Introduction
Hepatoid adenocarcinomas (HACs) have been reported
in gastrointestinal tract organs, including the gallbladder (4%),
pancreas (4%), uterus (4%), lung (5%) and ovary (10%); however, the
stomach (63%) is the most common origin of tumors according to a
study of 261 HAC cases (1). This is
due to the fact that the gastric system and liver were derived from
the primitive foregut of the embryo (2). Bourreille et al firstly reported
a case of α-fetoprotein-producing gastric carcinoma (AFPPGC) with
liver metastasis in 1970 (3), and
Ishikura et al termed this type of gastric cancer ‘hepatoid
adenocarcinoma of the stomach (GHAC)’ (4). The majority of patients with GHAC
demonstrate an elevated serum AFP level; however, 46% of GHAC
tissues were negatively stained with AFP (2). GHAC is a rare type of gastric
adenocarcinoma, with an incidence of only 0.38–1% among all gastric
cancers (5,6). In view of the high incidence of liver
metastasis, GHAC has a relatively poorer prognosis than common
gastric cancer. It is difficult to distinguish GHAC with liver
masses from primary hepatocellular carcinoma (HCC). To present the
clinicopathological features, and to evaluate the therapeutic
regimen and outcomes for patients with GHAC, we retrospectively
analyzed nine cases which were treated in the First Affiliated
Hospital of Zhejiang University, China, from January 2009 to
December 2013.
Case report
Clinical data
Relevant clinical data are provided in Table I. Eight patients were male and one was
female (median age, 63 years; range, 47–72 years). Eight patients
had epigastric discomfort that had persisted for a certain time.
Case 5 had an elevated serum AFP level that had persisted for two
years; ultrasonographic and computed tomography (CT) scans had
detected multiple hepatic nodules, so the patient was initially
misdiagnosed as having primary HCC. Then hepatic artery digital
subtraction angiography and transcatheter arterial
chemoembolization (TACE) were performed, followed by a gastric
biopsy which revealed gastric carcinoma. The hepatic nodes in this
case were confirmed as angioma. Gastroscopy and biopsy revealed
that gastric adenocarcinoma was present in all patients. The tumors
of six cases were located in the antrum, two in the cardia and one
in the corpus. All patients were serologically negative for
hepatitis B surface antigen and hepatitis C antibody, and they did
not reveal any imaging signs of cirrhosis. Cases 2 and 7 had a
history of alcohol abuse, and the others did not. The laboratory
investigation revealed that the serum AFP levels of eight patients
were notably elevated, with a median level of 916.8 ng/ml (range,
4.4–8455.9 ng/ml; Table I).
 | Table I.Preoperative clinical features in nine
cases of hepatoid adenocarcinoma. |
Table I.
Preoperative clinical features in nine
cases of hepatoid adenocarcinoma.
| Case | Gender/age | Site/size (cm) | Pre-/postoperative
AFP (ng/ml) | Liver/lung
metastases | Clinical
presentation | Endoscopic Borrmann
type |
|---|
| 1 | M/47 | Stomach, gastric
body/1.5×1.3 | 4.4/7.7 | No | Epigastric
discomfort | II |
| 2 | M/63 | Antrum/5.0×3.0 | 916.8/441.9 | No | Epigastric
tenderness | III |
| 3 | F/76 |
Cardia/7.0×5.0×3.0 | 448.6/63.0 | No | Upper abdominal
pain | II |
| 4 | M/61 | Antrum/6.5×4.0 | 3633.9/3.3 | No | Epigastric
discomfort | I |
| 5 | M/69 | Antrum/3.0×2.5 | 5333.2/58.2 | No | Elevated serum
AFP | II |
| 6 | M/57 | Antrum/3.0×4.0 | 42.3/5.1 | No | Upper abdominal
pain | II |
| 7 | M/67 | Cardia/4.0×3.2 | 270.0/32.9 | No | Epigastric
discomfort | II |
| 8 | M/58 | Antrum/4.5×4.0 | 8455.9/471.1 | No | Upper abdominal
pain | II |
| 9 | M/72 | Antrum/4.0×6.0 | 1079.3/n.a. | No | Epigastric
tenderness | II |
Treatment procedures and
prognosis
A CT scan revealed that three patients (cases 2, 4
and 6) had multiple retroperitoneal area and perigastric lymph node
enlargement, so the three cases received neo-adjuvant chemotherapy.
None of these patients developed liver or lung metastases. Due to
the notably elevated serum AFP level, case 5 was misdiagnosed as
HCC and received TACE treatment at first admission until gastric
endoscopy identified gastric adenocarcinoma. All patients received
radical gastrectomy, among whom seven received subtotal
gastrectomy, and two (cases 1 and 7) received total gastrectomy.
According to the American Joint Committee on Cancer (2010AJCC)
pathological tumor-node-metastasis (pTNM) staging classification
for carcinoma of the stomach, stages I and II were observed in one
patient, respectively, and stage III was observed in seven
patients. These patients recovered following surgery without any
notable postoperative complications. Seven patients underwent
adjuvant postoperative chemotherapy. In case 5, after four cycles
of chemotherapy regimen with S-1 and oxaliplatin, the serum AFP
increased from 58.2 to 1449.0 ng/ml, and a magnetic resonance
imaging scan revealed multiple hepatic metastases 6 months after
surgery. Case 9 demonstrated multiple hepatic metastases one month
after surgery and succumbed 5 months after surgery. Case 2
demonstrated a partial response following six cycles of
chemotherapy with FOLFOX regimen, but was observed to have multiple
liver metastases, so the patient was administered TS-1 as
chemotherapy as well as liver radiofrequency ablation (RFA). Case 4
was observed to have a liver metastasis 18 months after surgery,
following four cycles of chemotherapy with a combination of
capecitabine plus paclitaxel; this case underwent liver tumor
resection. Case 8 had lung metastasis 22 months after surgery and
received paclitaxel plus carboplatin as the chemotherapy regimen,
demonstrating a partial response. Until present, only one patient
has succumbed, four patients have liver metastases, one has lung
metastasis and four remain disease-free. Relevant treatment
procedures and prognosis data are shown in Table II. Written informed consent was
obtained from the patient's family and this study was approved by
the ethics committee of First Affiliated Hospital of Zhejiang
University, Hangzhou, China.
 | Table II.Treatment and prognosis of nine cases
of hepatoid adenocarcinoma. |
Table II.
Treatment and prognosis of nine cases
of hepatoid adenocarcinoma.
| Case | Neo/adjuvant
chemotherapy | Surgery/R0+D2 | Status | DFS (months) | OS (months) |
|---|
| 1 | No/SOXx6 | Yes | Disease-free | 7 | 7 (censored) |
| 2 |
(FOLFOXx2)/(FOLFOXx4), TS-1 | Yes | Liver metastases | 4 | 7 (censored) |
| 3 | No | Yes | Disease-free | 6 | 6 (censored) |
| 4 | (SOXx2)/(SOXx4),
capecitabine plus paclitaxel | Yes | Liver metastases | 18 | 28 (censored) |
| 5 | No/(SOXx4) | Yes | Liver metastases | 11 | 15 (censored) |
| 6 | (SOXx2)/(SOXx4) | Yes | Disease-free | 32 | 34 (censored) |
| 7 | No/(SOXx6) | Yes | Disease-free | 36 | 36 (censored) |
| 8 | No/(SOXx6),
paclitaxel plus carboplatin | Yes | Lung metastases | 22 | 43 (censored) |
| 9 | No | Yes | Liver metastases | 1 | 5 |
Pathological and immunohistochemical
features
The pathological diagnostic criteria of GHAC was
that tumor cells histologically demonstrate features resembling
HCC. There was no particular quantity requirement for histological
hepatoid differentiation, and patients with focal hepatoid or
intermingled with sarcoma were also diagnosed with GHAC. Of the
nine GHAC cases, six were confirmed to be HAC with complete
hepatocyte-like regions; HAC intermingled with signet-ring cell
components was observed in one case, with sarcoma cell components
in one case and with tubular adenocarcinoma components in one case,
respectively. Most of the patients (8/9) had poorly differentiated
tumors. Eight patients had lymph node metastasis. Six had
endovascular tumor emboli. Tumor cells were arranged in a
trabecular pattern and resembled HCC, with abundant blood sinus.
Polygonal cells with eosinophilic cytoplasm and hyperchromatic
nucleoli indicated hepatoid differentiation. Immunohistochemistry
revealed that the neoplastic cells in hepatoid areas of primary
tumors and metastases demonstrated positivity for AFP, with the
exception of cases 1 and 9. Specifically, the tumors in two cases
were stained positively for synaptophysin and chromogranin A, which
indicated neuroendocrine differentiation. The histopathological and
immunohistochemical features of the nine cases are shown in
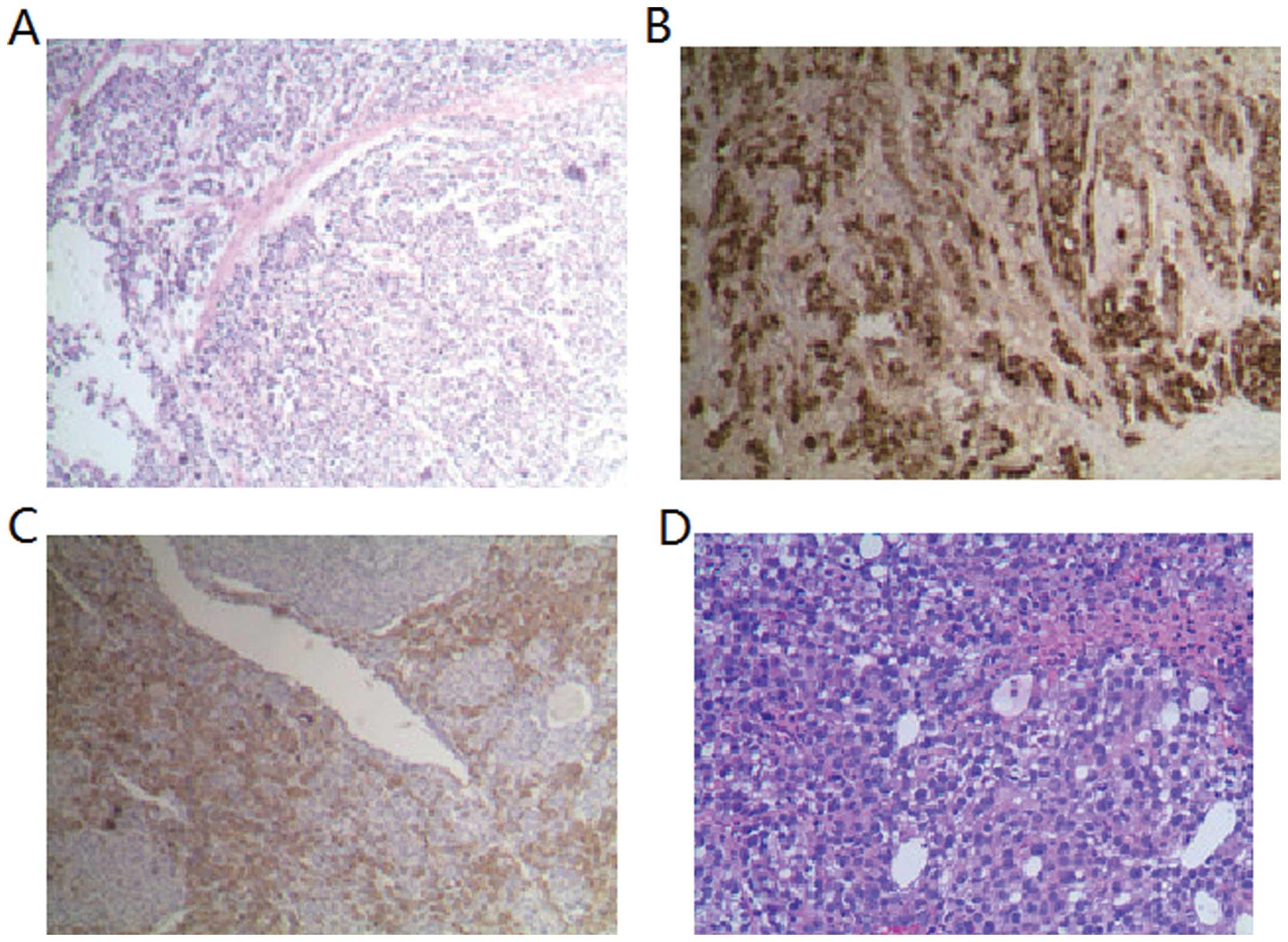
Table III. Immunohistochemical
features of hepatoid adenocarcinoma of the stomach are showed in
Fig. 1
 | Table III.Histopathological and
immunohistochemical features in nine cases of hepatoid
adenocarcinoma. |
Table III.
Histopathological and
immunohistochemical features in nine cases of hepatoid
adenocarcinoma.
| Case |
Histopathlogy/differentiation | AFP | CK | CgA | SYN | Vascular
invasion | pTNM
stage/pstage |
|---|
| 1 | Hepatoid with
signet-ring cell carcinoma/P | – | CK2+ | n.a. | n.a. | + | pT2N3aM0/IIIA |
| 2 | Hepatoid/P | + | CK2+ | + | + | + | pT4aN3bM0/IIIC |
| 3 | Carcinosarcoma | + | n.a. | n.a. | n.a. | n.a. | pT1bN0M0/IA |
| 4 | Hepatoid/P | + | CK7+CK19+CK20- | n.a. | n.a. | – | pT4aN2M0/IIIB |
| 5 | Hepatoid/P | + | CK20+ | + | + | – | pT3N1M0/IIB |
| 6 | Hepatoid/M-P | + | CK20- | – | – | + | pT4aN3M0/IIIC |
| 7 | Hepatoid with
tubular adenocarcinoma/P | + | CK19+ | – | – | + | pT4aN3M0/IIIC |
| 8 | Hepatoid/M-P | + | CK7-CK19+CK20+ | – | – | + | pT4aN2M0/IIIB |
| 9 | Hepatoid/P | – | CK14-CK20+ | – | – | + | PT4aN2M0/IIIB |
Discussion
The GHAC cases in our study were characterized
histologically by hepatoid differentiation and shared clinical
features, including elevated serum AFP, predilection for elderly
male patients and location in the antrum, aggressive behavior, and
preferential metastases to the lymph nodes and liver, which is
similar to the results of previous studies (6–9). GHAC
patients usually have an elevated serum AFP level, so it is often
misdiagnosed as primary HCC, particularly in GHAC patients with
simultaneously occurring liver-occupying lesions. Generally,
neighboring cirrhotic lesions are frequently observed in primary
HCC, while they seldom occur in GHAC with liver metastases.
Although metastatic lesions in the liver demonstrate a histological
appearance similar to that of HCC, the clinical background and
immunohistochemical examination still aid the differential
diagnosis, since HCC often develops from liver cirrhosis to HCC,
frequently accompanied by positive HepPar1 (10). The clinicopathological entity of GHAC
is a tumor composed of polygonal cells arranged in a solid or
trabecular manner that resembles HCC; hence, the pathological
diagnostic criteria of GHAC is that tumor cells histologically
demonstrate hepatoid features (11,12).
Although two tumor cases in our study did not express AFP, the
morphology and immunophenotype were consistent with GHAC.
Immunohistochemical staining is conducive to distinguishing HCC
from GHAC with liver metastases; however, its success has been
limited by the lack of a reliable positive marker for
hepatocellular differentiation. GHAC is frequently positive for AFP
(91%), cytokeratin (CK)18/CK19 (100%), CK20 (25%), pancytokeratin
(AE1/AE3) (92.3%) and α1-antitrypsin (91%) (13), and glypican-3 has been reported to be
useful in the differential diagnosis between GHAC and HCC (1). In addition to these markers, palate,
lung and nasal epithelium carcinoma-associated protein represents a
promising marker in distinguishing HAC from HCC, since it is
detected in liver metastases of GHAC, but not in HCC (14). However, none of these markers are
sensitive or specific enough. The serum AFP level was not
associated with the dimension, size, stage or prognosis of GHAC
(15); however, patients with
AFP-positive gastric cancer have a significantly higher tendency to
develop liver metastasis and have a shorter long-term survival than
patients with AFP-negative gastric cancers (16), since AFP's ability to suppress
lymphocyte DNA synthesis inhibits lymphocyte transformation
(17). Koide et al reported
that AFP-producing gastric cancers have high malignant potential
(high proliferative activity, weak apoptosis and rich
neovascularization) compared with that of AFP-negative gastric
cancers (18). Patients with normal
AFP levels may represent a subtype of GHAC, with a more positive
biological behavior (19). The
measurement of the serum AFP level is also helpful during the
follow-up period, as it usually falls sharply following adequate
surgical treatment, while persistence of AFP elevation following
active multimodality treatment including tumor resection may
indicate regional or distant metastasis. Kumashiro et al
reported that the histogenesis of HAC was strongly associated with
the intestinal phenotype, and its hepatoid component was in some
way associated with reduced CDX2 expression. High levels of CD10
and low levels of CDX2 expression may be associated with the
aggressive biological behavior of GHAC (5).
Although there is no standard therapy protocol for
GHAC, the diseases primary treatment modality can be based on that
of common gastric adenocarcinoma. The majority of patients (6/9) in
our study received first-line postoperative adjuvant chemotherapy.
The liver metastases should undergo tumor resection if metastatic
tumors are resectable; certain patients gain a survival benefit
from this (case 4). Additionally, RFA is a safe and effective
alternative for the partly unresectable liver metastases (case 2).
TACE with doxorubicin transiently arrests the progression of
recurrent liver metastases (20).
Systemic chemotherapy demonstrated good effects in our cases.
The poor prognosis is associated with tendency of
venous invasion, lymphatic permeation, lymph node metastasis, and
synchronous and metachronous metastasis of the liver or other
organs. One study reveals that a higher expression of c-Met may be
associated with the poor prognosis of AFP-producing gastric cancer
(21). Survival is closely associated
with the pTNM stage. Baek et al reported that the median
overall survival of patients with stages I–III and stage IV was
28.0 and 8.2 months, respectively (9). Relatively longer survival requires
accurate diagnosis at an earlier stage as well as active
multimodality treatment, including radical gastrectomy and adjuvant
chemotherapy.
In conclusion, GHAC usually occurs with an elevated
serum AFP level, and has unique pathological and
immunohistochemical characteristics with notable morphological
similarities to primary HCC. GHAC is associated with liver and
lymph node metastases, indicating that the prognosis is poorer than
with ordinary gastric cancer. It is essential to differentiate
metastatic liver lesions of GHAC from primary HCC.
References
|
1
|
Metzgeroth G, Strobel P, Baumbusch T,
Reiter A and Hastka J: Hepatoid adenocarcinoma - review of the
literature illustrated by a rare case originating in the peritoneal
cavity. Onkologie. 33:263–269. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nagai E, Ueyama T, Yao T and Tsuneyoshi M:
Hepatoid adenocarcinoma of the stomach. A clinicopathologic and
immunohistochemical analysis. Cancer. 72:1827–1835. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bourreille J, Metayer P, Sauger F, Matray
F and Fondimare A: Existence of alpha feto protein during
gastric-origin secondary cancer of the liver. Presse Med.
78:1277–1278. 1970.(in French). PubMed/NCBI
|
|
4
|
Ishikura H, Fukasawa Y, Ogasawara K,
Natori T, Tsukada Y and Aizawa M: An AFP-producing gastric
carcinoma with features of hepatic differentiation. A case report.
Cancer. 56:840–848. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kumashiro Y, Yao T, Aishima S, et al:
Hepatoid adenocarcinoma of the stomach: histogenesis and
progression in association with intestinal phenotype. Hum Pathol.
38:857–863. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liu X, Cheng Y, Sheng W, et al: Analysis
of clinicopathologic features and prognostic factors in hepatoid
adenocarcinoma of the stomach. Am J Surg Pathol. 34:1465–1471.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gao YB, Zhang DF, Jin XL and Xiao JC:
Preliminary study on the clinical and pathological relevance of
gastric hepatoid adenocarcinoma. J Dig Dis. 8:23–28. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang JF, Shi SS, Shao YF and Zhang HZ:
Clinicopathological and prognostic features of hepatoid
adenocarcinoma of the stomach. Chin Med J (Engl). 124:1470–1476.
2011.PubMed/NCBI
|
|
9
|
Baek SK, Han SW, Oh DY, Im SA, Kim TY and
Bang YJ: Clinicopathologic characteristics and treatment outcomes
of hepatoid adenocarcinoma of the stomach, a rare but unique
subtype of gastric cancer. BMC Gastroenterol. 11:562011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Maitra A, Murakata LA and Albores-Saavedra
J: Immunoreactivity for hepatocyte paraffin 1 antibody in hepatoid
adenocarcinomas of the gastrointestinal tract. Am J Clin Pathol.
115:689–694. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ishikura H, Kanda M, Ito M, Nosaka K and
Mizuno K: Hepatoid adenocarcinoma: a distinctive histological
subtype of alpha-fetoprotein-producing lung carcinoma. Virchows
Arch A Pathol Anat Histopathol. 417:73–80. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Galvez-Munoz E, Gallego-Plazas J,
Gonzalez-Orozco V, Menarguez-Pina F, Ruiz-Macia JA and Morcillo MA:
Hepatoid adenocarcinoma of the stomach - a different histology for
not so different gastric adenocarcinoma: a case report. Int Semin
Surg Oncol. 6:132009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Su JS, Chen YT, Wang RC, Wu CY, Lee SW and
Lee TY: Clinicopathological characteristics in the differential
diagnosis of hepatoid adenocarcinoma: a literature review. World J
Gastroenterol. 19:321–327. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sentani K, Oue N, Sakamoto N, et al: Gene
expression profiling with microarray and SAGE identifies PLUNC as a
marker for hepatoid adenocarcinoma of the stomach. Mod Pathol.
21:464–475. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Inoue M, Sano T, Kuchiba A, Taniguchi H,
Fukagawa T and Katai H: Long-term results of gastrectomy for
alpha-fetoprotein-producing gastric cancer. Br J Surg.
97:1056–1061. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chang YC, Nagasue N, Abe S, Taniura H,
Kumar DD and Nakamura T: Comparison between the clinicopathologic
features of AFP-positive and AFP-negative gastric cancers. Am J
Gastroenterol. 87:321–325. 1992.PubMed/NCBI
|
|
17
|
Yachnin S: The immunosuppressive
properties of alpha-fetoprotein: a brief overview. Ann N Y Acad
Sci. 417:105–107. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Koide N, Nishio A, Igarashi J, Kajikawa S,
Adachi W and Amano J: Alpha-fetoprotein-producing gastric cancer:
histochemical analysis of cell proliferation, apoptosis and
angiogenesis. Am J Gastroenterol. 94:1658–1663. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Papatsimpas G, Kamposioras K, Goula K, et
al: Hepatoid pancoast tumor. A case report and review of the
literature. Lung Cancer. 77:239–245. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lu CC, De-Chuan C, Lee HS and Chu HC: Pure
hepatoid adenocarcinoma of the stomach with spleen and lymph-node
metastases. Am J Surg. 199:e42–e44. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Amemiya H, Kono K, Mori Y, et al: High
frequency of c-Met expression in gastric cancers producing
alpha-fetoprotein. Oncology. 59:145–151. 2000. View Article : Google Scholar : PubMed/NCBI
|