Introduction
Bladder urothelial carcinoma is the most common
malignant urinary tract tumor worldwide, and is characterized by a
high level of morbidity and mortality (1,2). Of
bladder tumors, 90% are transitional cell carcinomas (TCCs)
(1). Approximately 80% of TCCs are
non-muscle-invasive (pTa-pT1), while 20% of TCCs are
muscle-invasive (pT2-pT4). Between 10–30% of non-muscle-invasive
TCCs will develop into muscle-invasive TCCs, and 50–70% of
non-muscle-invasive TCCs recur post-operatively (3). The 5-year overall survival rate of
non-muscle-invasive TCCs is 90%, while it is ~60% for pT2 TCCs, 35%
for pT3 TCCs, and 25% for pT4 TCCs (4,5). The
prognosis of TCC primarily depends on the clinical stage and the
histological grade at the time of diagnosis (6). Although surveillance strategies and
therapeutic options for patients with TCCs have developed in recent
years, these advances have not diminished the mortality or
recurrence rates of TCCs. In addition, the molecular mechanisms
underlying the development and patterns of recurrence of TCCs,
require further evaluation. Indeed, the identification of novel
molecular targets is necessary in order to devise improved
therapeutic approaches.
Dynamic phosphorylation events are involved in
signaling transduction, and are tightly regulated by protein
kinases and phosphatases. Protein tyrosine phosphatases (PTPs)
serve as inhibitors of tyrosine kinase signaling, and are involved
in tumor suppression (7). PTPs have
also been reported to exhibit functions in cellular physiology and
carcinogenesis (8,9). PTP non-receptor type 12 (PTPN12) is a
member of the PTP family and is located at 7q11.23 (10). PTPN12 has recently been identified as
a tumor suppressor gene, which is associated with progression in
human breast cancer (11). Another
recent study suggested that decreased expression of PTPN12 leads to
the upregulation of HER2/EGFR activity in HER2-negative breast
cancer, and that PTPN12 may act as a tumor suppressor gene in
HER2-negative breast cancer (12).
There is clear evidence of reduced expression of PTPN12 in various
human tumors, including esophageal squamous cell cancer and colon
cancer (13,14). Adhesion, migration and invasion of
tumor cells may increase the metastasis of malignant tumors, and
are also important factors in tumor recurrence. A number of studies
have indicated that PTPN12 is a ubiquitously expressed cytosolic
phosphatase, and is a key stimulator of cell migration and invasion
(15,16). PTPN12 has also been reported to
inhibit multiple oncogenic tyrosine kinases, in addition to
mediating the regulation of cell adhesion, migration and metastasis
(17). Furthermore, suppressing
PTPN12 expression may enhance the migration of colon (14) and ovarian (10) cancer cells, which suggests that PTPN12
may lead to metastasis in human malignancies. The effect of PTPN12
on the recurrence of TCCs is unclear, as TCC is a malignancy with a
high recurrence rate. Therefore, the expression of PTPN12 and its
function in TCC requires further elucidation.
The current study investigated the expression of
PTPN12 and its effect on proliferation and recurrence in human TCC.
The results indicated that PTPN12 expression is decreased in TCC,
and that decreased PTPN12 expression suppresses the proliferation,
adhesion, migration and invasion of TCC cells. Thus, loss of PTPN12
activity appears to be involved in the progression and recurrence
of TCC, and PTPN12 may be a suitable biomarker for TCC recurrence.
Furthermore, the present findings also suggested that restoring
PTPN12 activity may represent a therapeutic target for TCC. The
current study investigated the expression of PTPN12 and its effect
on proliferation and recurrence in human TCC. PTPN12 may be a
suitable biomarker in targeting TCC to prevent recurrence.
Materials and methods
Patients and samples
This study was approved by the Ethics Committee of
Affiliated Hospital of YanBian University Hospital (Yanji, China).
One hundred and sixty-four patients with TCC, who were treated in
the Department of Urology between 2008 and 2013 were randomly
selected. No patients with TCC received chemotherapy or
radiotherapy pre-operatively. Determination of the histological
cell type of the resected bladder cancer samples was conducted by
an experienced pathologist. All of the tumors in the present study
were from conventional TCCs. In addition, normal urothelial samples
were obtained from 146 patients who underwent bladder surgery due
to diseases other than TCC, such as urinary trauma and benign
prostatic hyperplasia. The histological grade was evaluated
according to the World Health Organization (WHO) 2004 grading
system for TCC (18). The tumor stage
was also assessed according to the Union for International Cancer
Control (UICC) 2009 TNM classification system (www.uicc.org/resources/tnm). All TCC and normal
bladder tissues were formalin-fixed and paraffin-embedded.
Furthermore, all samples in the current study were immediately
frozen in liquid nitrogen following resection, and then maintained
at −90°C prior to protein and total RNA extraction. Written
informed consent was obtained from all patients prior to the
study.
Cell culture
Four TCC cell lines, 253 J, T24, RT112 and TCCSUP,
were used in the current study. All cell lines were purchased from
the American Type Culture Collection (Manassas, VA, USA). TCC cells
were cultured with complete medium consisting of RPMI-1640 medium
(Gibco, Glasgow, Scotland) with 10% heat-inactivated fetal bovine
serum, and supplemented with 2 mM L-glutamine, 25 mM HEPES, 1%
non-essential amino acids, 100 µg/ml streptomycin, and 100 units/ml
penicillin (All from Sigma-Aldrich, St. Louis, MO, USA). All TCC
cell lines were incubated as monolayers in a 10-cm plastic dish and
cultured in a humidified atmosphere at 37°C with 5%
CO2.
Immunohistochemistry
Paraffin slices (4-µm) were deparaffinized in xylene
and then rehydrated with graded alcohol. Endogenous peroxidase
activity was suppressed with 0.3% hydrogen peroxide for 30 min. All
slices were also blocked with 20% rabbit serum for 20 min prior to
3 h of incubation with the appropriate primary antibody.
Subsequently, all slices were incubated with a polyclonal rabbit
anti-human PTPN12 antibody (1:500 dilution; ab90641) or monoclonal
mouse anti-human β-actin (1:5,000 dilution; ab6276) purchased from
Abcam (Cambridge, MA, USA) at 4°C for 16 h. The slices were washed
three times with Tris-buffered saline and incubated with
biotinylated goat anti-rabbit immunoglobulins (1:3,000 dilution;
E0432; DAKO, Glostrup, Denmark) at 4°C for 16 h. Antigen retrieval
was conducted by microwave heating of tissue sections attached to
microscope slides to temperatures up to 100°C. Determination of the
antibody reaction was conducted with a streptavidin-biotin complex.
The results of the immunohistochemical analysis were examined using
a CX31 binocular light microscope (Olympus Corporation, Tokyo,
Japan). PTPN12 immunostaining was semi-quantitatively assessed for
positive intensity using the following grades: -, negative; +,
weak; ++, moderate; and +++, strong.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from TCC and normal bladder
samples with a Quick PrepmRNA purification kit (GE Healthcare,
Buckinghamshire, UK), according to the manufacturer's instructions.
The first-strand cDNA was then produced, using a synthesis kit
(Amersham Biosciences, Little Chalfont, UK) for reverse
transcription. RT-PCR was also performed according to the
manufacturer's instructions. PCR products were evaluated using
agarose gel electrophoresis. qPCR was performed using LC FastStart
DNA Master SYBR Green I (Roche, Indianapolis, IN, USA). The cycling
conditions for the PCR were as follows: Denaturation (95°C for 5
min), annealing (30 cycles of 95°C for 30 sec, 60°C for 30 sec and
72°C for 1 min) and extension (72°C for 10 min). The PCR products
were then quantified with a Light Cycler (Roche). The primers for
human PTPN12 were designed by Primer Premier, version 5.0 (Premier
Biosoft International, Toronto, Canada), and the sequences were as
follows: Forward: 5′-AATACTGCAGCCACCGGAA-3′ and reverse:
5′-CAACACTGGCTTTGGATG-3′. The product size was 124 bp. GAPDH was
used as an internal control, and the primer sequences were as
follows: Forward: 5′-TCAAGAAGGTGGTGAAGCAG-3′ and reverse:
5′-GTGGAGGAGTGGGTGTC-3′.
Western blotting
Western blotting was performed according to the
manufacturer's instructions. Total protein was extracted using
lysis buffer and the protein concentration was detected using the
Bradford dye-binding protein assay (Bio-Rad Laboratories, Richmond,
CA, USA). SDS polyacrylamide gel electrophoresis was then
conducted. The rabbit anti-PTPN12 polyclonal antibody was obtained
from Abcam, and an anti-β-actin monoclonal antibody (Abcam) was
used as an internal control. The immune complexes were detected
using an ECL system (Amersham, Aylesbury, UK).
RNA interference (RNAi) and
transfection
All small interfering RNA (siRNA) oligonucleotide
sequences for PTPN12 were designed using siDirect software, version
2.0 (http://sidirect2.rnai.jp/). TCC cells
were seeded in 10-cm culture dishes with complete medium (no
antibiotics) until they reached 50% confluence. TCC cells were
subsequently transfected with siRNA oligonucleotides (Shanghai
Shenggong Biology Engineering Technology Service, Ltd., Shanghai,
China) using Lipofectamine 2000® (Invitrogen Life Technologies, CA,
USA). Following culture for 2 days, the expression of PTPN12 was
examined by RT-qPCR. The human cDNA coding sequence of PTPN12 was
cloned by RT-PCR, using a normal bladder sample as a substrate. The
PCR products were then sub-cloned into a pcDEF3 vector
(Sigma-Aldrich). The four TCC cell lines were also stably
transfected with a pcDEF3 vector containing the full-length cDNA of
PTPN12, using Lipofectamine 2000. Monoclonal TCC cell lines were
selected with G418 (Sigma-Aldrich) and the expression of PTPN12 was
also measured using RT-qPCR.
Proliferation analysis
The effect of PTPN12 on the proliferative ability of
TCC cells was analyzed by a WST-1 assay. Exponentially growing TCC
cells (2×103) were harvested and seeded into 96-well
microtiter plates. Following continuous incubation for 1, 2 or 3
days, 10 µl of WST-1 (Roche, Penzberg, Germany) was added to each
well and the incubation was continued for a further 2 h. The
absorbance of each well, which represented the cell count, was
examined using a microculture plate reader (Immunoreader, Tokyo,
Japan) at 450 nm.
Animal xenograft experiments
Thirty male BALB/C nude mice (4–6 weeks old; weight,
16–20 g) were maintained under specific pathogen-free conditions at
25°C in a relative humidity of 55%, with free access to food and
water. The mice were randomly divided into two groups: Control and
PTPN12 vector. A total of 4×108 TCC cells were injected
into the backs of nude mice. The mice were then observed
continuously for 5 weeks. The volume of each TCC tumor was measured
once per week. After 5 weeks, the mice were sacrificed under deep
anesthesia by intraperitoneal injection of pentobarbital sodium (80
mg/kg), and the final volume of each TCC tumor was recorded.
Adhesion, migration and invasion
assays
Adhesion assay
24-well culture plates were coated with 40 µg of
Matrigel/well (BD Biosciences, San Jose, CA, USA) and air-dried for
60 min. A total of 6×104 TCC cells were suspended with
RPMI-1640, containing 0.6% bovine serum albumin, and added to each
well. TCC cells were incubated for 2 h and washed three times in
order to remove unattached cells. The cells attached to the bottom
of the culture plate were stained with hematoxylin and counted
under a microscope.
Migration assay
The migration assay was performed with a 24-well
Transwell system (Poretics Corp., Livermore, CA, USA), which
contained 8 µm-pore polycarbonate membrane filters. TCC cells were
seeded into the upper wells at a density of 2×104
cells/well and incubated at 37°C for 24 h. The polycarbonate
membranes were removed, fixed with 70% ethanol and stained with
hematoxylin for 60 min. The upper sides of the membranes were
scraped to remove cells that had attached, but not migrated, and
the membranes were then mounted onto a microscope slide. Chemotaxis
was analyzed in each well by counting the number of TCC cells in 15
randomized fields under a microscope.
Invasion assay
The invasion assay was performed with a Transwell
system (Corning, Inc., Corning, NY, USA). The diameter of the
filter membrane was 6.5 mm and the pore size was 8 µm. Matrigel was
added to the filter, forming a thin gel layer, and 2×105
TCC cells, suspended with serum-free medium, were seeded into the
upper Transwell chamber. Following incubation for 24 h, TCC cells
on the upper filter were removed, while cells that had penetrated
to the lower filter were stained with hematoxylin. Cells in 15
randomized fields were counted under a microscope.
Statistical analysis
Statistical calculations were performed using SPSS
version 16.0 (SPSS, Inc., Chicago, IL, USA). All experiments were
conducted in triplicate and the results are presented as the mean ±
standard deviation. Data were analyzed using Student's
t-test. A χ2 test was also conducted, in order to
assess the correlation between the expression of PTPN12 and the
clinical characteristics of patients with TCC. P<0.05 was
considered to indicate a statistically significant difference.
Results
Patient characteristics
Data from 106 male and 58 female patients were
obtained in the current study. Patient ages ranged from 36–88 years
(median, 58 years) and tumor size ranged from 0.5–21 cm (median,
1.5 cm). Eighty-six patients were diagnosed with primary TCC
disease, and 78 patients had previously been treated with
cystectomy or transurethral resection. Of 164 patients with TCC, 67
were classified as grade I, 55 as grade II and 42 as grade III,
according to the WHO 2004 grading system for TCC. Clinical stage
was evaluated according to the UICC 2009 TNM staging system. The
numbers of patients with disease at each stage were as follows: T1,
46; T2, 42; T3, 41; and T4, 25 (Table
I). The follow-up period was 25–59 months. Presenting symptoms
included abdominal pain (21 patients), hematuria (113 patients),
palpable masses (3 patients) and urinary discomfort (48 patients).
Fourteen patients were anemic, 6 patients had metastatic TCC and 41
patients had >2 concomitant diseases at the time of diagnosis,
including angina pectoris, urolithiasis and diabetes mellitus.
 | Table I.Association between PTPN12 expression
and patient characteristics. |
Table I.
Association between PTPN12 expression
and patient characteristics.
| Characteristic | Number | PTPN12 mRNA (mean ±
SD) | P-value | PTPN12 protein |
|---|
|
|---|
| – (n) | + (n) | ++ (n) | +++ (n) | P-value |
|---|
| TCC | 164 |
3.24±0.36 |
| 24 | 64 | 59 | 17 |
|
| NT | 146 |
7.71±0.61 | <0.05 | 2 | 4 | 74 | 66 | <0.05 |
| Gender |
|
| >0.05 |
|
|
|
| >0.05 |
|
Male | 106 |
3.25±0.39 |
| 16 | 41 | 38 | 11 |
|
|
Female | 58 |
3.22±0.37 |
| 8 | 23 | 21 | 6 |
|
| Age (years) |
|
| >0.05 |
|
|
|
| >0.05 |
|
<60 | 99 | 3.28±0.36 |
| 15 | 41 | 33 | 10 |
|
|
≥60 | 65 |
3.18±0.33 |
| 9 | 23 | 26 | 7 |
|
| Tumor size
(cm) |
|
| <0.05 |
|
|
|
| <0.05 |
|
<3 | 84 |
4.89±0.48 |
| 6 | 21 | 45 | 12 |
|
| ≥3 | 80 |
1.52±0.17 |
| 8 | 43 | 14 | 5 |
|
| Histologic
grade |
|
| <0.05 |
|
|
|
| <0.05 |
| I | 67 |
4.49±0.42 |
| 3 | 12 | 38 | 14 |
|
| II | 55 |
3.14±0.32 |
| 5 | 31 | 16 | 3 |
|
|
III | 42 |
1.37±0.15 |
| 16 | 21 | 5 | 0 |
|
| Clinical stage |
|
| <0.05 |
|
|
|
| <0.05 |
|
T1-T2 | 88 |
4.58±0.43 |
| 5 | 23 | 47 | 13 |
|
|
T3-T4 | 76 |
1.68±0.17 |
| 19 | 41 | 12 | 4 |
|
| Primary or
recurrence |
|
| <0.05 |
|
|
|
| <0.05 |
|
Primary | 86 |
4.69±0.39 |
| 4 | 20 | 48 | 14 |
|
|
Recurrence | 78 |
1.56±0.18 |
| 20 | 44 | 11 | 3 |
|
IHC analysis of PTPN12 expression in
TCC
The expression of PTPN12 protein in normal human
urothelium and TCC tissues was determined by immunohistochemistry.
The expression of PTPN12 in TCC tissue samples was significantly
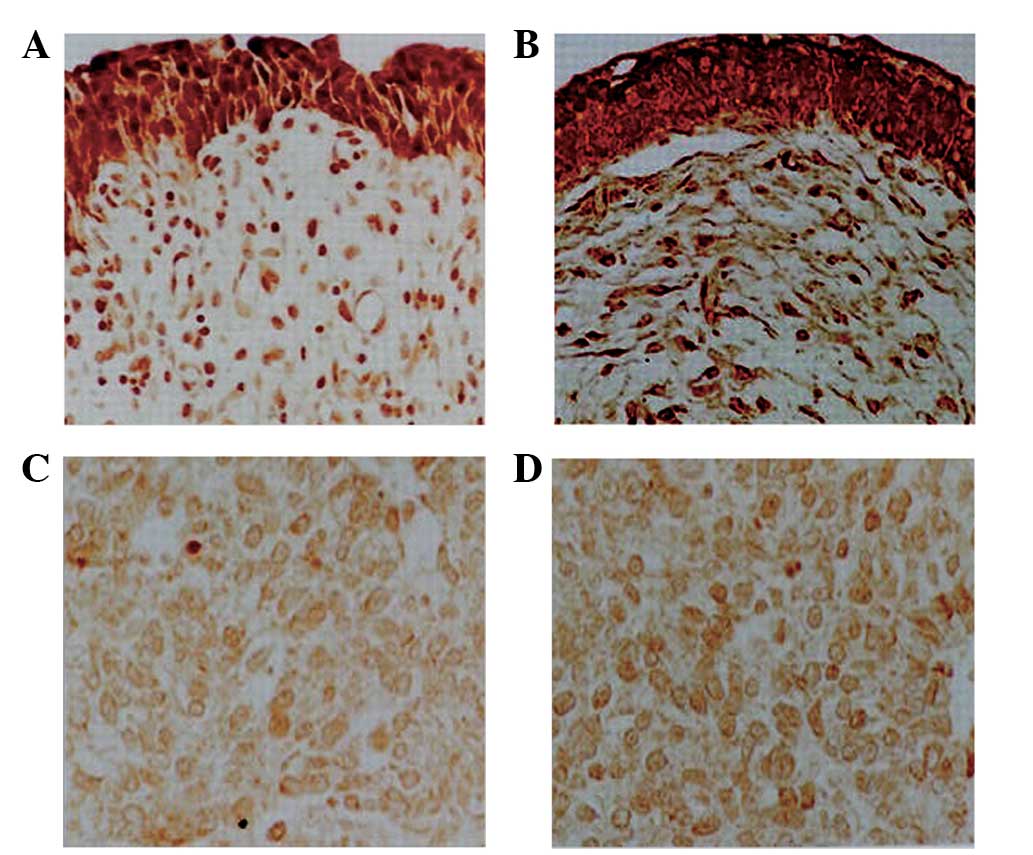
decreased compared with that in normal urothelium (Fig. 1). PTPN12 expression was detected in
140 of 164 TCC specimens (85.4%). While 46.3% of TCC specimens
exhibited medium or strong expression of PTPN12. By contrast,
PTPN12 expression was detected in 144 of 146 normal urothelium
tissues (98.6%) and 94.5% of TCC exhibited medium or strong PTPN12
expression. With respect to the correlation between clinical
characteristics and PTPN12 expression, PTPN12 expression was found
to be negatively associated with tumor size, pathological grade,
clinical stage and tumor recurrence, when assessed using
χ2 analysis. However, gender and age were not correlated
with PTPN12 expression (Table I).
These findings demonstrated that a reduction in PTPN12 protein
expression may be involved in the pathogenesis of human TCC.
RT-qPCR and western blot analysis of
PTPN12 expression in TCC
PTPN12 expression in normal human urothelium and TCC
tissues was also determined by RT-qPCR and western blotting. The
relative level of PTPN12 expression is represented as a ratio to
that of the internal control. PTPN12 expression was significantly
downregulated in TCC tissues compared with that in normal
urothelium tissues, and PTPN12 expression was similar to the levels
detected using immunohistochemistry. In addition, PTPN12 expression
in TCC tissues was negatively associated with tumor size,
pathological grade, clinical stage and tumor recurrence.
Representative results of selected TCC and corresponding normal
urothelium tissues are shown in Fig.
2.
Loss of PTPN12 activity increases the
proliferation of TCC cells
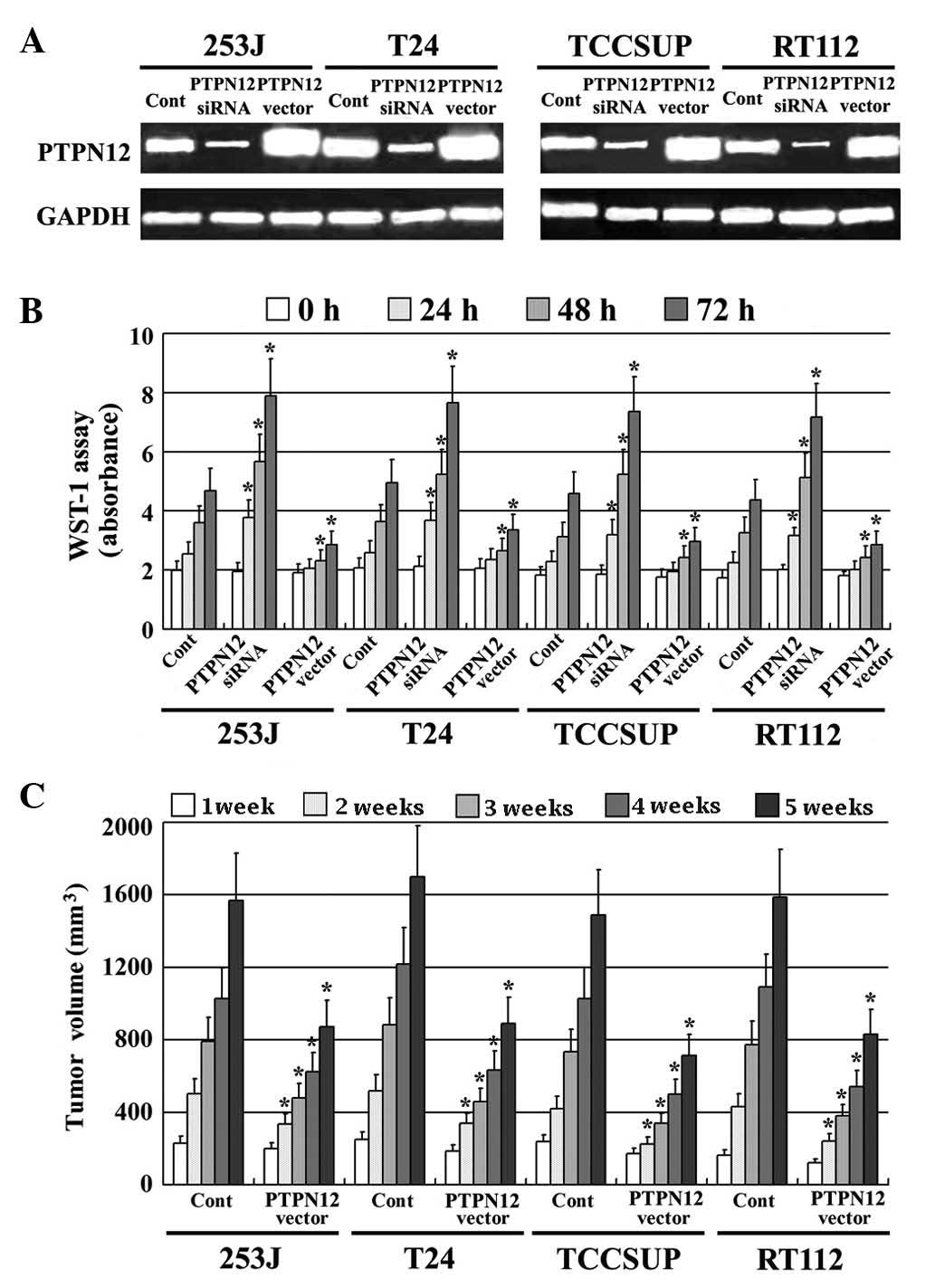
A pcDEF3 expression vector, which contained the full
length cDNA for PTPN12, was stably transfected into four TCC cell
lines (253J, T24, TCCSUP and RT112). PTPN12 expression was also
reduced using RNAi technology. PTPN12 expression in all transfected
TCC cell lines was confirmed by RT-PCR; PTPN12 mRNA expression was
significantly increased by the PTPN12 vector insert and decreased
by RNAi (Fig. 3A). The effect of
PTPN12 on the proliferation of TCC cells in vitro was
assessed by a WST-1 assay. TCC cells expressing high PTPN12,
exhibited significantly decreased proliferative ability compared
with the control cells. By contrast TCC cells expressing low PTPN12
expression exhibited a high proliferative ability compared with the
control group (Fig. 3B). In addition,
the effect of PTPN12 on the proliferation of TCC cells was
confirmed in vivo using xenografts in BALB/C nude mice
(Fig. 3C). These findings suggested
that decreased PTPN12 expression may be involved in the
proliferation of human TCC.
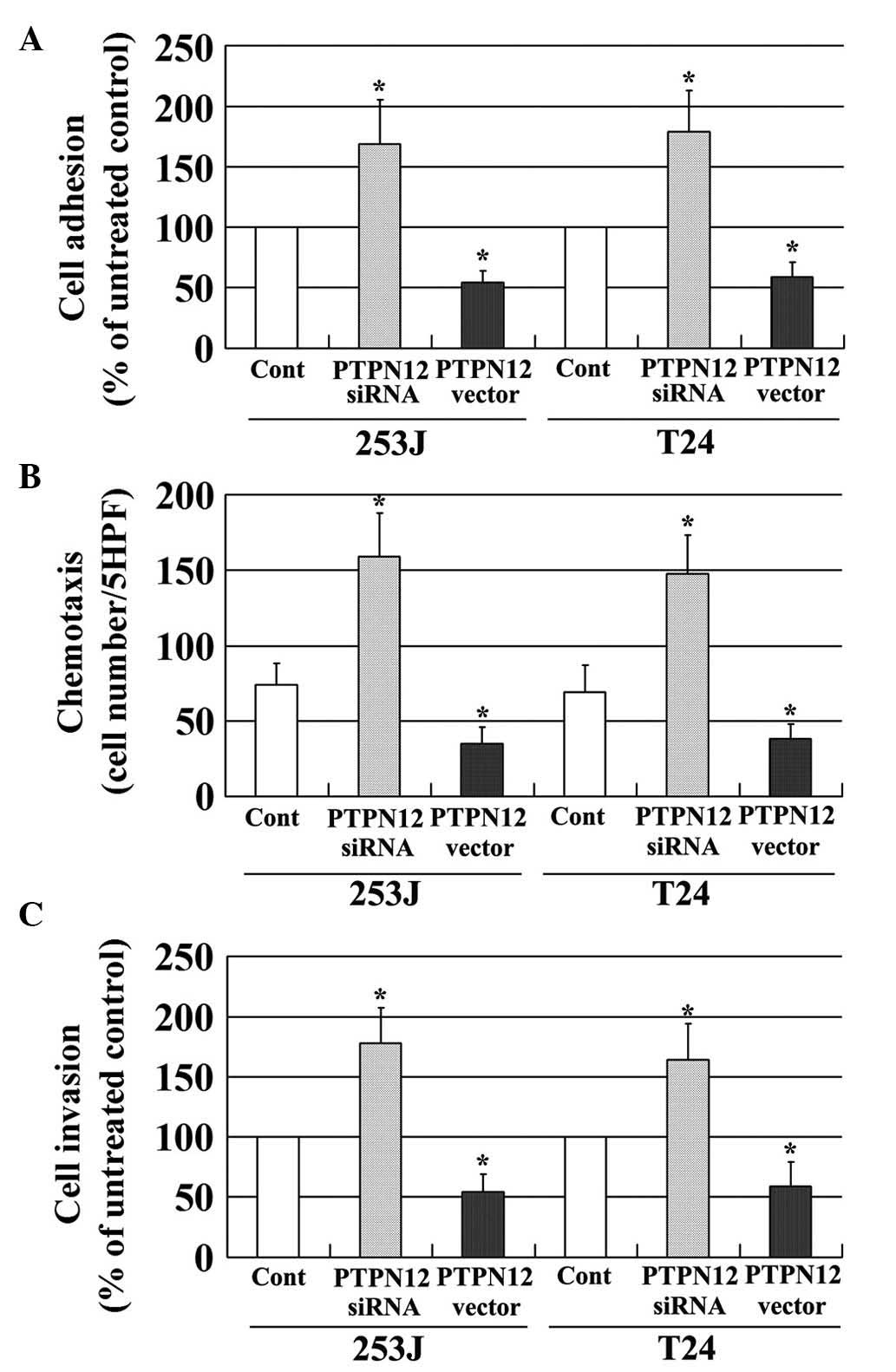
Loss of PTPN12 activity increases the
adhesion, migration and invasion of TCC cells
Recurrence, an important cause of mortality in
patients with malignancy, is a complex multistep process, which
includes changes in cell adhesion, migration and invasion (19). The effects of PTPN12 on the adhesion,
migration and invasion of TCC cells were evaluated in the present
study. The expression of PTPN12 in two TCC cell lines was decreased
using RNAi, and PTPN12 expression was increased by transfection
with the pcDEF3 vector, which contained the full length cDNA of
PTPN12. PTPN12 expression was evaluated using RT-PCR (Fig. 3A). As shown in Fig. 4, TCC cells with a low expression of
PTPN12 due to siRNA treatment, exhibited significantly increased
levels of adhesion (Fig. 4A),
migration (Fig. 4B) and invasion
(Fig. 4C) compared with untreated
controls. By contrast, TCC cells with a high expression of PTPN12,
exhibited reduced levels of adhesion (Fig. 4A), migration (Fig. 4B) and invasion (Fig. 4C) compared with control cells. These
findings suggested that decreased PTPN12 expression may enhance the
adhesion, migration and invasion of TCC cells, and that PTPN12 is
involved in the recurrence of TCC.
Discussion
Bladder urothelial carcinoma is the ninth most
common malignancy in humans, and the incidence is nearly 3-fold
higher in males than females (20).
TCC is a life-long disease, as the rate of recurrence is high and
thus, patients require regular out-patient check-ups for the rest
of their lives. Certain patients may undergo repeated surgery and
postoperative bladder perfusion chemotherapy. Therefore, routine
intravesical instillation with different chemotherapeutic agents is
performed in order to prevent recurrence of TCC following
transurethral resection, and radical cystectomy is usually
recommended for patients with muscle-invasive and high-frequency
recurrent disease (21). Although TCC
is sensitive to chemotherapy, no agents are currently available,
which specifically eliminate recurrence (22,23). The
identification of novel molecular mechanisms and therapeutic
targets for TCC are required.
Recently, PTPN12 has been hypothesized to be a tumor
suppressor gene. Loss of PTPN12 phosphatase activity has been
reported to cause cellular transformation in mammary epithelial
cells and aberrant acinar morphogenesis in human breast cancer
(12). In recent years, decreased
expression of PTPN12 has been observed in human breast cancer, and
this is associated with tumor progression and a poor prognosis
(11). PTPN12 expression is also
significantly decreased in hepatocellular carcinoma (HCC) tissues
compared with normal liver tissues, and PTPN12 is an independent
predictor of reduced cancer-specific and recurrence-free survival
in patients with HCC (24). Although
these studies have demonstrated that PTPN12 is involved in the
development of human tumors, the function of PTPN12 in TCC is
unclear, and the effect of PTPN12 on the proliferation of TCC cells
requires further elucidation. To the best of our knowledge, the
current study is the first to focus on PTPN12 expression in human
TCC. The present study analyzed the expression of PTPN12 in human
TCC, and the results of immunohistochemical analysis demonstrated
that its expression is significantly decreased in TCC tissues
compared with normal urothelium tissues. In addition, PTPN12
expression was significantly associated with tumor size,
pathological grade, clinical stage and tumor recurrence. PTPN12
expression in TCC was also measured using RT-qPCR and western
blotting, and the results of these analyses were in accordance with
those obtained using immunohistochemistry. The effect of PTPN12 on
the proliferation of TCC cells was also measured. The results
showed that reduced expression of PTPN12 significantly increased
the proliferative capacity of TCC cells in vitro. Similar
results were obtained using xenografts in BALB/C nude mice.
Therefore, the current study demonstrated that PTPN12 is an
important gene in carcinogenesis and is involved in the progression
of TCC.
Although a number of studies have been conducted in
order to better predict recurrence in patients with TCC, the
mechanism of tumor recurrence remains unknown (25–27). The
PTPN12 protein is an important regulator of cell adhesion, motility
and invasion as a result of its inhibition of, and interaction,
with multiple oncogenic tyrosine kinases (28). Therefore, it is hypothesized that
PTPN12 may be involved in the recurrence of TCC. A previous study
reported that the recurrence of TCC is associated with spread by
intraluminal seeding (19), and tumor
recurrence is known to be a complex multistep biological behavior
that includes cell adhesion, migration and invasion (29). Therefore, the effect of PTPN12 on the
adhesion, migration and invasion of TCC cells was investigated in
the current study. It was demonstrated that reduced expression of
PTPN12 enhances the adhesion, migration and invasion properties of
TCC cells. Furthermore, PTPN12 expression was significantly
decreased in the group with recurrent TCC, compared with the
primary TCC group. These finding suggest that PTPN12 may enhance
the adhesion, migration and invasion of TCC cells, thereby
facilitating the recurrence of TCC.
It was recently reported that PTPN12 acts as a
suppressor of epithelial cell motility by regulating the assembly
of adherent junctions and the activity of Rho GTPase in colon
cancer (14). Another study concluded
that PTPN12 acts as a negative regulator of cell motility via the
regulation of FAK activity by HER2 (10). Promoter hypermethylation is known to
be a key molecular mechanism, which leads to the gene silencing of
tumor suppressor genes. A recent study reported that
hypermethylation in the promoter CpG island is frequently detected,
and is related to low PTPN12 expression, which suggests that
hypermethylation in the promoter CpG island may be an important
mechanism regulating the expression of PTPN12 (11). Although the correlation between PTPN12
expression and progression in tumors has been analyzed in various
studies, the molecular mechanisms underlying the effects of PTPN12
in TCC remain elusive and require further investigation.
In conclusion, the present results showed that
PTPN12 expression is downregulated in human TCC, and that decreased
expression of PTPN12 may lead to the progression and recurrence of
this disease. It may be that patients with a low level of PTPN12
expression are at increased risk of the progression and recurrence
of TCC, and PTPN12 may be a useful predictive factor for TCC
recurrence. The current study also suggested that restoring PTPN12
expression may be a novel therapeutic strategy, and that the
molecular mechanisms underlying the effects of PTPN12 in TCC
require further elucidation.
Acknowledgements
This study is supported by grant of China State
Scholarship Fund (grant no. 201408220020) and Jilin Province 125
Scientific and Technological Research Project and YanBian
University Science and Technology Development Item (grant no.
2014039).
References
|
1
|
Ploeg M, Aben KK and Kiemeney LA: The
present and future burden of urinary bladder cancer in the world.
World J Urol. 279:289–293. 2009. View Article : Google Scholar
|
|
2
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jacobs BL, Lee CT and Montie JE: Bladder
cancer in 2010: How far have we come? CA Cancer J Clin. 60:244–272.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shariat SF, Karakiewicz PI, Palapattu GS,
et al: Outcomes of radical cystectomy for transitional cell
carcinoma of the bladder: A contemporary series from the Bladder
Cancer Research Consortium. J Urol. 176:2414–2422. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Morgan TM and Clark PE: Bladder cancer.
Curr Opin Oncol. 22:242–249. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kumar V, Abbas AK and Fausto N: 8th
Philadelephia: Elsevier. Pathologic Basis of Disease. 976–980.
2010.
|
|
7
|
Hsu JL, Huang SY, Chow NH and Chen SH:
Stable-isotope dimethyl labeling for quantitative proteomics. Anal
Chem. 75:6843–6852. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yarden Y and Sliwkowski MX: Untangling the
ErbB signalling network. Nat Rev Mol Cell Biol. 2:127–137. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hunter T: Tyrosine phosphorylation: Thirty
years and counting. Curr Opin Cell Biol. 21:140–146. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Villa-Moruzzi E: Tyrosine phosphatases in
the HER2-directed motility of ovarian cancer cells: Involvement of
PTPN12, ERK5 and FAK. Anal Cell Pathol (Amst). 34:101–112. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xunyi Y, Zhentao Y, Dandan J and Funian L:
Clinicopathological significance of PTPN12 expression in human
breast cancer. Braz J Med Biol Res. 45:1334–1340. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sun T, Aceto N, Meerbrey KL, et al:
Activation of multiple proto-oncogenic tyrosine kinases in breast
cancer via loss of the PTPN12 phosphatase. Cell. 144:703–718. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cao X, Li Y, Luo RZ, He LR, et al:
Tyrosine-protein phosphatase nonreceptor type 12 is a novel
prognostic biomarker for esophageal squamous cell carcinoma. Ann
Thorac Surg. 93:1674–1680. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Espejo R, Rengifo-Cam W, Schaller MD,
Evers BM and Sastry SK: PTPPEST controls motility, adherens
junction assembly and Rho GTPase activity in colon cancer cells. Am
J Physiol Cell Physiol. 299:C454–C463. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zheng Y, Yang W, Xia Y, et al: Ras-induced
and extracellular signal-regulated kinase 1 and 2
phosphorylation-dependent isomerization of protein tyrosine
phosphatase (PTP)-PEST by PIN1 promotes FAK dephosphorylation by
PTP-PEST. Mol Cell Biol. 31:4258–4269. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Davidson D, Shi X, Zhong MC, Rhee I and
Veillette A: The phosphatase PTP-PEST promotes secondary T cell
responses by dephosphorylating the protein tyrosine kinase Pyk2.
Immunity. 33:167–180. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Streit S, Ruhe JE, Knyazev P, et al:
PTP-PEST phosphatase variations in human cancer. Cancer Genet
Cytogenet. 170:48–53. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kapur U, Antic T, Venkataraman G,
Durazo-Arvizu R, Quek MM, Flanigan RC and Wojcik EM: Validation of
World Health Organization/International Society of Urologic
Pathology 2004 classification schema for bladder urothelial
carcinomas using quantitative nuclear morphometry: Identification
of predictive features using bootstrap method. Urology.
70:1028–1033. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Streit S, Ruhe JE, Knyazev P, et al:
PTP-PEST phosphatase variations in human cancer. Cancer Genet
Cytogenet. 170:48–53. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lerner SP: Bladder cancer clinical trials.
Urol Oncol. 23:275–279. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mitsumori K, Tsuchiya N, Habuchi T, et al:
Early and large-dose intravesical instillation of epirubicin to
prevent superficial bladder carcinoma recurrence after
transurethral resection. BJU Int. 94:317–321. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sylvester RJ, Oosterlinck W and van der
Meijden AP: A single immediate postoperative instillation of
chemotherapy decreases the risk of recurrence in patients with
stage Ta T1 bladder cancer: A meta-analysis of published results of
randomized clinical trials. J Urol. 171:2186–2190. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Brausi M, Collette L and Kurth K:
Variability in the recurrence rate at first follow up cystoscopy
after TUR in stage Ta T1 transitional cell carcinoma of the
bladder: A combined analysis of seven EORTC studies. Eur Urol.
41:523–531. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Luo RZ, Cai PQ, Li M, et al: Decreased
expression of PTPN12 correlates with tumor recurrence and poor
survival of patients with hepatocellular carcinoma. PLoS One.
9:e855922014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ajili F, Darouiche A, Chebil M and
Boubaker S: The efficiency of the EORTC scoring system for the
prediction of recurrence and progression of non-muscle-invasive
bladder cancer treated by bacillus Calmette-Guerin immunotherapy.
Ultrastruct Pathol. 37:249–253. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mares J, Szakacsova M, Soukup V, Duskova
J, Horinek A and Babjuk M: Prediction of recurrence in low and
intermediate risk non-muscle invasive bladder cancer by real-time
quantitative PCR analysis: cDNA microarray results. Neoplasma.
60:295–301. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Xu T, Zhu Z, Zhang X, Wang X, Zhong S,
Zhang M and Shen Z: Predicting recurrence and progression in
chinese patients with nonmuscle-invasive bladder cancer using EORTC
and CUETO scoring models. Urology. 82:387–393. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hafner C, Knuechel R, Zanardo L, et al:
Evidence for oligoclonality and tumor spread by intraluminal
seeding in multifocal urothelial carcinomas of the upper and lower
urinary tract. Oncogene. 20:4910–4015. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shang D, Liu Y, Yang P, Chen Y and Tian Y:
TGFBI-promoted adhesion, migration and invasion of human renal cell
carcinoma depends on inactivation of von Hippel-Lindau tumor
suppressor. Urology. 79:e1–e7. 2012. View Article : Google Scholar : PubMed/NCBI
|