Introduction
Hepatocellular carcinoma (HCC), the most common
primary malignancy of the liver, is the sixth most common type of
cancer worldwide and the third leading cause of cancer-related
mortality (1). The number of cases
diagnosed with HCC is expected to increase worldwide, particularly
in East and Southeast Asia (1).
Several lines of study demonstrate that the major risk factors of
HCC include chronic hepatitis B or C virus infection, diabetes,
non-alcoholic fatty liver disease, excessive alcohol consumption
and dietary exposure to aflatoxin (2,3). Despite
substantial and accelerated research in the area of HCC, the 5-year
survival rate for advanced disease remains <10% (4). The first line of therapy consists of
ablative therapy, surgical resection and transplantation (5). However, due to a shortage of donor
livers, and patients' advanced tumor stage or liver dysfunction,
less than 20% of HCC patients are eligible for these treatments
(6). Therefore, there is a need to
develop novel therapeutic approaches to improve the outcomes of
patients with HCC.
Hepatocarcinogenesis is a complex, multi-step
process which occurs as a result of a combination of epigenetic and
genetic alterations. It has been reported that apoptosis, which
triggers a range of extrinsic and intrinsic signals leading to cell
shrinkage, blebbing of the plasma membrane, chromatin condensation
and DNA laddering, plays a role in the development of neoplastic
transformation and tumor growth (7).
Accumulating evidence suggests that the dysregulation of the
apoptotic process plays a crucial role in the occurrence and
progression of human HCC (8).
Therefore, a number of potential anticarcinogens, which induce the
apoptotic process in various ways, may be applied in the management
of HCC (9,10).
In recent years, traditional Chinese medicines have
slowly gained considerable attention as a new source of anticancer
drugs. Although their curative mechanisms remain largely unknown,
certain drugs have been used to treat cancer (11). Wogonin
(5,7-dihydroxy-8-methoxy-2-phenylchromen-4-one,
C16H12O5), a flavonoid extracted
from the root of the traditional Chinese herb Scutellaria
baicalensis, has been considered to be responsible for the
therapeutic actions of Scutellaria baicalensis due to its
observed pharmacological actions including anti-inflammation and
anticancer properties, reduction of total cholesterol level and
anti-HIV activity (12–14). Due to its therapeutic potential,
wogonin has been recognized as a new source of anticancer drug and
a new chemotherapy adjuvant to enhance the efficacy of chemotherapy
and to ameliorate the side effects of cancer chemotherapies
(13–16). Wogonoside (wogonin-7-glucuronide,
C22H20O11), a metabolite of
wogonin, is also derived from Scutellaria baicalensis,
sharing the same flavone backbone as wogonin (17). Accumulating evidence demonstrates that
wogonoside is involved in various biological and pathological
processes including anti-inflammation, anti-angiogenesis, cell
cycle arrest and induction of autophagy (18–21). The
difference between the two chemical structures is the presence and
placement of 7-glucuronic acid, indicating that wogonoside and
wogonin may affect similar biochemical processes. Wogonin has been
reported to kill HCC cells in vitro and inhibit tumor growth
in vivo in a number of mouse tumor models (15,16,22,23).
All the properties of wogonoside mentioned above indicate that
wogonoside may be involved in the anticancer process. However, as a
metabolite of wogonin, relatively few studies have investigated the
potential effects of wogonoside on HCC, and its antitumor
properties have not been elucidated.
In the present study, the effects of wogonoside on
cell apoptosis were evaluated in the human HCC cell line Bel-7402.
The potential regulation pathway involved in its apoptotic effect
was also investigated.
Materials and methods
Cell lines and reagents
The human liver cancer cell line Bel-7402 was
donated by the State Key Laboratory of Medical Genetics of Central
South University, Changsha, China. It was cultured in RPMI-1640
medium (Gibco, Grand Island, NY, USA) supplemented with 15% fetal
bovine serum (Invitrogen Life Technologies, Carlsbad, CA, USA), 100
U/ml penicillin and 100 µg/ml streptomycin in a humidified
incubator under 95% air and 5% CO2 at 37°C. Wogonoside,
kindly provided by the Pharmacy College of Central South
University, was first dissolved in phosphate-buffered saline (PBS)
to prepare 10 mg/ml store solution and then serially diluted to
various concentrations prior to experiments. The present study was
approved by the ethics committee of Xiangya Hospital, Central South
University (Changsha, China).
Measurement of cell viability
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium
bromide (MTT) assay was used to evaluate the cell viability
according to the manufacturer's instructions (Sigma-Aldrich, St.
Louis, MO, USA). Briefly, 1×104 cells per well were
plated onto 96-well plates and incubated for 3 h. The cells were
then treated with wogonoside at the indicated concentrations (1, 2,
4, 8, 16, 32, 64, 128, 256 and 512 µM, and 1 and 2 mM) for 48 h.
Each experiment was performed in triplicate. MTT reagent was added.
Following incubation for 4 h at 37°C, the absorbance, which is
directly proportional to the number of viable cells in cultures,
was measured at 570 nm using a microplate reader (Mithras LB940
multilabel reader; Berthold Technologies, Bad Wildbad, Germany).
The cell viability was expressed as a percentage value of control
cells cultured with medium alone. The test was run three times and
the inhibition rate was calculated with the formula: Inhibition
rate = 1 - [(TreatmentA570 - BlankA570) /
(ControlA570 - BlankA570)] × 100% to produce
an inhibition curve and derive the half maximal inhibitory
concentration (IC50) of wogonoside.
DNA ladder assay was also performed as previously
described (24). Briefly, Bel-7402
cells were cultured in a 25-mm2 flask with 4, 8, 12 and
16 µM wogonoside at ~2×106 cells per group for DNA
sample extraction. The control group were treated with 100 µM
5-fluorouracil (5-FU; Sigma-Aldrich). Cells were harvested at 12,
24, 36 and 48 h after treatment. DNA was electrophoresed in 1.2%
agarose gels at 10 V/cm for 2 h. The analysis of DNA fragmentation
was carried out using the manufacturer's apoptotic DNA ladder kit
(Calbiochem, Billerica, CA, USA).
Flow cytometry for cell cycle
detection
Cells were plated in 35-mm dishes at concentrations
determined to yield 60–70% confluence within 48 h and then treated
with wogonoside at the indicated concentrations (4, 8, 12 and 16
µM) for 48 h. The adherent and floating cells were harvested, and
the cells were resuspended in PBS, and fixed with 70% ethanol at
−20° overnight. The cells were first incubated with RNaseA (20
U/ml; Sigma-Aldrich) at 37°C for 30 min and then labeled with
propidium iodide (50 µg/ml) and incubated at room temperature in
the dark for 30 min. DNA content was then analyzed using a FACScan
instrument equipped with FACStation running CellQuest software
(Becton-Dickinson, San Jose, CA, USA).
Western blotting of Bcl-2, Bax and p53
expression
Western blotting was performed to assess Bcl-2, Bax
and p53 expression as previously described (25). Briefly, a total of 106
cells were sedimented and lysed for 15 min in ice-cold lysis buffer
[0.1% sodium dodecyl sulphate (SDS), 1% NP-40, 50 mM HEPES, pH 7.4,
2 mM ethylenediaminetetraacetic acid, 100 mM NaCl, 5 mM sodium
orthovanadate, 40 µM p-nitrophenyl phosphate and 1% protease
inhibitor mixture set I; Calbiochem, Billerica, MA, USA]. After
removing the cell debris by centrifugation at 16,200 × g for 15
min, equal amounts of proteins were separated on 12% SDS
polyacrylamide gel, blotted onto a nitrocellulose membrane (GE
Healthcare, Little Chalfont, UK) and blocked with 5% nonfat dry
milk in PBS/Tween (0.05% Tween-20 in PBS). Bcl-2, Bax and p53
antibodies were used (all from Santa Cruz Biotechnology, Santa
Cruz, CA, USA). The membranes were then incubated with the
appropriate horseradish peroxidase-conjugated secondary antibodies
(1:2000). The immunoreactive protein bands were developed by
enhanced chemiluminescence.
Statistical analysis
Data are expressed as the means ± standard
deviation. Statistical analysis was performed using SPSS software
version 17.0 (SPSS, Inc., Chicago, IL, USA). The difference between
two groups was analyzed by Student's t-test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Proliferative inhibition and cell
cycle arrest by wogonoside
To examine the effect of wogonoside on the cell
viability in HCC cells, MTT assay was performed first. Cell
viability was dose-dependently reduced in Bel-7402 liver cancer
cells following wogonoside treatment at the indicated
concentrations for 24 h (Fig. 1).
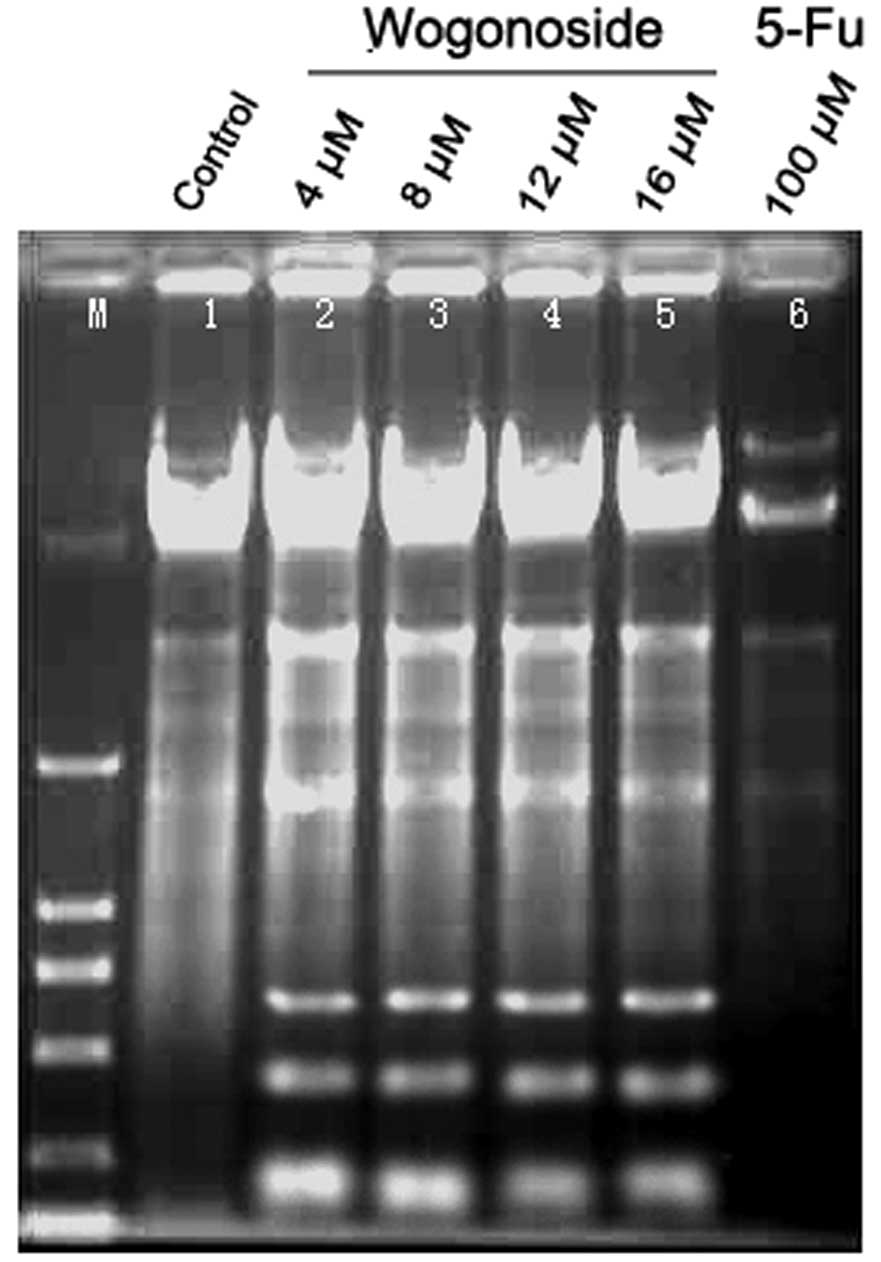
Wogonoside inhibited Bel-7402 cells with an IC50 value
of 8 µM. The results of the DNA ladder assay revealed that cells
from the control group presented an intact band of genomic DNA
whereas the 5-FU and wogonoside treatment group did not (Fig. 2). The effect of wogonoside on the cell
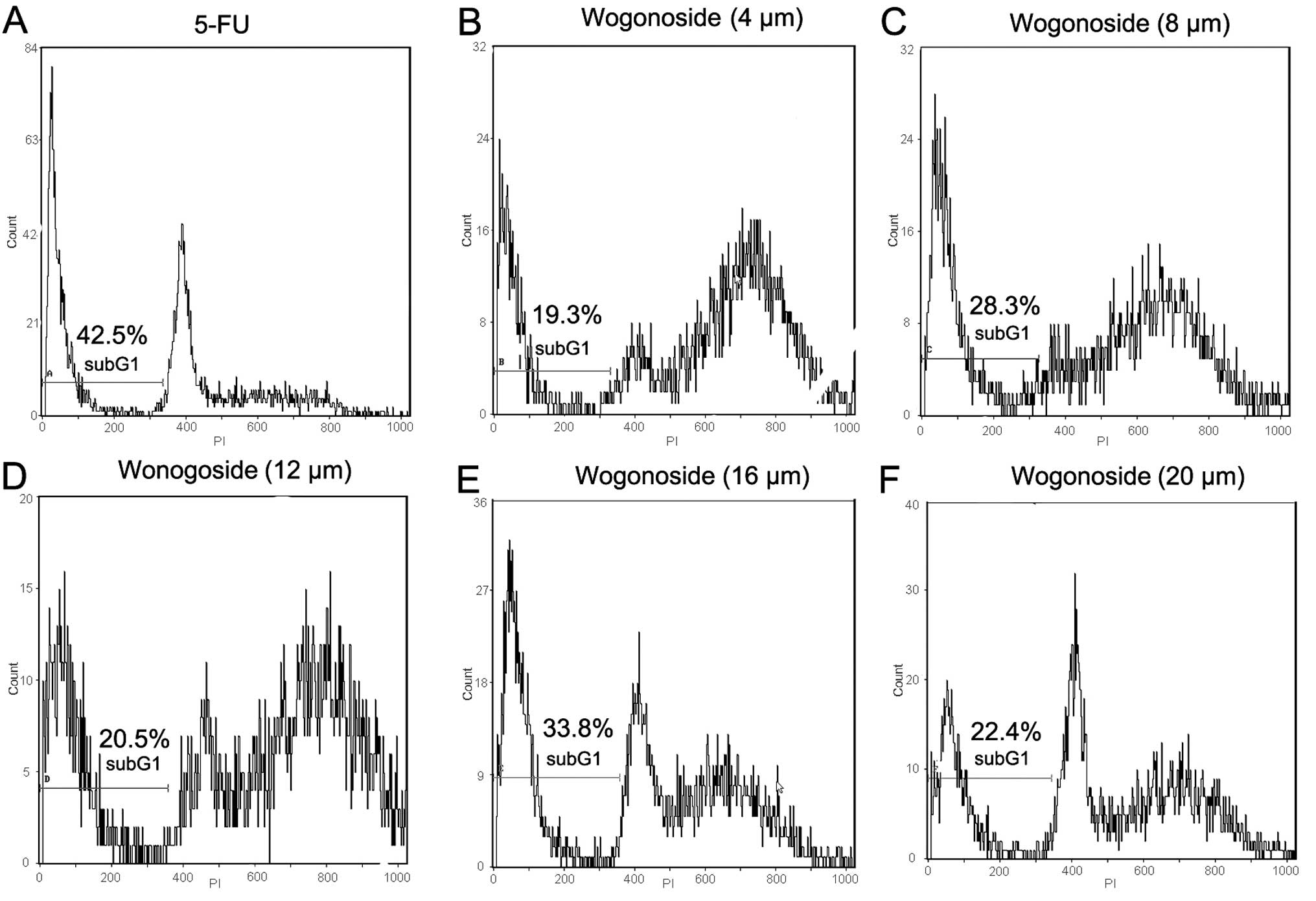
cycle progression of Bel-7402 was also examined. Cell cycle
analysis revealed that exposure to wogonoside (4 µM) for 24 h
induced the accumulation of a significant proportion of cells in
the G2/M phase (Fig. 3). The data
above suggest that wogonoside treatment induced apoptosis in the
HCC cell line.
Inhibition of Bcl-2 protein enhances
the apoptosis-inducing activity of wogonoside
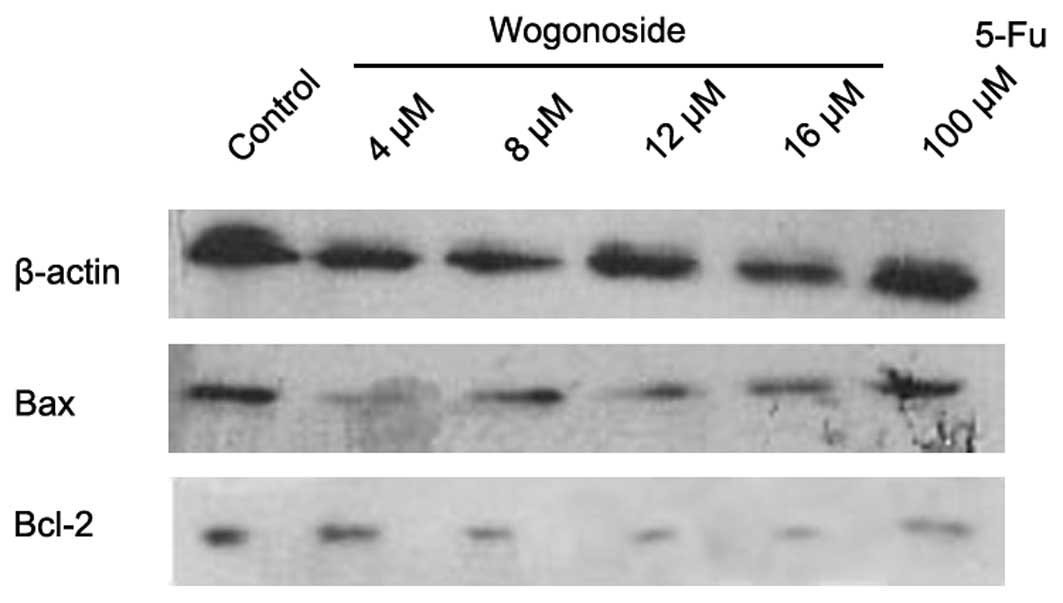
The potential effects of wogonoside were
investigated in HCC cells. The pro-apoptotic Bax protein is among a
number of key regulators of apoptosis. Therefore, the effects of
wogonoside were investigated in this regulatory protein. We
observed that wogonoside increased Bax expression in HCC cells in
the western blotting assay (Fig. 4),
which suggests that wogonoside promotes HCC cell apoptosis via the
Bax protein regulatory pathway. The expression of anti-apoptotic
protein Bcl-2 was also examined in this study. Bcl-2 expression was
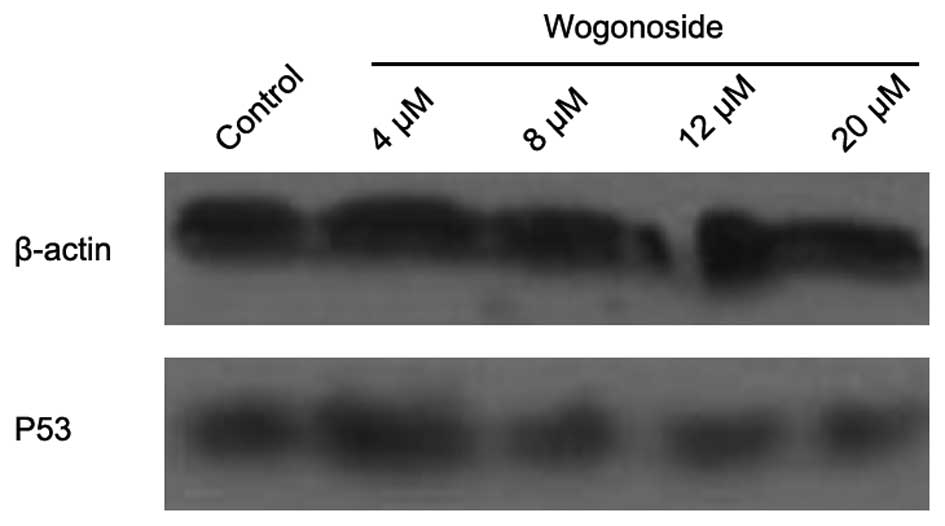
revealed to be decreased in the western blotting assay (Fig. 4). The expression of p53 protein was
also detected in the study (Fig. 5).
Western blotting assay did not reveal any significant change in p53
expression.
Discussion
Herbs have been used successfully in traditional
Chinese medicine for centuries; however, their therapeutic
mechanism remains unknown. The present study demonstrates that
wogonoside, a major constituent of Scutellaria baicalensis,
attenuated in vitro proliferation of the HCC cell line
Bel-7402 by inducing apoptosis and downregulating Bax/Bcl-2
signaling pathways. These results reveal that an adjuvant therapy
of wogonoside may have potential therapeutic benefits for HCC.
Scutellaria baicalensis is often used for the
treatment of inflammation, cardiovascular disease, and respiratory
and gastrointestinal infection (26).
The anticancer effect of Scutellaria baicalensis extract has
recently become a topic of interest. Moreover, a number of in
vitro studies suggest that the extract of Scutellaria
baicalensis inhibits the growth of various cancer cell lines
through specific biological signaling pathways, including
apoptosis, angiogenesis and inhibition of the androgen receptor
(15,16,18–23).
Increasing evidence has demonstrated that
wogonoside, a major extract of Scutellaria baicalensis,
induces autophagy in MDA-MB-231 cells by regulating the MAPK-mTOR
pathway (19). It also participates
in cell cycle arrest and differentiation by regulating the
expression and subcellular localization of PLSCR1 in AMl cells
(20). Moreover, wogonoside induces
the inhibition of lipopolysaccharide-induced angiogenesis in
vitro and in vivo via toll-like receptor 4 signal
transduction (21). The present study
confirmed that wogonoside possesses the ability to induce cycle
arrest and apoptosis in HCC cells, indicating that the glucuronide
metabolite wogonoside, like its aglycone wogonin, possesses
biological activity.
Apoptosis represents a physiological means of
eliminating excess cells during liver development and regeneration
(27). Apoptotic cell death is
initialized through the extrinsic or intrinsic signaling pathways
that are ultimately coupled to the activation of the effect of
caspases. The extrinsic pathway is largely controlled by the
pro-apoptotic (e.g. Bax, Bad, Bid and Bak) and anti-apoptotic (e.g.
Bcl-2 and Bcl-xL) Bcl-2 family proteins. It has been reported that
the induction of mitochondrial apoptosis requires the involvement
of the Bcl-2 family (28). The Bcl-2
family is comprised of proteins that share a Bcl-2 homology region
and undergo heterodimerization or homodimerizaton (29). The ratio between anti- and
pro-apoptotic proteins is considered a determinant for tissue
homeostasis since it influences the sensitivity of cells to
inducers of apoptosis (30).
In this study, it was demonstrated that the
induction of apoptosis by wogonoside in HCC cells is associated
with increased expression of the pro-apoptotic protein Bax and
decreased expression of the anti-apoptotic protein Bcl-2. A
wogonin-induced decreased ratio of Bcl-2 to Bax and increased
expression of cleaved caspase 3 and 9 has been demonstrated in
human breast cancer cells (31).
Wogonoside, derived from Scutellaria baicalensis, shares the
same flavone backbone as wogonin. Similar results were obtained in
our study. Therefore, it is reasonable to assume that
wogonoside-induced apoptosis of HCC cells is mediated by the
mitochondrial pathway by altering the ratio of Bcl-2 to Bax.
The p53 protein, which acts as a guardian of the
genome, is one of the key factors controlling cell proliferation,
suppressing the growth and transformation of cells. Mutations in
the p53 tumor suppressor gene are among the most common alterations
observed in HCC (10). Various
chemotherapeutic agents require p53 to induce apoptosis. Thus, we
investigated p53 protein expression induced by wogonoside in HCC
cells. There was no significant alteration observed in the western
blotting assay. The results revealed that there may not be a
correlation between wogonoside-induced apoptosis and the p53
pathway in HCC cells.
In conclusion, wogonoside may act as an effective
drug with anti-proliferative and apoptotic activity in HCC cells.
This study also presented a molecular mechanism responsible for the
effects; namely, downregulation of the Bcl-2/Bax signaling pathway
in wogonoside-induced apoptosis. The results of this study suggest
that wogonoside may represent a potential therapeutic agent against
HCC.
Acknowledgements
This study was supported by grants from the National
Natural Science Foundation of China (nos. 81201420, 81272034 and
81472130), the Provincial Science Foundation of Hunan (no.
14JJ3032), the Scientific Research Project of the Development and
Reform Commission of Hunan Province [no. (2013)1199], the
Scientific Research Project of the Science and Technology Office of
Hunan Province (no. 2013SK2018), the Doctoral Scientific Fund
Project of the Ministry of Education of China (no. 20120162110036),
and the Fundamental Research Funds for the Central Universities of
Central South University (no. 2013zzts319).
References
|
1
|
Forner A, Llovet JM and Bruix J:
Hepatocellular carcinoma. Lancet. 379:1245–1255. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bridges JF, Joy SM, Gallego G, Kudo M, Ye
SL, Han KH, Cheng AL and Blauvelt BM: Needs for hepatocellular
carcinoma control policy in the Asia-Pacific region. Asian Pac J
Cancer Prev. 12:2585–2591. 2011.PubMed/NCBI
|
|
3
|
Sherman M: Hepatocellular carcinoma:
epidemiology, surveillance and diagnosis. Semin Liver Dis. 30:3–16.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Olsen SK, Brown RS and Siegel AB:
Hepatocellular carcinoma: review of current treatment with a focus
on targeted molecular therapies. Therap Adv Gastroenterol. 3:55–66.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
EI-Serag HB: Hepatocellular carcinoma. N
Engl J Med. 365:1118–1127. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Davila JA, Duan Z, McGlynn KA and El-Serag
HB: Utilization and outcomes of palliative therapy for
hepatocellular carcinoma: a population-based study in the United
States. J Clin Gastroenterol. 46:71–77. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Riedl SJ and Salvesen GS: The apoptosome:
signalling platform of cell death. Nat Rev Mol Cell Biol.
8:405–413. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fabregat I, Roncero C and Fernández M:
Survival and apoptosis: a dysregulated balance in liver cancer.
Liver Int. 27:155–162. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fransvea E, Angelotti U, Antonaci S and
Giannelli G: Blocking transforming growth factor-beta up-regulates
E-cadherin and reduces migration and invasion of hepatocellular
carcinoma cells. Hepatology. 47:1557–1566. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hussain SP, Schwank J, Staib F, Wang XW
and Harris CC: TP53 mutations and hepatocellular carcinoma:
insights into the etiology and pathogenesis of liver cancer.
Oncogene. 26:2166–2176. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Normile D: Asian medicine. The new face of
traditional Chinese medicine. Science. 299:188–190. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yoon SB, Lee YJ, Park SK, Kim HC, Bae H,
Kim HM, Ko SG, Choi HY, Oh MS and Park W: Anti-inflammatory effects
of Scutellaria baicalensis water extract on LPS-activated
RAW 264.7 macrophages. J Ethnopharmacol. 125:286–290. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li-Weber M: New therapeutic aspects of
flavones: the anticancer properties of Scutellaria and its
main active constituents Wogonin, Baicalein and Baicalin. Cancer
Treat Rev. 35:57–68. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Burnett BP, Jia Q, Zhao Y and Levy RM: A
medicinal extract of Scutellaria baicalensis and Acacia
catechu acts as a dual inhibitor of cyclooxygenase and
5-lipoxygenase to reduce inflammation. J Med Food. 10:442–451.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhao Q, Wang J, Zou MJ, Hu R, Zhao L,
Qiang L, Rong JJ, You QD and Guo QL: Wogonin potentiates the
antitumor effects of low dose 5-fluorouracil against gastric cancer
through induction of apoptosis by down-regulation of NF-kappaB and
regulation of its metabolism. Toxicol Lett. 197:201–210. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Enomoto R, Koshiba C, Suzuki C and Lee E:
Wogonin potentiates the antitumor action of etoposide and
ameliorates its adverse effects. Cancer Chemother Pharmacol.
67:1063–1072. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li C, Lin G and Zuo Z: Pharmacological
effects and pharmacokinetics properties of Radix
Scutellariae and its bioactive flavones. Biopharm Drug Dispos.
32:427–445. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yang YZ, Tang YZ and Liu YH: Wogonoside
displays anti-inflammatory effects through modulating inflammatory
mediator expression using RAW264.7 cells. J Ethnopharmacol.
148:271–276. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sun Y, Zou M, Hu C, Qin Y, Song X, Lu N
and Guo Q: Wogonoside induces autophagy in MDA-MB-231 cells by
regulating MAPK-mTOR pathway. Food Chem Toxicol. 51:53–60. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen Y, Hui H, Yang H, Zhao K, Qin Y, Gu
C, Wang X, Lu N and Guo Q: Wogonoside induces cell cycle arrest and
differentiation by affecting expression and subcellular
localization of PLSCR1 in AMl cells. Blood. 121:3682–3691. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen Y, Lu N, Ling Y, Gao Y, Wang L, Sun
Y, Qi Q, Feng F, Liu W, Liu W, You Q and Guo Q: Wogonoside inhibits
lipopolysaccharide-induced angiogenesis in vitro and in vivo via
toll-like receptor 4 signal transduction. Toxicology. 259:10–17.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chow SE, Chang YL, Chuang SF and Wang JS:
Wogonin induced apoptosis in human nasopharyngeal carcinoma cells
by targeting GSK-3β and Δ Np63. Cancer Chemother Pharmacol.
68:835–845. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Baumann S, Fas SC, Giaisi M, Müller WW,
Merling A, Gülow K, Edler L, Krammer PH and Li-Weber M: Wogonin
preferentially kills malignant lymphocytes and suppresses T-cell
tumor growth by inducing PLCgamma1-and Ca2+-dependent apoptosis.
Blood. 111:2354–2363. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Roy M, Chakraborty S, Siddiqi M and
Bhattacharya RK: Induction of apoptosis in tumor cells by natural
phenolic compounds. Asian Pac J Cancer Prev. 3:61–67.
2002.PubMed/NCBI
|
|
25
|
Dang YM, Huang G, Chen YR, Dang ZF, Chen
C, Liu FL, Guo YF and Xie XD: Sulforaphane inhibits the
proliferation of the BIU87 bladder cancer cell line via IGFBP-3
elevation. Asian Pac J Cancer Prev. 15:1517–1520. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yu J, Liu H, Lei J, Tan W, Hu X and Zou G:
Antitumor activity of chloroform fraction of Scutellaria
barbata and its active constituents. Phytother Res. 21:817–822.
2007. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Guicciardi ME and Gores GJ: Apoptosis: a
mechanism of acute and chronic liver injury. Gut. 54:1024–1033.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Brunelle JK and Letai A: Control of
mitochondrial apoptosis by the Bcl-2 family. J Cell Sci.
122:437–441. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Steinbach JP and Weller M: Apoptosis in
Gliomas: Molecular Mechanisms and Therapeutic Implications. J
Neurooncol. 70:247–256. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Korsmeyer SJ: BCL-2 gene family and the
regulation of programmed cell death. Cancer Res. 59:(Suppl 7).
1693–1700. 1999.
|
|
31
|
Chung H, Jung YM, Shin DH, Lee JY, Oh MY,
Kim HJ, Jang KS, Jeon SJ, Son KH and Kong G: Anticancer effects of
wogonin in both estrogen receptor-positive and -negative human
breast cancer cell lines in vitro and in nude mice xenografts. Int
J Cancer. 122:816–822. 2008. View Article : Google Scholar : PubMed/NCBI
|