Introduction
Gastric neuroendocrine carcinoma (G-NEC) is a
malignant subtype of gastroenteral neuroendocrine tumor (GE-NET).
It is a rare neoplasm known for its malignant biological behavior,
neuroendocrine characteristics and poor prognosis (1). G-NECs account for 0.1–0.2% of all
gastric malignancies; however, due to a lack of typical clinical
presentation, it is difficult to diagnose prior to surgery
(1). The incidence of G-NEC has been
increasing each year and surgery is the only effective treatment
option.
Duodenal Brunner's gland adenoma (BGA) is a rare,
benign lesion that accounts for 10.6% of all benign duodenal tumors
(2). The original description of BGA
was provided by Salvioli (3) in 1876,
and since then, only ~200 cases have been reported in China
(4). BGA is typically associated with
no symptoms; however, in symptomatic patients, BGA presents with
hemorrhagic or obstructive indicators of disease. The treatment of
BGA varies according to the size of the tumor, the symptoms
exhibited and the risk of malignancy (3). The pathogenesis of BGA is unclear, but
malignant transformation to duodenal cancer has been reported in
the literature (5). To the best of
our knowledge, no cases of G-NEC coexisting with BGA have
previously been reported. The present study therefore reports the
first case of G-NEC combined with BGA. Written informed consent was
obtained from the patient's family.
Case report
A 67-year-old female presented to a private hospital
with a two-month history of vague abdominal discomfort, without
nausea, vomiting, acid reflux or eructation. The patient had no
significant past medical history, including no family history of
cancer or surgery. An intraluminal mass measuring 2.0×1.5×1.1 cm
was observed in the anterior wall of the duodenum.
Esophagogastroduodenoscopy showed a large mucosal bulge measuring
5.0×3.5×1 cm, with ulceration, in the antral lesser curvature of
the stomach. The mass exhibited clear boundaries and a wide tumor
pedicle. The patient was transferred to Ren Ji Hospital, Shanghai
Jiao Tong University School of Medicine (Shanghai, China), due to
frequent abdominal pain. Laboratory tests and tumor biomarker
analysis for α-fetoprotein, carcinoembryonic antigen, carbohydrate
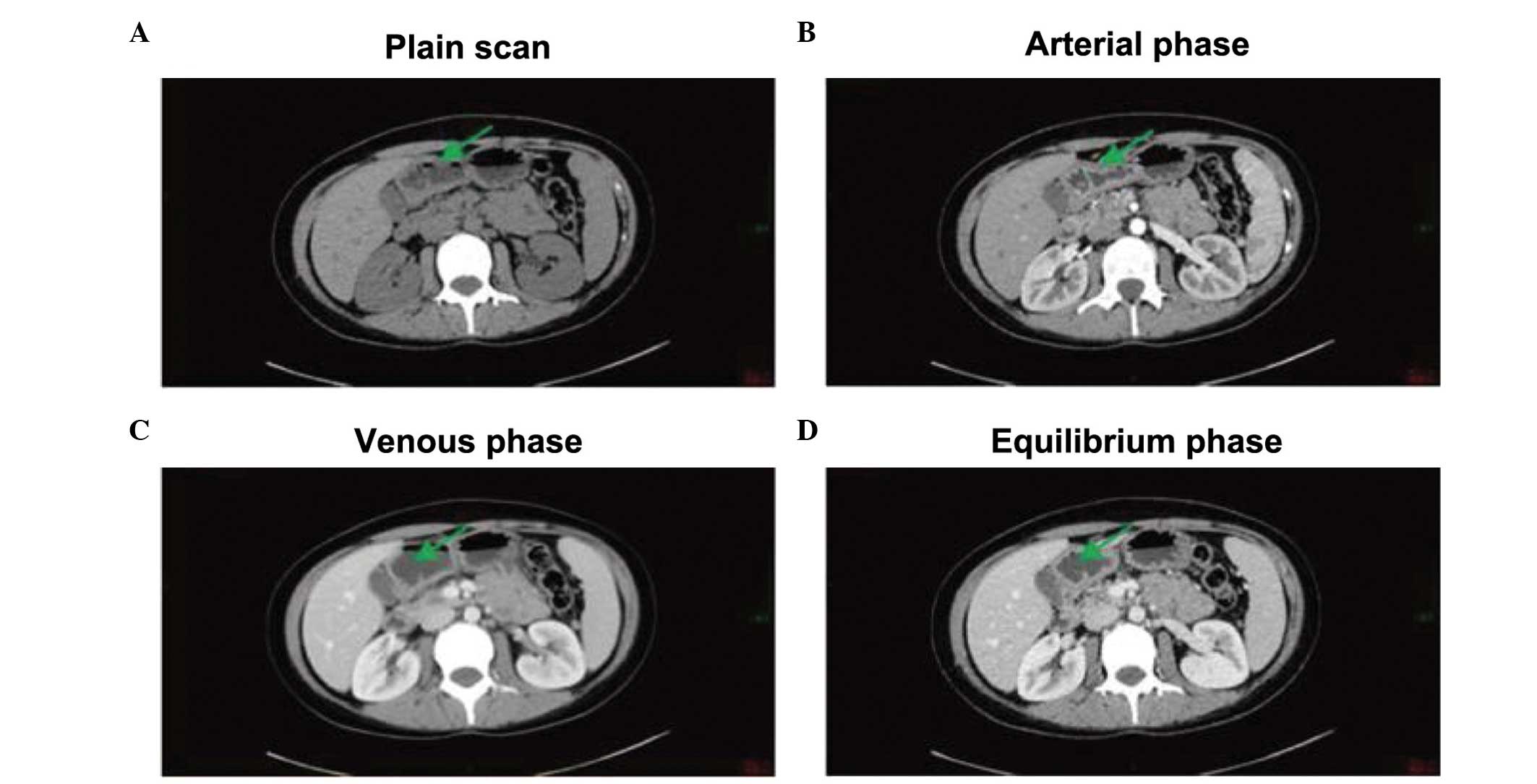
antigen 19-9 were normal. An abdominal plain computed tomography
(CT) scan revealed an equal density shadow combined with uneven
thickening. During three phases of intravenous contrast-enhanced
CT, the lesions showed marked enhancement at the arterial phase and
loss of enhancement at the venous and equilibrium phases. No liver
metastases were detected (Fig.
1).
The patient underwent a radical distal gastrectomy,
D2 lymphadenectomy and Billroth-I gastroenterostomy. Hematoxylin
and eosin (HE) staining of the resected specimen revealed a mixture
of atypical small and large G-NEC cells, with a rose-like
distribution, reduced cytoplasm and homogeneous fine nuclear
chromatin (Fig. 2). Mitotic figures
were common. Deep myometrial invasion and fuzzy boundaries were
observed. One out of two lymph nodes in the lesser curvature of the
stomach was positive for metastasis. The tumor was staged as
T2N1M0, stage IIIB, according to the European Neuroendocrine Tumor
Society guidelines (6). In addition,
an intraluminal pedicle mass measuring 2.0×1.5×1.1 cm was observed
in the anterior wall of the duodenum, with a clear boundary and
wide tumor pedicle. The surface of the mass was smooth, without
ulceration or erosion, and was covered by intestinal mucosa. The
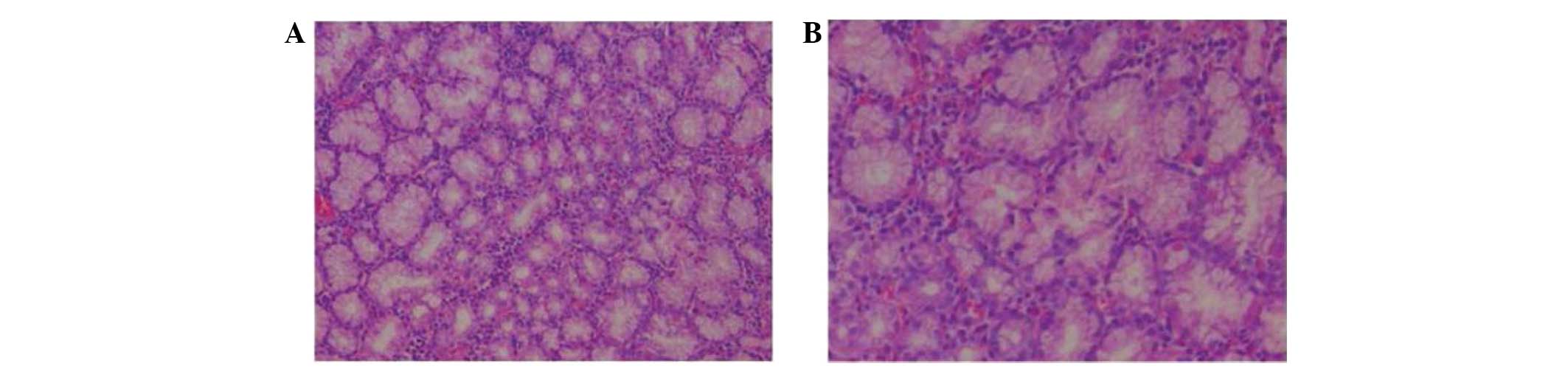
tumor section was sallow, lobulated and soft. HE staining revealed
that the tumor lobules were composed of normal-appearing Brunner's
glands, with the usual mixture of normal tissues, including ducts,
adipose tissue and lymphoid tissue. The observed gland hyperplasia
with chronic inflammation was consistent with the diagnosis of BGA
(Fig. 3).
Immunohistochemical analysis of the antral tumor
specimen was performed following the surgery. In all tumours,
immunostaining was classified as membranous, cytoplasmic,
membranous and cytoplasmic (heterogeneous tissue labelling) or
negative. Results were considered to be negative (−) when no
immunostaining was detected, very weak (+) when staining was
present in <20% of tumor cells, weak (++) when staining was
present in 20–50% of tumor cells, and strong (+++) when staining
was present in >50% of tumor cells. Immunohistochemical analysis
demonstrated positive staining for cytokeratin (CK)7(+), CK8(+),
cluster of differentiation 56(+), chromogranin A (CgA)(+),
Ki-67(+++) and p53(+++). The tumor was negative for p63.
Immunohistochemical staining for synaptophysin (Syn) and CgA were
positive, and revealed diffuse cytoplasmic distribution for each
protein (Fig. 4). These results are
consistent with a diagnosis of G-NEC.
The patient did not receive radiotherapy or
chemotherapy. Abdominal CT, magnetic resonance imaging and
endoscopy were performed every six months for the first two years
after surgery. For the next two years, abdominal CT and endoscopy
were performed every 12 months. No metastatic lesions were found
during the four-year follow-up period.
Discussion
Gastrointestinal neuroendocrine tumors, also
referred to as GE-NETs, are a rare low-grade malignancy, accounting
for ~2% of all gastrointestinal malignancies (7). The incidence of GE-NETs has been
increasing in recent years. Currently, there are no uniform grading
standards for GE-NETs. According to the latest classification
defined by the World Health Organization in 2010, neuroendocrine
tumors are classified into three grades based on the Ki-67 labeling
index (8). Grade 1 tumors have a
Ki-67 labeling index of ≤4%, grade 2 tumors have an index of 3–20%
and grade 3 tumors have an index of ≥20%. G-NECs, the more
malignant subtype of GE-NETs, account for 0.1–0.2% of all gastric
malignancies (9). Immunohistochemical
analysis of the tumor from the present patient revealed a Ki-67
labeling index of 67%, resulting in a diagnosis of grade 3
G-NEC.
G-NEC exhibit no specific early clinical
manifestations, while the late clinical manifestations include
upper abdominal pain and progressive dysphagia. A few cases of
G-NEC have been reported with blood in the stool and anemia
(10); thus, the tumors may be
confused with gastric adenocarcinoma or gastric lymphoma. Indeed,
the present patient was misdiagnosed with gastric cancer prior to
surgery. The diagnosis of G-NEC relies on the morphological
characteristics of the tumor and immunohistochemical analysis.
G-NECs secrete the neural markers Syn and CgA. Any patient with
positive expression of these markers can be diagnosed with a G-NEC
(11).
Surgery is required for patients diagnosed with
G-NEC (12). Other treatment options
include biological therapy, molecular targeted therapy,
chemotherapy and radiation therapy. Novel biological and targeted
therapies have been the focus of G-NEC treatment in recent years.
Biological therapy has focused on somatostatin (SST), which
specifically binds the SST receptor (SSTR) expressed on the surface
of NET cells, thereby inhibiting the secretion of various bioactive
substances, including 5-hydroxytryptophan, insulin and gastrin
(13), resulting in the improvement
of clinical symptoms. SST suppresses tumor growth by blocking cells
in the G1 phase of the cell cycle, regulating immunity
through an SSTR independent mechanism, inhibiting angiogenesis and
promoting apoptosis (14). It has
been reported that large doses of SST analogs can lead to tumor
cell apoptosis and inhibit tumor growth, but these results remain
controversial (15). Tyrosine kinase
inhibitors are the most commonly used agents in targeted therapy
and the mammalian target of rapamycin (mTOR) receptor has been the
focus of several studies. In the phase III RADIANT-3 clinical
trial, everolimus, an inhibitor of the PI3K/AKT/mTOR signaling
pathway, significantly extending the time to progression for
patients in the drug arm compared with those in the placebo arm of
the study. The patient in the present study did not receive
biological treatment prior to or following surgery, as no symptoms
developed that were associated with carcinoid syndrome.
Additionally, no post-operative chemotherapy was administered. The
patient remains alive and free of symptoms of carcinoid syndrome at
four years post-surgery.
BGA is usually asymptomatic, but may exhibit
clinical manifestations that can range from non-specific symptoms
to gastrointestinal bleeding or obstruction only detectable by an
upper gastrointestinal endoscopy (16,17). It
has been suggested that BGA is not a true tumor, but a lesion of
nodular hyperplasia or hamartoma (18). BGA generally arises in individuals
aged 50–60 years. The tumor is often small, ranging in size from
1–10 cm, with an average size of 1–2 cm. At present, the etiology
of BGA is not clear, but it is believed to be caused by duodenal
inflammation or hyperacidity, leading to hyperplasia of Brunner's
glands and hamartoma (19).
In conclusion, BGA is a rare benign lesion which may
be diagnosed by histopathology. BGA has a good prognosis, however,
we hypothesize that lesions must be resected if their shape or size
changes significantly. In the present patient, BGA was discovered
during the surgery for G-NEC. Further investigation is required to
determine if the pathogenesis of BGA in this patient was associated
with the neuroendocrine characteristics of the G-NEC or if it was
just a coincidence.
References
|
1
|
Nishikura K, Watanabe H, Iwafuchi M,
Fujiwara T, Kojima K and Ajioka Y: Carcinogenesis of gastric
endocrine cell carcinoma: analysis of histopathology and p53 gene
alteration. Gastric Cancer. 6:203–209. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gao YP, Zhu JS and Zheng WJ: Brunner's
gland adenoma of duodenum: a case report and literature review.
World J Gastroenterol. 10:2616–2617. 2004.PubMed/NCBI
|
|
3
|
Coskun A and Erkan N: Giant Brunner's
gland adenoma as an unusual cause of anaemia: Report of a case.
Radiol Oncol. 45:129–131. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Stewart ZA, Hruban RH, Fishman EF and
Wolfgang CL: Surgical management of giant Brunner's gland
hamartoma: case report and literature review. World J Surg Oncol.
7:682009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Masaru Koizumi, Sata N, Yoshizawa K,
Kurihara K and Yasuda Y: Carcinoma arising from Brunner's gland in
the duodenum after 17 years of observation - a case report and
literature review. Case Rep Gastroenterol. 1:103–109. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rindi G, Klöppel G, Alhman H, et al: all
other Frascati Consensus Conference participants; European
Neuroendocrine Tumor Society (ENETS): TNM staging of foregut
(neuro)endocrine tumors: a consensus proposal including a grading
system. Virchows Arch. 449:395–401. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Toumpanakis CG and Caplin ME: Molecular
genetics of gastroenteropancreatic neuroendocrine tumors. Am J
Gastroenterol. 103:729–732. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rindi G, Arnold R, Bosman FT, Capella C,
Klimstra DS, Klöppel G, et al: Nomenclature and classification of
neuroendocrine neoplasms of the digestive systemWHO Classification
of Tumors of the Digestive System. Bosman FT, Carneiro F, Hruban RH
and Theise ND: 4th. IARC Press; Lyon, France: pp. 13–14. 2010
|
|
9
|
Uchiyama C, Tamura S, Nakatsuka S, et al:
Immunohistochemical consistency between primary tumors and lymph
node metastases of gastric neuroendocrine carcinoma. World J Surg
Oncol. 10:1152012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Massironi S, Sciola V, Peracchi M, et al:
Neuroendocrine tumors of the gastro-entero-pancreatic system. World
J Gastroenterol. 14:5377–5384. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Solcia E, Klöppel G and Sobin LH: WHO
International Histological Classification of Tumors: Histological
Typing of Endocrine Tumors. 2nd. Springer-Verlag; Berlin: pp.
56–70. 2000
|
|
12
|
Fendrich V and Bartsch DK: Surgical
treatment of gastrointestinal neuroendocrine tumors. Langenbecks
Arch Surg. 396:299–311. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Susini C and Buscail L: Rationale for the
use of somatostatin analogs as antitumor agents. Ann Oncol.
17:1733–1742. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mejias M, Garcia-Pras E, Tiani C, et al:
The somatostatin analogue octreotide inhibits angiogenesis in the
earliest, but not in advanced, stages of portal hypertension in
rats. J Cell Mol Med. 12:1690–1699. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Panzuto F, Di Fonzo M, Iannicelli E, et
al: Long term clinical outcome of somatostatin analogues for
treatment of progressive, metastatic, well-differentiated
entero-pancreatic endocrine carcinoma. Ann Oncol. 17:461–466. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Matsumoto T, Iida M, Matsui T, et al: A
large Brunner's gland adenoma removed by endoscopic polypectomy.
Endoscopy. 22:192–193. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rocco A, Borriello P, Compare D, De
Colibus P, Pica L, Iacono A and Nardone G: Large Brunner's gland
adenoma: case report and literature review. World J Gastroenterol.
12:1966–1968. 2006.PubMed/NCBI
|
|
18
|
Rufenacht H, Kasper M, Heitz PU, et al:
‘Brunneroma’: hamartoma or tumor? Pathol Res Pract. 181:107–111.
1986. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Levine JA, Burgart LJ, Batts KP and Wang
KK: Brunner's gland hamartomas: clinical presentation and
pathological features of 27 cases. Am J Gastroenterol. 90:290–294.
1995.PubMed/NCBI
|