptor gamma
and beta-catenin signaling during adipogenesis. A glycogen synthase
kinase 3beta phosphorylation-defective mutant of beta-catenin
inhibits expression of a subset of adipogenic genes. J Biol Chem.
279:45020–45027. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Atug O, Tahan V, Eren F, Tiftikci A,
Imeryuz N, Hamzaoglu HO and Tozun N: Pro12Ala polymorphism in the
peroxisome proliferator-activated receptor-gamma (PPARgamma) gene
in inflammatory bowel disease. J Gastrointestin Liver Dis.
17:433–437. 2008.PubMed/NCBI
|
|
34
|
Shrestha UK, Karimi O, Crusius JB, Zhou F,
Wang Z, Chen Z, van Bodegraven AA, Xiao J, Morré SA, Wang H, et al:
Distribution of peroxisome proliferator-activated receptor-gamma
polymorphisms in Chinese and Dutch patients with inflammatory bowel
disease. Inflamm Bowel Dis. 16:312–319. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Jennewein C, von Knethen A, Schmid T and
Brüne B: MicroRNA-27b contributes to lipopolysaccharide-mediated
peroxisome proliferator-activated receptor gamma (PPARgamma) mRNA
destabilization. J Biol Chem. 285:11846–11853. 2010. View Article : Google Scholar : PubMed/NCBI
|