Introduction
Oral cancer accounts for ~3% of all malignancies
worldwide, with ~500,000 new cases diagnosed annually and a
five-year survival rate of ~50% (1).
The development of oral cancer is usually preceded by the
occurrence of precancerous lesions. Oral leukoplakia (OLK), also
known as a common oral precancerous lesion, is a type of oral
mucosal epithelial keratosis abnormality. The global prevalence of
OLK is ~1% for all ages, with an increasing prevalence in adults,
and the malignant transformation rate of OLK is 2–3% (2). Leukoplakia usually occurs ~5 years prior
to the development of oral cancer (3). At present, no specific treatment has
been identified for OLK, thus preventing the development and
progression of OLK is important as it may reduce the incidence of
oral cancer. Previous studies have demonstrated that oxidative
stress damage may be involved in the pathogenesis of OLK (4,5).
Peroxiredoxin 1 (Prx1) is a major 2-Cys member of the peroxiredoxin
family, which is abundant and ubiquitously distributed in tissues
and is expressed at higher levels in numerous types of malignant
tumor, including oral squamous cell carcinoma (6–8). The
primary biochemical function of Prx1 appears to be as a
peroxide-detoxifying enzyme scavenging reactive oxygen species
(ROS), and studies have identified its functional switching from a
peroxidase enzyme to a molecular chaperone, which regulates cell
proliferation, differentiation and apoptosis under stress
conditions (9,10). Apoptosis signal-regulating kinase 1
(ASK1) is well known as a proapoptotic, stress-acivated signaling
molecule, which participates in the c-Jun N-terminal kinase (JNK)
and p38-mitogen activated protein kinase (MAPK) signaling cascades
(11). It has been reported that Prx1
interacts with ASK1 via the thioredoxin-binding domain of ASK1, and
that this action is highly inducible by H2O2
(12). However, to the best of our
knowledge, no information is currently available regarding the role
of Prx1 in OLK. In the present study, for the first time, to the
best of our knowledge, the role of Prx1 in ASK1-induced apoptosis
by oxidative stress in OLK was investigated, in order to provide
valuable clues for the prevention and treatment of OLK.
Materials and methods
Patients and specimens
A total of 20 OLK patients with clinical and
pathological diagnosis of OLK, with epithelial mild or
mild-moderate dysplasia, at the Capital Medical University School
of Stomatology (Beijing, China) were randomly selected for use in
the present study. The OLK tissues of these patients were taken via
biopsy, and 10 samples of normal oral mucosa were obtained from
maxillofacial plastic surgery procedures for use as negative
controls. Amongst the 20 OLK cases, 12 were female and 8 were male,
aged 45–84 years (mean age, 64 years), including 13 cases of buccal
mucosa, 1 case of lip mucosa and 6 cases of tongue mucosa. The
present study was approved by the Human Research Ethics Committee
of Capital Medical University School of Stomatology, and all
patients signed an informed consent forms.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
For quantification of messenger RNA (mRNA)
expression, total RNA was extracted from human OLK and control
tissues using TRIzol (Invitrogen Life Technologies, Carlsbad, CA,
USA) according to the manufacturer's instructions. Complementary
DNA (cDNA) was synthesized by reverse transcribing 2 µg RNA with
the High-Capacity cDNA Reverse Transcription kit (Applied
Biosystems, Foster City, CA, USA). Aliquots of cDNA (1 µl) were
used as templates and SYBR Green Dye reagent (Applied Biosystems,
Foster City, CA, USA) was used to quantify the products formed
during the RT-qPCR reaction. For data analysis, the
2−∆∆Ct method was used, and the raw data was normalized
to the housekeeping gene GAPDH (13).
The experiment was performed in triplicate. The sequences of
primers were: GAPDH-forward (F), 5′-aggtcggtgtgaacggatttg-3′ and
reverse (R), 5′-tgtagaccatgtagttgaggtca-3′; Prx1-F,
5′-gggtattcttcggcagatca-3′ and Prx1-R, 5′-tccccatgtttgtcagtgaa-3′;
ASK1-F, 5′-aagtcccaacccatagaaattcct-3′ and ASK1-R,
5′-agccagtcggtaagttcagaatctt-3′; p38-F,
5′-gagctgaagattctggattttgg-3′ and p38-R,
5′-tagccacgtagccggtcatt-3′.
Cell culture
Human oral precancerous cell line DOK (presented by
Professor Chen Xiaoxin, Cancer Research Program, Julius L. Chambers
Biomedical/Biotechnology Research Institute, North Carolina Central
University, Durham, NC, USA) were maintained in Dulbecco's modified
Eagle's medium-nutrient mixture F-12, supplemented with 15% (v/v)
fetal bovine serum (FBS; Gibco Life Technologies, Carlsbad, CA,
USA) containing 100 U/ml penicillin and 100 µg/ml streptomycin, in
a 5% CO2 atmosphere at 37°C.
Plasmids and cell transfection
The pEZ-M02-ASK1 and pEZ-M02-Prx1 plasmids were
obtained from GeneCopoeia, Inc. (Rockville, MD, USA), while Prx1
short hairpin RNA (shRNA) plasmid and control shRNA Plasmid-A were
obtained from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA). DOK
cells were plated in six-well plates at 1×106
cells/well, then transfected with 2 µg plasmids using
Lipofectamine® 2000 (Invitrogen Life Technologies) according to the
manufacturer's instructions.
Flow cytometry for cell apoptosis
detection
Following transfection of Prx1 shRNA plasmid for 48
h, the cells were stimulated with 5 mM H2O2
for various time-periods (15, 30 and 45 min). Apoptotic cell death
was measured by flow cytometry (FACSCalibur; BD Biosciences,
Franklin Lakes, NJ, USA) with Annexin V/fluorescein isothiocyanate
and propidium iodide staining (R&D Systems, Inc., Minneapolis,
MN, USA).
Western blot analysis
Cells and tissues were rinsed three times with
ice-cold phosphate-buffered saline (PBS; Hyclone, Logan, UT, USA)
and lysed in immunoprecipitation assay buffer [50 mM Tris-Cl (pH
7.4), 1% NP40, 150 Mm NaCl, 1 mM EDTA, 1 M phenylmethylsulfonyl
fluoride, 10 µg each of aprotinin and leupeptin, and 1 mM
Na3VO4] (Invitrogen Life Technologies), to
which the protease inhibitor mix Complete™ (Roche Diagnostics,
Basel, Switzerland) was added. Following centrifugation at 12,000 ×
g for 30 min, the supernatant was collected, and the protein
concentration was determined using the Lowry method (14). Equal quantities of protein were
separated on 12% SDS-PAGE gels and blotted onto nitrocellulose
membranes (Pierce Biotechnology, Inc., Appleton, WI, USA). The
blots were subsequently incubated with rabbit polyclonal anti-human
Prx1 antibody (1:1,000; cat. no. ab41906; Abcam, Cambridge, UK),
rabbit polyclonal anti-human ASK1 (1:1,000; cat. no. 3762s; Cell
Signaling Technology, Inc., Danvers, MA, USA), rabbit polyclonal
anti-human p-ASK1 (1:1,000; cat. no. 3765s; Cell Signaling
Technology, Inc.), rabbit monoclonal anti-human p38 (1:1,000; cat.
no. ab7952; Abcam) and rabbit monoclonal anti-p-human p38 (1:1,000;
cat. no. ab178867; Abcam) at 4°C overnight. Rabbit polyclonal
anti-human β-actin antibody (1:1,000; cat. no. A2066;
Sigma-Aldrich, St. Louis, MO, USA) was used as a loading control.
Immunoreactive bands were detected by 1-h incubation at room
temperature with goat anti-rabbit (cat. no. ab136636) and goat
anti-mouse (cat. no. ab979023) horseradish peroxidase
(HRP)-conjugated secondary antibodies (1:1,000; Abcam) and enhanced
chemiluminescence reagents (GE Healthcare Life Sciences, Chalfont,
UK). Each experiment was repeated a minimum of three times.
Co-immunoprecipitation assay
To examine the association between Prx1 and ASK1, a
co-immunoprecipitation assay was used to detect the interaction
between Prx1 with ASK1 in vitro. Cell extract from
H2O2-treated DOK cells was collected
following centrifugation at 14,000 × g for 15 min at 4°C and boiled
for 5 min with 2X loading buffer for examination by 4–12% SDS-PAGE.
Whole cell extracts were incubated with the rabbit monoclonal
anti-human Prx1 (1:1,000; cat. no. ab109506; Abcam) or rabbit
polyclonal anti-human β-actin (1:1,000; cat. no. A2066;
Sigma-Aldrich) antibodies at 4°C overnight and incubated with the
HRP-conjugated secondary antibodies for 30 min at room temperature.
For protein precipitation, protein A-Agarose beads (Invitrogen Life
Technologies) in extract buffer were added prior to incubation with
gentle mixing for 16 h at 4°C. Subsequently, the beads were
pelleted by centrifugation at 14,000 × g for 15 min at 4°C and
washed three times with extract buffer. The protein was eluted from
the beads at 100°C using 1% SDS-PAGE sample buffer supplemented
with 50 mM dithiothreitol and resolved with SDS-PAGE.
Glutathione-S-transferase (GST)
pull-down assays
To further investigate whether Prx1 protein
interacts with ASK1 protein directly in vitro, a GST
pull-down assay kit (21516; Thermo Fisher Scientific, Waltham, MA,
USA) was used. Cell lysates were prepared and centrifuged at 15,000
× g for 15 min and the supernatants were collected. Histidine
(His)-Prx1 (Prx1 protein with a 6-His tag, prepared in our
laboratory) were incubated with GST or GST-fused ASK1 conjugated to
sepharose beads in reaction buffer (50 mM Tris-HCl, pH 7.5, 150 mM
NaCl, 10% glycerol, 1.5 mM MgCl2, 5 mM NaF, 1% Triton
X-100 and protease inhibitor mixture Complete; Roche Diagnostics)
at 4°C for 12 h. Following centrifugation at 500 × g for 5 min at
4°C, the proteins bound to sepharose beads were washed with
ice-cold PBS, mixed with 2X SDS sample buffer and eluted by the
addition of 10 µl glutathione elution buffer (G-Biosciences, St.
Louis, MO, USA). The sepharose beads were suspended and incubated
at room temperature for 5 min. They were then centrifuged for at
500 × g for 5 min at 4°C to sediment the sepharose beads, and the
supernatants were transferred to fresh tubes for SDS-PAGE. Western
blot analysis was then performed to examine Prx1 binding to ASK1
using rabbit monoclonal anti-human Prx1 antibody (1:1,000; cat. no.
ab109506; Abcam).
Statistical analysis
Data were expressed as the mean ± standard
deviation. Comparisons were performed by two independent Student's
t-test (independent 2-sample t-test) using SPSS 17.0 (SPSS, Inc.,
Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
Prx1 expression is enhanced in OLK
tissues
Prx1 mRNA expression was significantly higher in 20
OLK tissues than that of normal oral mucosa samples, with
approximately 2-fold greater expression than that of the normal
group (P=0.047; Fig. 1A). Closely
correlated with the mRNA levels, the protein expression of Prx1 was
also enhanced in 20 cases of OLK, compared with that of the normal
group, and the mean relative Prx1 content was 1.11 in the OLK group
(Fig. 1B).
Expression levels of ASK1, p-ASK1, p38
and p-p38 are increased in OLK tissues
The mRNA expression levels of ASK1 and p38 were both
significantly higher in OLK tissues than that in the normal oral
mucosa, with ~3- (P=0.024) (Fig. 2A)
and 4-fold (P=0.022) (Fig. 2B)
increase compared with the normal group, respectively. The protein
expression of ASK1 and p-ASK1 were slightly increased in the OLK
tissues, and the mean relative content of ASK1 and p-ASK1 was 1.06
and 1.11, respectively, in the OLK group (Fig. 2C). The protein expression of p38 and
p-p38 in OLK group were also both increased slightly, and the mean
relative p38 and p-p38 content was 1.09 and 1.11, respectively, in
the OLK group (Fig. 2D) The results
of the western blot were not statistically analyzed.
Expression of Prx1, ASK1 and p38 in
DOK cells treated with H2O2
Western blot analysis with anti-Prx1 antibody was
performed in DOK cells treated with 5 mM H2O2
for the indicated time-periods (Fig.
3A). The expression of Prx1 was not markedly altered until 45
min. To investigate whether endogenous Prx1 affected ASK1
expression and ASK1-induced apoptosis, the suppression of
endogenous Prx1 expression was induced by transfecting Prx1 shRNAs
into DOK cells (Fig. 3B). The
expression of phosphorylated ASK1 and p38 were subsequently
evaluated by immunoblotting assay. As shown in Fig. 3C, upon stimulation with
H2O2, the levels of p-ASK1 rose rapidly and
strongly from 15 min. Following Prx1 knockdown, higher levels of
p-ASK1 and p-p38 were observed, compared with that of control
(mock-transfected) DOK cells. In Prx1-knockdown DOK cells, the
expression of phosphorylated ASK1 and p38 gradually increased in a
time-dependent manner, and then decreased at 45 min.
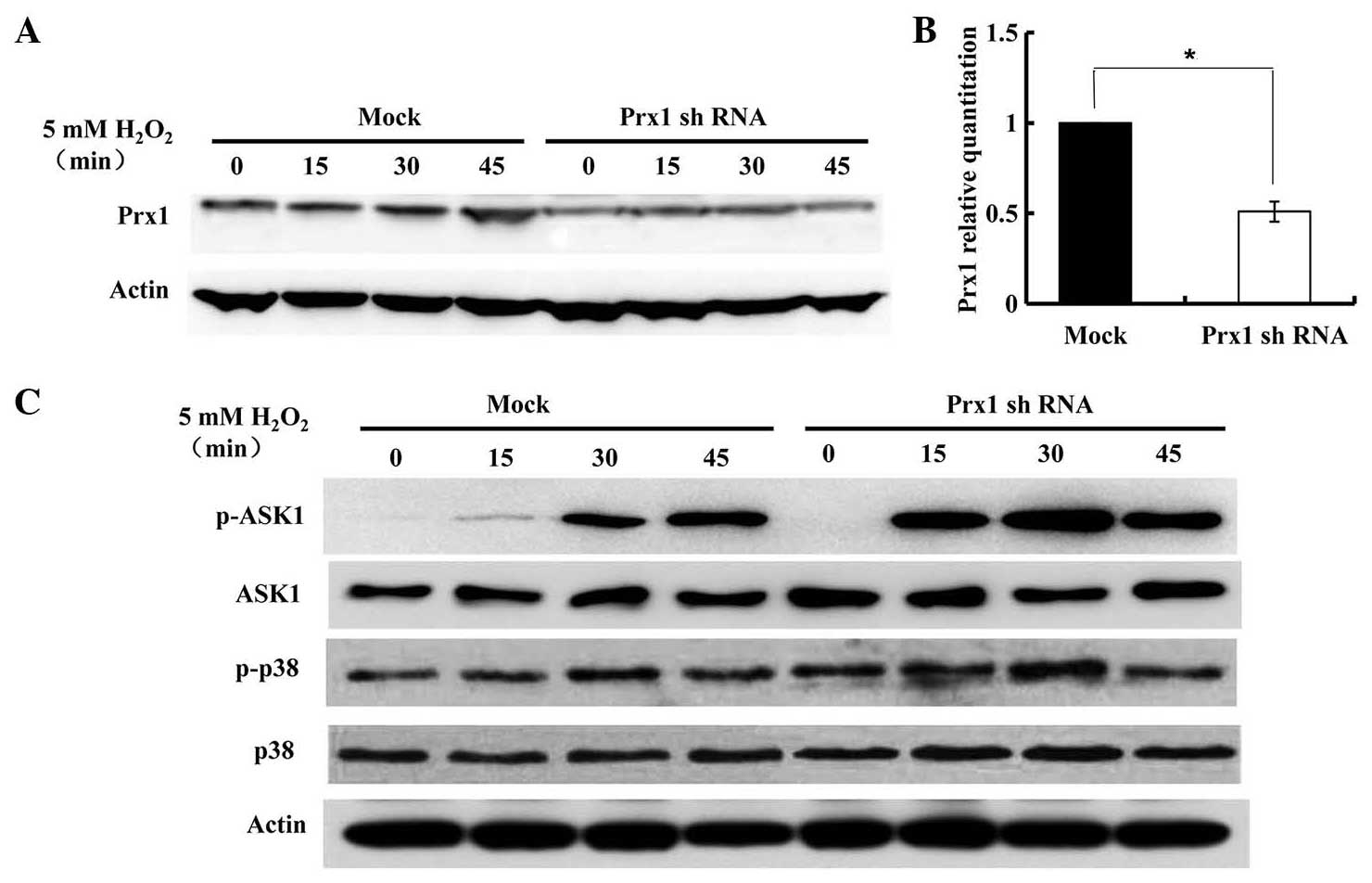 | Figure 3.Expression of Prx1 and its regulation
of the ASK1-mediated signaling pathway for the activation of p38.
DOK cells were transfected with control and Prx1 shRNA vector.
Following transfection for 48 h, cells were treated with 5 mM
H2O2 for the indicated times, and cell
lysates were subjected to immunoblot analysis with anti-Prx1,
anti-p-Thr845 ASK1, anti-ASK1, anti-p-p38, anti-p38 or anti-β-actin
antibodies. (A) Expression of Prx1 in
H2O2-treated DOK cells, with or without Prx1
shRNA transfection. (B) Prx1 expression was suppressed by shRNA
transfection, *P=0.046. (C) The expression of ASK1, p-ASK1, p38 and
p-p38 in DOK cells with or without Prx1 shRNA transfection. Prx1,
peroxiredoxin 1; mRNA, messenger RNA; OLK, oral leukoplakia; ASK1,
apoptosis signal-regulating kinase 1; p, phosphorylated; shRNA,
short hairpin RNA. |
Prx1 suppresses apoptosis in DOK cells
treated with H2O2
Subsequently, the functional roles of Prx1 in
H2O2-induced apoptosis were examined. DOK
cells were transiently transfected with Prx1 shRNA. Following
transfection for 48 h, the cells were treated with 5 mM
H2O2 (0, 15, 30 and 45 min)and apoptotic cell
death was measured by flow cytometry. Notably,
H2O2-induced apoptosis was significantly
enhanced in DOK cells transfected with Prx1 shRNA compared with
that of the mock-transfected cells (Fig.
4), particularly following treatment with 5 mM
H2O2 for 30 min (56.0±2.8 vs. 20.3±3.3%,
respectively; P=0.008).
No interaction is detected between
Prx1 and ASK1 in DOK cells treated with
H2O2
To investigate the association between Prx1 and
ASK1, whether Prx1 interacts with ASK1 in vitro was examined
(Fig. 5A). DOK cells were transiently
transfected with expression plasmids encoding ASK1 and Prx1.
Following co-transfection for 48 h, the cells were stimulated with
5 mM of H2O2 for 30 min, extracted and
immunoprecipitated with anti-Prx1 antibody. No ASK1 was detected in
immunoprecipitates of endogenous Prx1 under these conditions.
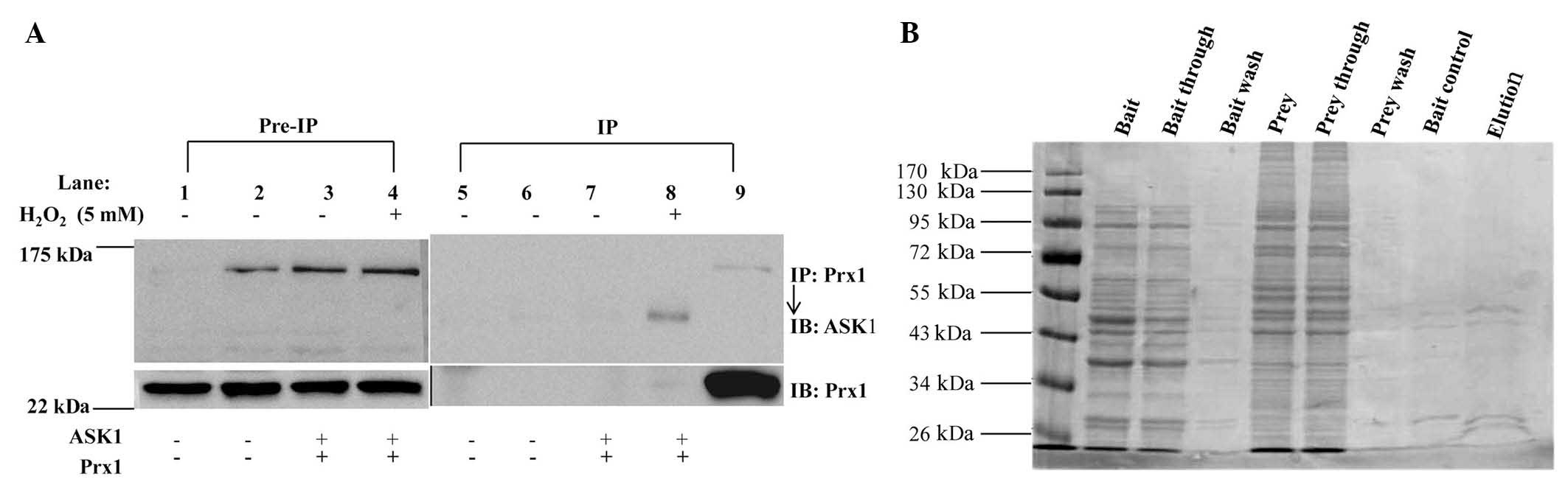 | Figure 5.No interaction was identified between
Prx1 and ASK1. (A) DOK cells were transfected for 48 h with
plasmids encoding Prx1 and ASK1, respectively. Cells were
stimulated with 5 mM H2O2 for 30 min. Cell
lysates were incubated with rabbit monoclonal antibodies against
Prxl, precipitated by Protein A/G beads and detected by western
blots using rabbit polyclonal antibodies against ASK1. Pre-immune
IgG was used as a negative control. Lane 1, blank cell control;
lane 2, transfection control; lane 3, Cells transfected with 2
vectors; lane 4, cells transfected with 2 vectors and treated with
H2O2; lane 5, positive control. (B) Direct
interaction between Prx1 and ASK1 proteins in vitro was
investigated by glutathione S-transferase pull-down assay. Prx1,
peroxiredoxin 1; ASK1, apoptosis signal-regulating kinase 1; IP,
immunoprecipitation; IB, immunoblotting; IgG, goat anti-rabbit
HRP-conjugated IgG. |
Whether Prx1 directly interacts with ASK1 in
vitro was further examined by GST pull-down assay. A GST
pull-down assay kit was used, and the results indicated that ASK1
did not markedly bind to Prx1 (Fig.
5B). These results suggested that Prx1 and ASK1 were not able
to directly interact in vitro under these conditions.
Discussion
Studies have shown that the pathogenesis of tumors
is closely associated with oxidative stress. The levels of ROS
reflect the intracellular redox state, and in turn, high levels of
ROS induce DNA damage or cell death (15,16).
Previous studies by our group revealed that the development of OLK
was also associated with oxidative stress (17). Peroxiredoxins are a family of
peroxidases, which catalyze the removal of
H2O2 and other hydroperoxides. Prx1 is a
major member of the peroxiredoxin family, and is localized to the
cytoplasm, functioning to protect cells from excessive ROS damage
(18). The expression of Prx1 is
increased in multiple malignant tumors, including oral cancer
(6,19). It was previously reported that the
overexpression of Prx1 was able to enhance the clonogenic survival
of irradiated cells and attenuate ionizing radiation-induced JNK
activation and apoptosis (10). Prx1
inhibits cell apoptosis by regulating the activation of the p38MAPK
signaling pathway in cisplatin-induced oxidative stress (7). A previous study by our group
demonstrated that the expression of Prx1 was significantly higher
in human OLK tissues than that in the normal oral mucosa (17). Prx1 expression is closely associated
with the apoptosis in OLK tissues (20). In the present study, compared with the
normal oral mucosa, the mRNA and protein expression levels of Prx1,
ASK1 and p38 were increased, and p-ASK1 and p-p38 were
overexpressed in OLK tissues (P<0.05). These results suggest
that Prx1 may be involved in the development of OLK, and is
associated with oxidative stress-induced apoptosis, however, the
specific molecular mechanism remains elusive.
ASK1, a kinase functioning upstream of the JNK
activating signaling cascade, performs distinct biological
functions in cell differentiation and regulation of apoptotic
signaling in response to external stimulation (21,22). The
p38 protein has a wide range of biological functions, including
roles in cell proliferation, differentiation, apoptosis, migration
and invasion. As a regulator of cell apoptosis, p38 exerts dual
functions in regulating cell survival or apoptosis depending on the
diversity of cell types and external stimuli (23). Certain studies have suggested that p38
is highly expressed and has significant roles in prostate, breast,
bladder and lung cancer, as well as follicular lymphoma and
leukemia (24). When cells are
subjected to oxidative stress, inflammation and other external
stimuli, ASK1 is phosphorylated and subsequently activates the JNK
and p38 downstream signaling pathways, p-JNK and p-p38, promoting
cell apoptosis (25,26). In thyroid cancer cells exposed to Prx1
inhibitor MG132, the expression levels of ASK1 and p38 were
increased (27). A recent report
demonstrated that, in human malignant breast epithelial cells,
silencing of the Prx1 gene and treatment with various
concentrations of H2O2, significantly
increased p-p38 protein expression (9). The expression of Prx1 in dopaminergic
neuronal cells inhibited 6-hydroxydopamine-induced apoptotic death
by reducing p38/caspase-3 activation (28).
In order to investigate whether endogenous Prx1
affected ASK1-mediated apoptosis induced by oxidative stress in
OLK, the effects of Prx1-knockdown on the activation of
ASK1-mediated signaling by H2O2 in DOK cells
was evaluated. Following stimulation of DOK cells with
H2O2, the levels of p-ASK1 were rapidly and
notably enhanced at 15 min, while p-ASK1 and p-p38 gradually
increased in a time-dependent manner. Interestingly, the levels
were subsequently reduced at 45 min. Following Prx1 knockdown,
higher levels of p-ASK1 and p-p38 were observed, compared with that
in the control (mock-transfected) DOK cells. The expression of
p-ASK1 and p-p38 were gradually increased in a time-dependent
manner. These results suggested that endogenous Prx1 may be a
negative regulator for ASK1-mediated signaling in the activation of
the p38 pathway in response to H2O2. In
addition, H2O2-induced apoptosis was
significantly enhanced in Prx1-knockdown DOK cells compared with
that in mock-transfected cells, which suggest that Prx1 may
function as an endogenous antagonist of ASK1-mediated apoptosis
induced by H2O2, and Prx1 may exert a
protective effect against ASK1-induced apoptosis mediated by
oxidative stress. However, the specific mechanisms underlying the
effects of Prx1, ASK1 and p38 in the development of OLK require
further study.
The interaction of Prx1 with ASK1 in DOK cells was
examined, and no direct interaction between ASK1 and Prx1 was
observed in co-immunoprecipitation or GST pull-down assays, which
differed to the results of a previous group, where interactions
were identified between Prx1 and ASK1 in response to
H2O2 in HEK293 cells (12). The results indicated that Prx1 may
interact with ASK1 indirectly, or that the formation of an
interaction between ASK1 and Prx1 occurred in a transient manner or
at a lower affinity that was undetectable in the specific
conditions of the present study (29).
In conclusion, the present results indicated that
Prx1 is involved in OLK pathogenesis. Prx1 may participate in
providing resistance against extracellular damage from oxidative
stress via inhibition of the ASK1-induced apoptotic signaling
pathway. Therefore, targeting Prx1 may offer a novel strategy for
the treatment of patients with OLK.
Acknowledgements
The present study was funded by the National Natural
Science Foundation (no. 81070836), the Beijing Natural Science
Foundation (no. 7102065), the National Natural Science Foundation
of China (no. 81470752) and the Beijing Municipal Administration of
Hospital Key Medical Development Project (no. ZYLX201407).
References
|
1
|
Messadi DV: Diagnostic aids for detection
of oral precancerous conditions. Int J Oral Sci. 5:59–65. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
van der Waal I: Oral potentially malignant
disorders: Is malignant transformation predictable and preventable?
Med Oral Patol Oral Cir Bucal. 19:e386–e390. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Neville BW and Day TA: Oral cancer and
precancerous lesions. CA Cancer J Clin. 52:195–215. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nayyar AS and Khan M: In search of
malignant transformation: A pilot study. J Cancer Res Ther.
8:277–281. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Metgud R and Bajaj S: Evaluation of
salivary and serum lipid peroxidation, and glutathione in oral
leukoplakia and oral squamous cell carcinoma. J Oral Sci.
56:135–142. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yanagawa T, Iwasa S, Ishii T, Tabuchi K,
Yusa H, Onizawa K, Omura K, Harada H, Suzuki H and Yoshida H:
Peroxiredoxin I expression in oral cancer: A potential new tumor
marker. Cancer Lett. 156:27–35. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cha MK, Suh KH and Kim IH: Overexpression
of peroxiredoxin I and thioredoxin1 in human breast carcinoma. J
Exp Clin Cancer Res. 28:932009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhao YH, Zhang M, Yan F, Casto BC and Tang
XF: Nicotine-induced upregulation of antioxidant protein Prx 1 in
oral squamous cell carcinoma. Chin Sci Bull. 58:1912–1918. 2013.
View Article : Google Scholar
|
|
9
|
Turner-Ivey B, Manevich Y, Schulte J,
Kistner-Griffin E, Jezierska-Drutel A, Liu Y and Neumann CA: Role
for Prdx1 as a specific sensor in redox-regulated senescence in
breast cancer. Oncogene. 32:5302–5314. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kim YJ, Lee WS, Ip C, Chae HZ, Park EM and
Park YM: Prx1 suppresses radiation-induced c-Jun NH2-terminal
kinase signaling in lung cancer cells through interaction with the
glutathione S-transferase Pi/c-Jun NH2-terminal kinase complex.
Cancer Res. 66:7136–7142. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Turjanski AG, Vaqué JP and Gutkind JS: MAP
kinases and the control of nuclear events. Oncogene. 26:3240–3253.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kim SY, Kim TJ and Lee KY: A novel
function of peroxiredoxin 1 (Prx-1) in apoptosis signal-regulating
kinase 1 (ASK1)-mediated signaling pathway. FEBS Lett.
582:1913–1918. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wu Z, Sheng H, Chen Y, Tang J, Liu Y, Chen
Q, Lu L and Jin W: Copy number variation of the Lipoprotein(a)
(LPA) gene is associated with coronary artery disease in a southern
Han Chinese population. Int J Clin Exp Med. 7:3669–3677.
2014.PubMed/NCBI
|
|
14
|
Lowry OH, Rosebrough NJ, Farr AL and
Randall RJ: Protein measurement with the Folin phenol reagent. J
Biol Chem. 193:265–275. 1951.PubMed/NCBI
|
|
15
|
Memon AA, Chang JW, Oh BR and Yoo YJ:
Identification of differentially expressed proteins during human
urinary bladder cancer progression. Cancer Detect Prev. 29:249–255.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xie Q, Zhou Y, Lan G, Yang L, Zheng W,
Liang Y and Chen T: Sensitization of cancer cells to radiation by
selenadiazole derivatives by regulation of ROS-mediated DNA damage
and ERK and AKT pathways. Biochem Biophys Res Commun. 449:88–93.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ge LH, Hou M, Yang J, Chen T and Tang XF:
Prx1 overexpression in human OLK. Beijing J Stomatology.
20:135–137. 2012.
|
|
18
|
Chatterjee S, Feinstein SI, Dodia C,
Sorokina E, Lien YC, Nguyen S, Debolt K, Speicher D and Fisher AB:
Peroxiredoxin 6 phosphorylation and subsequent phospholipase A2
activity are required for agonist-mediated activation of NADPH
oxidase in mouse pulmonary microvascular endothelium and alveolar
macrophages. J Biol Chem. 286:11696–11706. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tang XF, Zhang XY and Zhang M: The
differences expression of oxidative stress-related genes in oral
cancer and precancerous cells. Beijing J Stomatology. 16:308–311.
2008.
|
|
20
|
Zhang JF, Tang XF, Ge LH, Yang J, Niu WW,
Zhang M and Chen T: Role of apoptosis signal-regulating kinase 1 in
the cell apoptosis in oral leukoplakia. Beijing J Stomatology.
22:65–69. 2014.
|
|
21
|
Nakagawa H, Hirata Y, Takeda K, Hayakawa
Y, Sato T, Kinoshita H, Sakamoto K, Nakata W, Hikiba Y, Omata M, et
al: Apoptosis signal-regulating kinase 1 inhibits
hepatocarcinogenesis by controlling the tumor-suppressing function
of stress-activated mitogen-activated protein kinase. Hepatology.
54:185–195. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hayakawa Y, Hirata Y, Nakagawa H, Sakamoto
K, Hikiba Y, Kinoshita H, Nakata W, Takahashi R, Tateishi K, Tada
M, et al: Apoptosis signal-regulating kinase 1 and cyclin D1
compose a positive feedback loop contributing to tumor growth in
gastric cancer. Proc Natl Acad Sci USA. 108:780–785. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Koul HK, Pal M and Koul S: Role of p38 MAP
kinase signal transduction in solid tumors. Genes Cancer.
4:342–359. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Che JP, Li W, Yan Y, Liu M, Wang GC, Li
QY, Yang B, Yao XD and Zheng JH: Expression and clinical
significance of the nin one binding protein and p38 MAPK in
prostate carcinoma. Int J Clin Exp Pathol. 6:2300–2311.
2013.PubMed/NCBI
|
|
25
|
Takeda K, Matsuzawa A, Nishitoh H and
Ichijo H: Roles of MAPKKK ASK1 in stress-induced cell death. Cell
Struct Funct. 28:23–29. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Soqa M, Matsuzawa A and Ichijo H:
Oxidative stress-induced diseases via the ASK1 signaling pathway.
Int J Cell Biol. 2012:4395872012.PubMed/NCBI
|
|
27
|
Du ZX, Yan Y, Zhang HY, Liu BQ, Gao YY,
Niu XF, Guan Y, Meng X and Wang HQ: Suppression of MG132-mediated
cell death by peroxiredoxin 1 through influence on ASK1 activation
in human thyroid cancer cells. Endocr Relat Cancer. 17:553–560.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lee YM, Park SH, Shin DI, Hwang JY, Park
B, Park YJ, Lee TH, Chae HZ, Jin BK, Oh TH, et al: Oxidative
modification of peroxiredoxin is associated with drug-induced
apoptotic signaling in experimental models of Parkinson disease. J
Biol Chem. 283:9986–9998. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jarvis RM, Hughes SM and Ledgerwood EC:
Peroxiredoxin 1 functions as a signal peroxidase to receive,
transduce, and transmit peroxide signals in mammalian cells. Free
Radic Biol Med. 53:1522–1530. 2012. View Article : Google Scholar : PubMed/NCBI
|



















