Introduction
Early gastric cancer (EGC) is defined as invasive
gastric cancer that does not invade beyond the submucosa,
irrespective of lymph node metastasis. EGC has an excellent
prognosis, with a 5-year survival rate of >90% (1). There are two treatment options for EGC,
endoscopic submucosal dissection (ESD) or surgical resection with
lymph node dissection. Treatment methods are selected according to
the probability of lymph node metastasis (2). In addition to lymph node metastasis, the
size of the tumor, presence or absence of an ulcer, lymphovascular
invasion and histological type are all used to determine which
method of treatment should be undertaken (2).
Absolute indications for the use of ESD were defined
as differentiated mucosal cancer without ulceration and tumors
<2 cm in size. The expanded version of the guidelines included
the following criteria: i) differentiated mucosal cancer without
ulceration and tumor >2 cm in size; ii) differentiated mucosal
cancer with ulceration and a tumor <3 cm in size; or iii)
differentiated submucosal cancer with a tumor <3 cm in size and
a submucosal invasion depth of <500 µm (3). Based on a study by Hirasawa et al
(4), the guidelines for ESD were
further expanded and it is now accepted that carcinoma of the
undifferentiated type, without ulceration and with a tumor size of
<2 cm in size is included in the Japanese Gastric Cancer
Treatment Guidelines 2010 (ver. 3) (4,5).
ESD guidelines were previously based on hematoxylin
and eosin staining; however, numerous studies have reported that
the expression of cell adhesion molecules, cell surface molecules,
membrane-associated mucin phenotypes and collagen phenotypes are
associated with lymph node metastasis and prognosis of carcinomas
in multiple organs (6–15). Mucin 1, cell surface associated (MUC1)
is a transmembrane member of the mucin family and has been reported
to be associated with metastatic progression (16). The present study aimed to investigate
MUC1 expression using immunohistochemistry in endoscopic biopsy
specimens from submucosal invasive gastric carcinomas in order to
determine whether MUC1 was a potential predictor of lymph node
metastasis. In addition, the present study examined the association
between MUC1 expression and clinicopathological variables.
Materials and methods
Patient and tissue specimens
The present study included 144 patients with
submucosal invasive gastric carcinomas who underwent surgical
resection with lymph node dissection following endoscopic biopsies
between August 2005 and December 2012 at Konkuk University Medical
Center (Seoul, Korea). All cases were reviewed according to the
current guidelines from the World Health Organization 2010
classifications (17).
Immunohistochemical staining for MUC1 was performed in endoscopic
biopsy specimens and the association of MUC1 immunoreactivity with
the following clinicopathological characteristics of patients was
investigated: Tumor size, histological type, gross type, depth of
invasion, lymphovascular invasion, perineural invasion, Lauren
classification, mucin phenotype, p53 immunoreactivity, MUC1
immunoreactivity and lymph node metastasis in surgically resected
submucosal invasive gastric carcinomas. The present study was
approved by the Institutional Review Board of Konkuk University
Medical Center.
Immunohistochemistry
The formalin-fixed, paraffin-embedded tissue blocks
were sectioned at 3-µm thickness and immunohistochemical staining
was performed using an iVIEW DAB detection kit and a BenchMark XT
staining instrument (Ventana Medical System, Inc., Tucson, AR,
USA). Immunohistochemical staining was performed using rabbit
polyclonal MUC1 (dilution, 1:400; catalog no., RB9222P0) primary
antibodies in endoscopic biopsy specimens; and mouse monoclonal
CD10 (clone, 56C6; dilution, 1:50; catalog no., MS728S0), MUC2
(clone, 996/1; dilution, 1:2,000; catalog no., MS1729P0), MUC5AC
(clone, 45M1; dilution, 1:2,000; catalog no., MS145P0), MUC6
(clone, CLH5; dilution, 1:200; catalog no., MS1153S0) and p53
(clone, DO-7; dilution, 1:500; catalog no., MS186P0) primary
antibodies in surgically resected specimens. All primary antibodies
were purchased from Thermo Fisher Scientific (Fremont, CA, USA).
Mucin phenotypes were evaluated through CD10, MUC2, MUC5AC and MUC6
immunohistochemical staining in order to classify each as gastric
type, intestinal type, mixed type or unclassified type. Positive
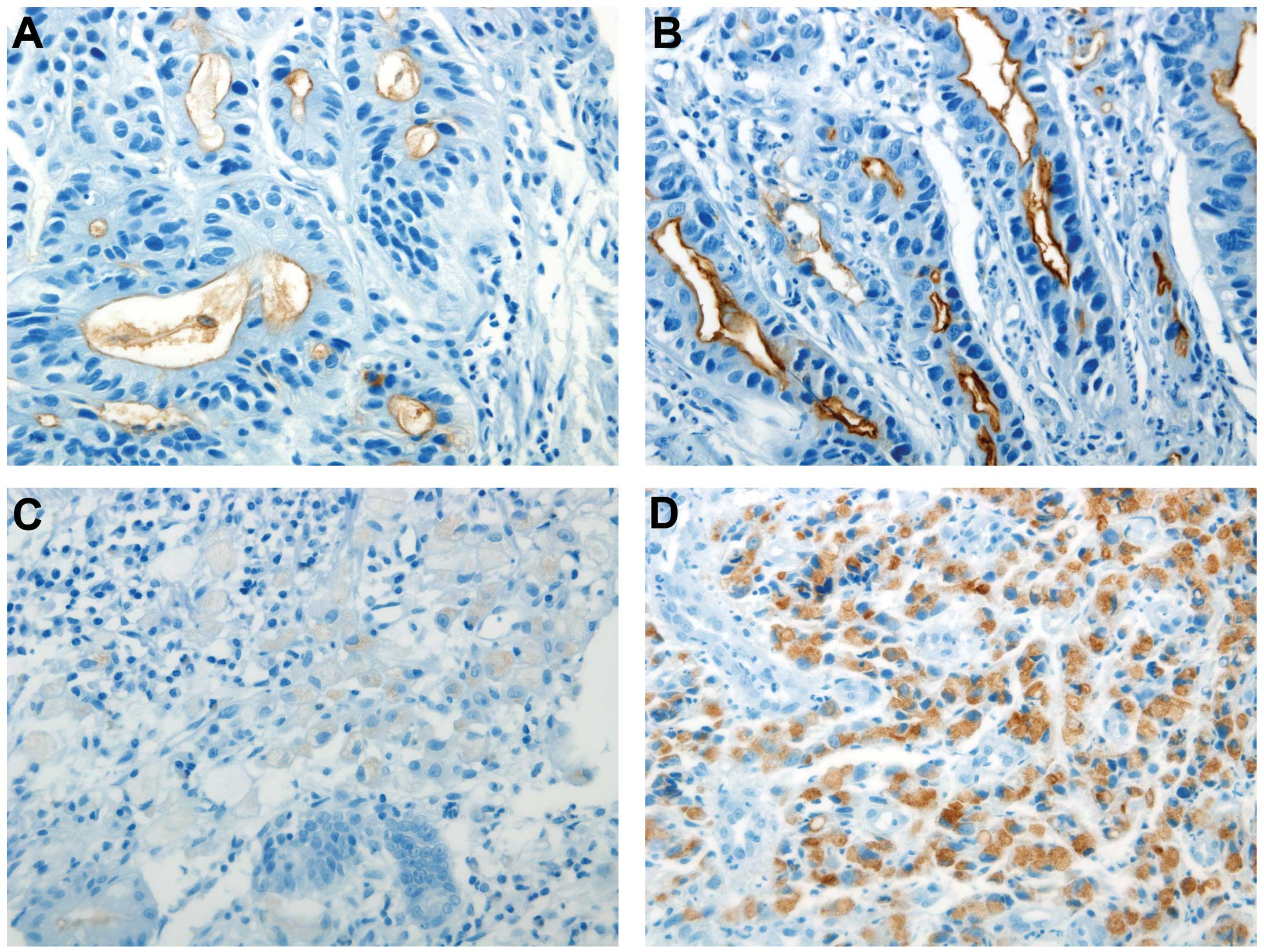
reactivity for MUC1 was defined as strong luminal immunoreactivity
or more than moderate cytoplasmic immunoreactivity in >5% of the
tumor cells (Fig. 1). In addition,
positive reactivity for CD10, MUC2, MUC5AC and MUC6 was defined as
positive immunoreactivity in >10% of tumor cells and for p53, in
>5% of tumor cells.
Statistical analysis
MUC1 immunoreactivity in association with
clinicopathological parameters was examined using the χ2
and Student's t tests. The lymph node metastasis in
association with MUC1 immunoreactivity and clinicopathological
parameters was examined using the χ2 test and Student's
t test. Multivariate analysis was performed using logistic
regression. P<0.05 was considered to indicate a statistically
significant difference between values. Values are presented as the
mean ± standard deviation. All statistical analyses were performed
using SPSS version 20.0 (International Business Machines, Armonk,
NY, USA).
Results
Clinicopathological variables
There were 17, 71, 24, 14 and 18 cases of well-,
moderately- and poorly-differentiated tubular adenocarcinomas,
mixed adenocarcinomas and poorly-cohesive carcinomas with biopsy
specimens, respectively. In total, there were 89, 40, 9, 5 and 1
cases of tubular adenocarcinomas, mixed adenocarcinomas,
poorly-cohesive carcinomas, medullary carcinomas and mucinous
carcinoma with surgically resected submucosal invasive cancers,
respectively. Of the total 144 submucosal invasive gastric
carcinomas, 6 cases were surgically resected following ESD due to a
submucosal invasion depth ≥500 µm or involvement of the ESD
resection margin. The mean age of the patients was 61.7 ± 11.5
years and the study group was comprised of 97 men and 47 women. The
gross types of EGCs were as follows: 11 type I, 26 type IIa, 25
type IIb, 69 type IIc and 13 type III, with a mean tumor size of
3.7±2.0 cm and a mean submucosal depth of invasion of 1,530±1,089
µm. There were 44, 38 and 56 cases of submucosal invasion level 1,
2 and 3, respectively. In 31 cases, the depth of submucosal
invasion was <500 µm and in 113 cases, the depth was ≥500 µm.
The level of submucosal invasion could not be measured in 6 cases
due to previous ESD. The poorly-differentiated carcinoma cells were
present in 35 cases and poorly-cohesive carcinoma cells were
present in 32 cases of the endoscopic biopsy specimens. According
to Japanese classification, undifferentiated type carcinoma
includes poorly differentiated adenocarcinoma, signet ring cell
carcinoma, and mucinous adenocarcinoma (5). There were 56 cases of undifferentiated
type, according to the Japanese classification and among them, 9
cases were included in the expanded guidelines of ESD. There were
24 cases of lymph node metastasis. Immunohistochemical staining for
MUC1 and p53 was positive in 70 and 58 cases, respectively. A total
of 48, 32, 26 and 34 cases were classified into gastric,
intestinal, mixed and unclassified mucin phenotypes. However,
immunohistochemistry was unable to be performed for p53 expression
in 6 cases and for evaluation of the mucin phenotype in 4 cases.
Furthermore, 85, 47 and 12 cases were intestinal, diffuse and mixed
type according to the Lauren classification, respectively. There
were 46, 5 and 4 cases of lymphatic, venous and perineural
invasion, respectively (Table I).
 | Table I.Univariable analysis of lymph node
metastasis and clinicopathological features in submucosal invasive
gastric carcinoma. |
Table I.
Univariable analysis of lymph node
metastasis and clinicopathological features in submucosal invasive
gastric carcinoma.
|
| Lymph node
metastasis |
|
|---|
|
|
|
|
|---|
| Clinicopathological
feature | Present (n=24) | Absent (n=120) | P-value |
|---|
| Mean age (SD) | 60 (14.0) | 62 (11.0) | 0.436 |
| Gender ratio,
male:female | 13:11 | 84:36 | 0.155 |
| Gross type |
|
| 0.267 |
| EGC
I | 3 | 8 |
|
| EGC
IIa | 5 | 21 |
|
| EGC
IIb | 1 | 24 |
|
| EGC
IIc | 12 | 57 |
|
| EGC
III | 3 | 10 |
|
| Endoscopic biopsy
histology |
|
| 0.246 |
|
Well-differentiated tubular
adenocarcinoma | 2 | 15 |
|
|
Moderately-differentiated
tubular adenocarcinoma | 9 | 62 |
|
|
Poorly-differentiated tubular
adenocarcinoma | 8 | 16 |
|
|
Poorly-cohesive carcinoma | 3 | 15 |
|
| Mixed
adenocarcinoma | 2 | 12 |
|
| Mean tumor size, cm
(SD) | 5.0 (2.4) | 3.4 (1.8) | 0.005 |
| Mean submucosal
invasion depth, µm (SD) | 1815 (997) | 1473 (1101) | 0.161 |
| Level of submucosal
invasion |
|
| 0.018 |
| SM1 | 6 | 38 |
|
| SM2 | 2 | 36 |
|
| SM3 | 15 | 41 |
|
| Level of submucosal
invasion by Japanese classification |
|
| 0.6 |
| <500
µm | 4 | 27 |
|
| ≥500
µm | 20 | 93 |
|
| Presence of poorly
differentiated cells |
|
| 0.038 |
| Yes | 10 | 25 |
|
| No | 14 | 95 |
|
| Presence of poorly
cohesive cells |
|
| 1.0 |
| Yes | 5 | 27 |
|
| No | 19 | 93 |
|
| Tumor differentiation
by Japanese classification |
|
| 0.111 |
|
Differentiated | 11 | 77 |
|
|
Undifferentiated | 13 | 43 |
|
| Endoscopic submucosal
dissection indication |
|
| 0.356 |
| Expended
indication | 0 | 9 |
|
|
Surgery | 24 | 111 |
|
| MUC1
immunohistochemistry |
|
| 0.024 |
|
Positive | 17 | 53 |
|
|
Negative | 7 | 67 |
|
| p53
immunohistochemistry |
|
| 0.254 |
|
Positive | 7 | 51 |
|
|
Negative | 16 | 64 |
|
| Mucin phenotype |
|
| 0.825 |
|
Gastric | 9 | 39 |
|
|
Intestinal | 5 | 27 |
|
|
Mixed | 3 | 23 |
|
|
Unclassified | 7 | 27 |
|
|
Intestinal | 10 | 75 |
|
| Lauren
classification |
|
| 0.027 |
|
Diffuse | 9 | 38 |
|
|
Mixed | 5 | 7 |
|
|
Intestinal vs. diffuse |
|
| 0.906a |
|
Intestinal vs. mixed |
|
| 0.053a |
| Diffuse
vs. mixed |
|
| 0.399a |
| Lymphatic
invasion |
|
| <0.001 |
|
Present | 20 | 26 |
|
| Not
identified | 4 | 94 |
|
| Vascular
invasion |
|
| 0.59 |
|
Present | 0 | 5 |
|
| Not
identified | 24 | 115 |
| Perineural
invasion |
|
| 0.522 |
|
Present | 1 | 3 |
|
| Not
identified | 23 | 117 |
|
Lymph node metastasis and
clinicopathologic features
Lymph node metastasis was identified in 24 cases and
was identified to be significantly associated with tumor size
(P=0.005), the level of submucosal invasion (P=0.018), the presence
of poorly-differentiated carcinoma cells (P=0.038), MUC1 expression
(P=0.024), Lauren classification (P=0.027) and lymphatic invasion
(P<0.001), through univariate analysis (Table I). However, lymph node metastasis was
not found to be associated with histologic type (P=0.246) (Table I). By contrast, following Bonferroni
correction, the Lauren classification was not significantly
associated with lymph node metastasis: Intestinal type vs. diffuse
type, P=0.906; intestinal vs. mixed, P=0.053; and diffuse vs.
mixed, P=0.339, according to the Bonferroni corrected P-value. The
following three factors, MUC1 expression (P=0.007), size of tumor
(P=0.018), and lymphatic invasion (P<0.001), were identified as
independent risk factors for lymph node metastasis (Table II).
 | Table II.Multivariable analysis of MUC1
expression, tumor size and lymph node metastasis in submucosal
invasive gastric carcinoma. |
Table II.
Multivariable analysis of MUC1
expression, tumor size and lymph node metastasis in submucosal
invasive gastric carcinoma.
|
| Odds ratio | 95% confidence
interval | P-value |
|---|
| MUC1
expression | 6.380 | 1.661–24.502 | 0.007 |
| Tumor size | 1.394 | 1.058–1.837 | 0.018 |
| Lymphatic
invasion | 17.443 | 4.849–62.739 | <0.001 |
MUC1 expression and
clinicopathological features
A total of 70 cases were positive for MUC1
immunohistochemical staining. The positive MUC1 expression was
significantly associated with the presence of poorly-differentiated
carcinoma cells (P=0.001), poorly-cohesive carcinoma cells
(P=0.015), undifferentiated type (P<0.001), diffuse type under
the Lauren classification (P<0.001) and lymph node metastasis
(P=0.024) (Table III). The
following two factors, diffuse type under the Lauren classification
(P<0.001) and lymph node metastasis (P=0.035), were identified
as independent factors for positive MUC1 expression (Table IV).
 | Table III.Univariable analysis of MUC1
expression and clinicopathological features in submucosal invasive
gastric carcinoma. |
Table III.
Univariable analysis of MUC1
expression and clinicopathological features in submucosal invasive
gastric carcinoma.
|
| MUC1
immunoreactivity |
|---|
|
|
|
|
|---|
|
| Positive
(n=70) | Negative
(n=74) | P-value |
|---|
| Presence of poorly
differentiated cells |
|
| 0.001 |
|
Yes | 26 | 9 |
|
| No | 44 | 65 |
|
| Presence of poorly
cohesive cells |
|
| 0.015 |
|
Yes | 22 | 10 |
|
| No | 48 | 64 |
|
| Tumor
differentiation |
|
| <0.001 |
|
Differentiated | 31 | 57 |
|
|
Undifferentiated | 39 | 17 |
|
| Lauren
classification |
|
| <0.001 |
|
Intestinal | 29 | 56 |
|
|
Diffuse | 34 | 13 |
|
| Mixed | 7 | 5 |
|
| Intestinal vs.
diffuse |
|
|
<0.001a |
|
Intestinal vs. mixed |
|
| 0.363a |
| Diffuse
vs. mixed |
|
| 1.000a |
| Lymph node
metastasis |
|
| 0.024 |
|
Yes | 17 | 7 |
|
| No | 53 | 67 |
|
 | Table IV.Multivariable analysis of MUC1
expression and clinicopathological features in submucosal invasive
gastric carcinoma. |
Table IV.
Multivariable analysis of MUC1
expression and clinicopathological features in submucosal invasive
gastric carcinoma.
|
| Odds ratio | 95% confidence
interval | P-value |
|---|
| Lauren
classification |
|
|
|
|
Intestinal vs. diffuse | 5.158 | 2.255–11.798 | <0.001 |
|
Intestinal vs. mixed | 2.028 | 0.548–7.501 | 0.289 |
| Lymph node
metastasis | 3.211 | 1.088–9.473 | 0.035 |
Discussion
Mucins are high-molecular-weight epithelial
glycoproteins that provide protection and lubrication to epithelial
surfaces; of note, the role of mucins in cell signaling has been
the focus of numerous studies (7,9,10,16). MUC1
is one of the membrane-associated type mucins that is known to
contribute to epithelial cell-to-cell interactions (7). It was demonstrated that the expression
of MUC1 was primarily located at the apical surface of ductal
epithelia. However, MUC1 is overexpressed in metastatic disease and
becomes localized throughout the cell (16). In numerous types of tumors, MUC1
expression was reported to be correlated with aggressiveness,
metastatic disease and poor prognosis (9–13,16,18). MUC1
expression was reported to accelerate tumor invasion and metastasis
via the impairment of E-cadherin (10), decrease the binding of p120 catenin to
E-cadherin (18), upregulate matrix
metalloproteinase 13 expression (13)
and activate Wnt/β-catenin abnormally (9). Furthermore, MUC1 expression was
demonstrated to be associated with metastatic progression in the
gastrointestinal system. However, in gastric cancer, it was
reported that the expression of MUC1 was not limited to metastatic
disease, but also highly expressed in the majority of isolated
cancer cells invading throughout the stroma of the primary tumor
(16). This therefore indicated that
MUC1 may be involved in initiating the spread of cancer.
In previous studies, MUC1 expression was identified
in >50% of differentiated and undifferentiated gastric
carcinomas. In addition, MUC1-positive staining appeared to be
associated with better tumor differentiation, as the majority of
studies suggest that MUC1 expression is associated with lymphatic
invasion, nodal metastasis and poor prognosis (19). In the present study, MUC1 expression
was identified in 31 cases of 88 differentiated type carcinomas and
in 39 cases of 56 undifferentiated type carcinomas (P<0.001).
These results differed from those of previous studies, this may be
due to the difference in immunohistochemical methods and analysis
(12,19). In the present study, MUC1 positivity
was significantly associated with the presence of
poorly-differentiated carcinoma cells (P=0.001), poorly-cohesive
carcinoma cells (P=0.015), the undifferentiated type according to
the Japanese classification system (P<0.001), the diffuse type
under the Lauren classification (P<0.001) and lymph node
metastasis (P=0.024), as determined using univariate analysis. The
results of the multivariate analysis demonstrated that MUC1
expression was associated with the diffuse type under the Lauren
classification system (P<0.001) and lymph node metastasis
(P=0.035). These results suggested that MUC1-positive staining may
be associated with poorly-differentiated carcinoma cells and
poorly-cohesive carcinoma cells, which invade throughout the stroma
of the primary tumor, as well as lymph node metastasis.
MUC1 immunohistochemical staining patterns are
divided into the luminal and cytoplasmic patterns. The present
study revealed 70 positive cases for MUC1 immunohistochemical
staining out of 144 total cases (48.6%). Among them, 39, 20 and 11
cases revealed cytoplasmic, luminal and mixed patterns of
immunohistochemical staining, respectively. Of the 39 total
undifferentiated type cases, 32 cases revealed cytoplasmic staining
and 7 cases exhibited mixed staining. In addition, 20 cases out of
the 31 total differentiated type cases revealed luminal staining, 7
cases showed cytoplasmic staining and 4 cases showed mixed
staining. For tumor differentiation, the immunohistochemical
staining pattern for MUC1 was significantly different (P<0.001)
and the undifferentiated type showed MUC1 cytoplasmic staining more
frequently. Yonezawa et al (19) reported that the MUC1
immunohistochemical staining pattern is different depending on
tumor differentiation; finding that stain mainly accumulates at the
apex in papillary adenocarcinoma, mucinous adenocarcinoma,
well-differentiated or moderately-differentiated adenocarcinoma,
while poorly-differentiated carcinoma and signet-ring cell
carcinoma demonstrated primarily cytoplasmic staining. The results
of the present study strongly supported this previous study, as
they indicated that the immunohistochemical pattern of MUC1 varied
according to tumor differentiation in gastric adenocarcinoma.
Of the 24 cases of submucosal invasive gastric
carcinoma with lymph node metastasis, 17 cases demonstrated
positive MUC1 expression in the endoscopic biopsy specimens. These
cases included 1, 4, 8, 1 and 3 cases of well-, moderately- and
poorly-differentiated adenocarcinoma, mixed adenocarcinoma and
poorly-cohesive cell carcinoma, respectively. A case of
well-differentiated adenocarcinoma and 3 cases out of the 4
moderately-differentiated adenocarcinomas revealed luminal MUC1
immunohistochemical patterns while the one remaining
moderately-differentiated adenocarcinoma demonstrated a mixed
pattern. In addition, 6 out of 8 cases of poorly-differentiated
adenocarcinomas, 1 case of mixed adenocarcinoma and all 3
poorly-cohesive cell carcinomas displayed a cytoplasmic pattern,
while the remaining 2 poorly differentiated adenocarcinomas
exhibited a mixed pattern. Positive MUC1 expression is important,
regardless of the immunohistochemical expression region.
Hirasawa et al (4) found there was no lymph node metastasis
in 310 patients with undifferentiated type mucosal cancer with
lesions ≤2 cm and without ulcers from a population of 1,442
patients with undifferentiated mucosal cancer without ulcers. The
Japanese Gastric Cancer Association accepted this study, and now
undifferentiated type mucosal cancer with lesions ≤2 cm without
ulcers are classified as expanded ESD indications in the Japanese
Gastric Cancer Treatment Guidelines (5). According to these guidelines, the most
important factors for predicting lymph node metastasis are as
follows: Tumor size, tumor differentiation, ulceration, depth of
submucosal invasion and lymphovascular invasion. The present study
included 9 cases that underwent expanded ESD and lymph node
metastasis was not identified in these cases. In the present study,
tumor size, level of submucosal invasion, presence of
poorly-differentiated carcinoma cells, MUC1 immunoreactivity and
lymphatic invasion were found to be significantly associated with
lymph node metastasis, as determined using univariate analysis. The
results of the multivariate analysis demonstrated that tumor size,
MUC1 immunoreactivity and lymphatic invasion were significantly
associated with lymph node metastasis. Of note, the level of
submucosal invasion was associated with lymph node metastasis;
however, the depth of submucosal invasion in accordance with the
Japanese classification (500 µm of submucosal invasion depth) was
not significantly associated with lymph node metastasis.
In conclusion, positive MUC1 expression in
endoscopic biopsy specimens may be a predictive factor of lymph
node metastasis in submucosal invasive gastric carcinoma. More
large studies, including cases of expanded ESD indication, are
required in order to determine whether MUC1 immunohistochemistry
may be used for selecting between ESD and surgical resection.
Acknowledgements
The present study was written as part of Konkuk
University's research support program for its faculty on sabbatical
leave in 2010.
References
|
1
|
Gotoda T, Iwasaki M, Kusano C, Seewald S
and Oda I: Endoscopic resection of early gastric cancer treated by
guideline and expanded. National cancer centre criteria. Br J Surg.
97:868–871. 2010.
|
|
2
|
Gotoda T, Yanagisawa A, Sasako M, Ono H,
Nakanishi Y, Shimoda T and Kato Y: Incidence of lymph node
metastasis from early gastric cancer: Estimation with a large
number of cases at two large centers. Grastric Cancer. 3:219–225.
2000. View Article : Google Scholar
|
|
3
|
Soetikno R, Kaltenbach T, Yeh R and Gotoda
T: Endoscopic mucosal resection for early cancers of the upper
gastrointestinal tract. J Clin Oncol. 23:4490–4498. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hirasawa T, Gotoda T, Miyata S, Kato Y,
Shimoda T, Taniguchi H, Fujisaki J, Sano T and Yamaguchi T:
Incidence of lymph node metastasis and the feasibility of
endoscopic resection for undifferentiated-type early gastric
cancer. Gastric Cancer. 12:148–152. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Japanese Gastric Cancer Association, .
Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric
Cancer. 14:113–123. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yoshii T, Miyagi Y, Nakamura Y, Kobayashi
O, Kameda Y and Ohkawa S: Pilot research for the correlation
between the expression pattern of E-cadherin-β-catenin complex and
lymph node metastasis in early gastric cancer. Tumori. 99:234–238.
2013.PubMed/NCBI
|
|
7
|
Hwang I, Kang YN, Kim JY, DO YR, Song HS
and Park KU: Prognostic significance of membrane-associated mucins
1 and 4 in gastric adenocarcinoma. Exp Ther Med. 4:311–316.
2012.PubMed/NCBI
|
|
8
|
Joo M, Lee HK and Kang YK: Expression of
E-cadherin, beta-catenin, CD44s and CD44v6 in gastric
adenocarcinoma: Relationship with lymph node metastasis. Anticancer
Res. 23:1581–1588. 2003.PubMed/NCBI
|
|
9
|
Wang Z, Sun J, Hu X and Huang S:
Interference of mucin 1 inhibits progression of colon carcinoma by
repression of Wnt/β-catenin signaling. DNA Cell Biol. 33:162–170.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ohno T, Aihara R, Kamiyama Y, Mochiki E,
Asao T and Kuwano H: Prognostic significance of combined expression
of MUC1 and adhesion molecules in advanced gastric cancer. Eur J
Cancer. 42:256–263. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ando H, Aihara R, Ohno T, Ogata K, Mochiki
E and Kuwano H: Prognostic significance of the expression of MUC1
and collagen type IV in advanced gastric carcinoma. Br J Surg.
96:901–909. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tamura Y, Higashi M, Kitamoto S, Yokoyama
S, Osako M, Horinouchi M, Shimizu T, Tabata M, Batra SK, Goto M, et
al: MUC4 and MUC1 expression in adenocarcinoma of the stomach
correlates with vessel invasion and lymph node metastasis: An
immunohistochemical study of early gastric cancer. PLoS One.
7:e492512012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ye Q, Yan Z, Liao X, Li Y, Yang J, Sun J,
Kawano T, Wang X, Cao Z, Wang Z, et al: MUC1 induces metastasis in
esophageal squamous cell carcinoma by upregulating matrix
metalloproteinase 13. Lab Invest. 91:778–787. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shibahara H, Higashi M, Koriyama C,
Yokoyama S, Kitazono I, Kurumiya Y, Narita M, Kuze S, Kyokane T,
Mita S, et al: Pathobiological implications of mucin (MUC)
expression in the outcome of small bowel cancer. PLoS One.
9:e861112014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kaira K, Okumura T, Nakagawa K, Ohde Y,
Takahashi T, Murakami H, Naito T, Endo M, Kondo H, Nakajima T, et
al: MUC1 expression in pulmonary metastatic tumors: A comparison of
primary lung cancer. Pathol Oncol Res. 18:439–447. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Horm TM and Schroeder JA: MUC1 and
metastatic cancer: Expression, function and therapeutic targeting.
Cell Adh Migr. 7:187–198. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lauwers GY, Franceschi S, Carneiro F,
Montgomery E, Graham DY, Tatematsu M, Curado MP and Hattori T:
Gastric carcinomaWHO classification of tumours of the digestive
system. Bosman FT, Carneiro F, Hruban RH and Theise ND: 4th. IARC
Press; Lyon, France: pp. 48–58. 2010
|
|
18
|
Liu X, Yi C, Wen Y, Radhakrishnan P,
Tremayne JR, Dao T, Johnson KR and Hollingsworth MA: Interactions
between MUC1 and p120 catenin regulate dynamic features of cell
adhesion, motility and metastasis. Cancer Res. 74:1609–1620. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yonezawa S, Kitajima S, Higashi M, Osako
M, Horinouchi M, Yokoyama S, Kitamoto S, Yamada N, Tamura Y,
Shimizu T, et al: A novel anti-MUC1 antibody against the MUC1
cytoplasmic tail domain: Use in sensitive identification of poorly
differentiated cells in adenocarcinoma of the stomach. Gastric
Cancer. 15:370–381. 2012. View Article : Google Scholar : PubMed/NCBI
|