Introduction
Gastric cancer (GC) is the most commonly-diagnosed
cancer with the second highest mortality rate worldwide (1). In Asian countries, the prognosis for
early-stage GC is good; however, the five-year survival rate is
only 40% (2). Furthermore, although
surgery, chemotherapy and radiation therapy are predominantly used
for the treatment of GC, the median overall survival is <1 year
(3). Due to the lack of currently
available effectual treatment strategies for patients with advanced
GC patients, the development of novel therapeutic approaches for
the treatment of this life-threatening disease is crucial (4). Plants are considered to be one of the
most important sources for the development of novel anticancer
agents, with plant-derived agents typically exhibiting relatively
fewer side-effects. Various plants have been used in clinical
practice in China for thousands of years as important alternative
treatments for a variety of diseases. Tulipa edulis Bak
(TEB), a Chinese herb, appears to exhibit a curative effect on
swelling and redness, removing toxicity and eliminating stagnation
(5). In addition, TEB has been used
to treat various diseases, such as furunculosis, as well as liver,
gastric and breast cancer (6).
However, the precise mechanism of the potential antitumor activity
of TEB in GC has yet to be investigated.
Anticancer agents commonly target cancer cells
through the induction of cell apoptosis, typically by targeting the
mitochondrial signaling pathway (7).
The B-cell lymphoma-2 (Bcl-2) family of proteins includes critical
apoptosis regulators, such as the apoptotic suppressor Bcl-2, and
promoters, such as Bcl-2-associated X protein (Bax) (8–12). Bax and
Bcl-2 are typically considered as the molecular hallmarks of
apoptosis (13). In mammalian cells,
intrinsic apoptotic signaling leads to mitochondrial outer membrane
(MOM) permeabilization, the release of apoptogenic factors (such as
cytochrome c) and the subsequent activation of caspase
protein expression (14). Thus, one
possible mechanism through which the proteins of the Bcl-2 family
regulate apoptosis is by altering the MOM permeability following
homo- or hetero-association (15).
During the early apoptosis of cancer cells, the MOM is known to
change; thus, the anti-apoptotic Bcl-2 protein may bind to active
Bax in order to prevent damage to the MOM (16). During the process of apoptosis, cell
fate appears to be determined by the ratio of active anti- and
pro-apoptotic Bcl-2 family members (17). This ratio is altered by aberrant
expression of Bax and Bcl-2 proteins, impairing the normal
apoptotic program and contributing to various apoptosis-associated
diseases. Therefore, controlling the permeability of mitochondria
using Bcl-2 family proteins ultimately results in mitochondrial
changes that induce cell apoptosis (18). Previously, it has been demonstrated
that treatment with ethanolic extract of TEB (EETEB) resulted in a
decreased number of microvilli and shrinkage of the cytoplasmic
organoid volume in human gastric carcinoma SGC-7901 cells,
indicating that TEB may induce apoptosis at the molecular level
(19).
Thus, the aims of the present study were to
investigate the effect of EETEB on the apoptosis of SGC-7901 human
GC cells and determine the possible molecular mechanisms underlying
this effect.
Materials and methods
TEB alcohol extraction
A preparation of dry TEB extract powder
(Tongrentang, Fuzhou, China) was weighed (58.7 g) and added to a
1,000-ml flask with 500 ml 95% (v/v) ethanol. The mixture was
refluxed at 95°C for 3 h. A second sample was mixed with an
additional 500 ml 95% (v/v) ethanol and a second reflux was
performed. The two samples were mixed and concentrated on a rotary
evaporator at a temperature of 50°C for 48 h. Subsequently, the
sample was successively washed two times with distilled water and
three times with petroleum ether to obtain the final extract
(EETEB), weighing 1.71 g.
Cell culture and cytotoxicity
analysis
SGC-7901 human GC cells, from the Tumor Hospital of
Fujian (Fujian, China) were grown and maintained in RPMI 1640
medium (Thermo Fisher Scientific, Inc., Waltham, MA, USA)
containing 10% fetal bovine serum, 80 U/ml penicillin and 80 U/ml
streptomycin (all from Thermo Fisher Scientific, Inc.) at 37°C in a
humidified atmosphere of 5% CO2. The SGC-7901 cells were
inoculated into 96-well plates at a concentration of 100 µl/well
and cultured for 24 h. Subsequently, the cells were treated with
various concentrations of EETEB (0.75, 1.0 and 1.5 mg crude
EETEB/ml); the control cells were treated with PBS. Following
incubation for 24 h, 50 µl MTT solution (1.1 mg/ml) was added to
each well and incubated for an additional 4 h. Finally, the medium
was replaced with 1.5 mg/ml MTT and incubated at 37°C for ~4 h. The
crystallization was resolved using dimethyl sulfoxide and the
absorbance was measured using a microplate spectrophotometer
(SpectraMax 190; Molecular Devices, LLC, Sunnyvale, CA, USA) at a
wavelength of 580 nm.
Apoptosis detection
SGC-7901 cells were seeded into 6-well plates in 2
ml medium and treated with various concentrations of EETEB (0.75,
1.0 and 1.5 mg/ml) for 24 h. Flow cytometric analysis with an
Annexin V-fluorescein isothiocyanate (FITC)/propidium iodide (PI)
kit (BD Biosciences, Franklin Lakes, NJ, USA) was used to detect
the apoptosis of SGC-7901 cells. The staining procedure was
performed according to the manufacturer's instructions. In the
apoptosis assay, an Annexin V/PI double-negative population
[labeled as LL in the fluorescence-activated cell sorting (FACS)
diagram] indicated the viable cells; by contrast, an Annexin
V-positive/PI-negative or Annexin V/PI double-positive population
represented cells undergoing early or late apoptosis,
respectively.
Apoptosis analysis using DAPI
staining
DAPI staining followed by laser scanning confocal
microscopy (LSCM; LSM 710; Carl Zeiss AG, Oberkochen, Germany) was
used to detect the apoptosis of the SGC-7901 cell nuclei. A minimum
of 5×104 cells treated with various concentrations of
EETEB (0.75, 1.0 and 1.5 mg/ml) were evaluated per chamber. A
488-nm, 10-MW argon laser beam was used for excitation of blue DAPI
fluorescence. Subsequently, LSCM was performed to observe the
nuclei of the SGC-7901 cells and detect apoptosis. The collected
data were analyzed using Microsoft Excel 2000 software (Microsoft
Research, Redmond, WA, USA).
Cell ultrastructure
The cell lysates were collected by centrifugation at
15,000 × g for 10 min at 25°C, the cells treated with various
concentrations of EETEB (0.75, 1.0 and 1.5 mg/ml) were fixed in
1.5% paraformaldehyde and 4% glutaraldehyde in 0.1 M
phosphate-buffered saline (PBS) buffer (pH 7.2–7.4) for 12 h at
4°C. Next, the cells were washed with PBS buffer and post-fixed
with 1% OsO4 in 0.1 M PBS buffer for 2 h. The cells were
then dehydrated in a graded ethanol alcohol series and embedded in
Epoxy resin 618 (E-51, Ganxi Chemical Co. Ltd., Jiangxi, China).
Ultrathin sections (100 nm) were cut using an ultramicrotome (EM
UC6; Leica Microsystems GmbH, Wetzlar, Germany). The sections were
stained in 2.0% uranyl acetate for 20 min and lead citrate for 15
min. Finally, transmission electron microscopy (TEM; H-7650;
Hitachi, Ltd., Tokyo, Japan) was used to examine and capture images
of the cell sections.
Measurement of mitochondrial membrane
potential
The mitochondrial membrane potential was detected
using a JC-1 fluorescent probe (Nanjing KeyGen Biotech Co., Ltd.,
Nanjing, China), which is a cationic dye that exhibits
potential-dependent accumulation in mitochondria. A change in JC-1
fluorescence emission from red to green indicates depolarization of
the mitochondrial membrane; thus, this dye can be used as an
indicator of changes in mitochondrial membrane potential. In the
current experiment, following trypsin digestion (Thermo Fisher
Scientific Inc.), SGC-7901 cells (1×106) treated with
various concentrations of EETEB (0.75, 1.0 and 1.5 mg/ml) were
resuspended in 1 ml medium and incubated with 10 µg/ml JC-1 at 37°C
in an atmosphere of 5% CO2 for 30 min. JC-1 fluorescence
was recorded using a FACSCalibur flow cytometer (Becton-Dickinson,
Franklin Lakes, NJ, USA) at emission wavelengths of 525 and 590 nm
for green and red fluorescence, respectively.
Reverse transcription-polymerase chain
reaction (RT-PCR)
SGC-7901 cells (1×106 cells/well) were
seeded into 6-well plates with 2 ml medium and treated with various
concentrations of EETEB (0.75, 1.0 and 1.5 mg/ml) for 24 h. Total
RNA was extracted from the SGC-7901 cells using a Beyzol reagent
kit (Shanghai Biyuntian Bio-Technology Co., Ltd., Shanghai, China).
Subsequently, oligo(dT)-primed RNA (1 µg) was reverse-transcribed
using SuperScript II® Reverse Transcriptase (Promega Corporation,
Madison, WI, USA), according to the manufacturer's instructions.
PCR was performed to determine the quantity of Bcl-2 and Bax mRNA
in the obtained cDNA samples. GAPDH was used as the internal
control and the DNA bands were examined using a Gel Documentation
system (Gel Doc 2000; Bio-Rad Laboratories, Hercules, CA, USA).
Western blotting
SGC-7901 cells (1×106) were seeded in
6-well plates in 2 ml medium and treated with various
concentrations of EETEB (0.75, 1.0 and 1.5 mg/ml) for 24 h. The
cells were washed twice with cold PBS and then lysed in
radioimmunoprecipitation assay buffer containing 1 mM phenylmethyl
sulfonyl fluoride (Roche Diagnostics GmbH, Mannheim, Germany).
Next, the lysates were centrifuged at 12,000 × g for 10 min at 4°C
to acquire the total Bax and Bcl-2 proteins from the mitochondria.
The cytosolic and mitochondrial fraction proteins were collected. A
single aliquot of the supernatant (50 mg protein) was subjected to
electrophoretic separation using SDS-PAGE and transferred to a
polyvinylidene fluoride (PVDF) membrane (EMD Millipore, Billerica,
MA, USA). The membranes were incubated in blocking buffer (non-fat
milk) and then incubated overnight at 4°C with rabbit polyclonal
antibodies against Bax (1:1,000, 20 kDa, Cell Signaling Technology,
Inc., Danvers, MA, USA), Bcl-2 (1:1,000, 26 kDa, Cell Signaling
Technology, Inc.) or β-actin (1:4,000, 43 kDa, Beyotime Institute
of Biotechnology, Haimen, China). The membranes were stringently
washed and incubated with HRP-conjugated secondary antibodies
(Proteintech Group, Chicago, IL, USA), for 1 h at room temperature.
Images were captured using a Kodak Image Station 400R (Eastman
Kodak Co., Rochester, NY, USA).
Statistical analysis
All the data are presented as the mean of three
experiments and were analyzed using SPSS software (version 16.0;
SPSS, Inc., Chicago, IL, USA). Statistically significant
differences between the control and treatment groups were obtained
by performing one-way analysis of variance. P<0.05 was
considered to indicate a statistically significant difference.
Results
EETEB inhibits the proliferation of
SGC-7901 cells
The effect of EETEB on the proliferation of SGC-7901
cells was determined by performing an MTT assay. As demonstrated in
Fig. 1, treatment with 0.75, 1.0 and
1.5 mg/ml EETEB resulted in cell viability of 71.99±7.26,
54.28±5.28 and 34.48±5.84%, respectively. These values were
significantly higher compared with the untreated control cells
(P<0.05), indicating that EETEB inhibited SGC-7901 cell
proliferation in a dose-dependent manner.
EETEB induces the apoptosis of
SGC-7901 cells
To determine whether the cell-growth suppressive
effect of EETEB was due to apoptosis, the pro-apoptotic activity of
EETEB on SGC-7901 cells was determined using Annexin-V/PI staining
followed by FACS analysis. As indicated in Fig. 2A and B, following treatment with 0,
0.75, 1.0 and 1.5 mg/ml EETEB, the percentage of cells undergoing
early or late apoptosis was 8.90±0.81, 14.31±1.13, 18.28±2.32 and
50.68±2.05%, respectively. EETEB concentrations of 1.0 and 1.5
mg/ml resulted in a significant increase in apoptosis compared with
the control group (P<0.05), indicating that EETEB promoted
SGC-7901 cell apoptosis in a dose-dependent manner.
 | Figure 2.Effect of EETEB on SGC-7901 cell
apoptosis. Cells were grown and treated with increasing
concentrations of EETEB for 24 h, collected and stained with
Annexin V/PI. (A) Representative fluorescence-activated cell
sorting (FACS) analysis scattergrams displaying four different cell
populations, labeled as: LL, double-negative stained cells = live
cell population; LR, Annexin V-positive/PI-negative stained cells -
early apoptosis; UR, Annexin V/PI double-positive stained cells -
late apoptosis; and UL, Annexin V-negative/PI-positive stained
cells - dead cells. Data are representative of three independent
experiments. (B) Quantification of FACS analysis. Data are
presented as the mean ± standard error of the mean of three
independent experiments (n=3). #P<0.05, vs. control
cells. UP, upper left; UR, upper right; LL, lower left; LR, lower
right; PI, propodium iodide; FITC, fluorescein isothiocyanate;
EETEB, ethanolic extract of Tulipa edulis Bak. |
The promotion of cellular apoptosis by EETEB
treatment was verified by examining nuclear morphological changes
following staining of cell nuclei with the DNA-binding dye, DAPI.
As indicated in Fig. 3, EETEB-treated
cells (Fig. 3B-D) exhibited typical
apoptotic morphological features, such as condensed chromatin and
fragmented nuclei. By contrast, the untreated cell nuclei (Fig. 3A) exhibited homogenous and less
intense staining.
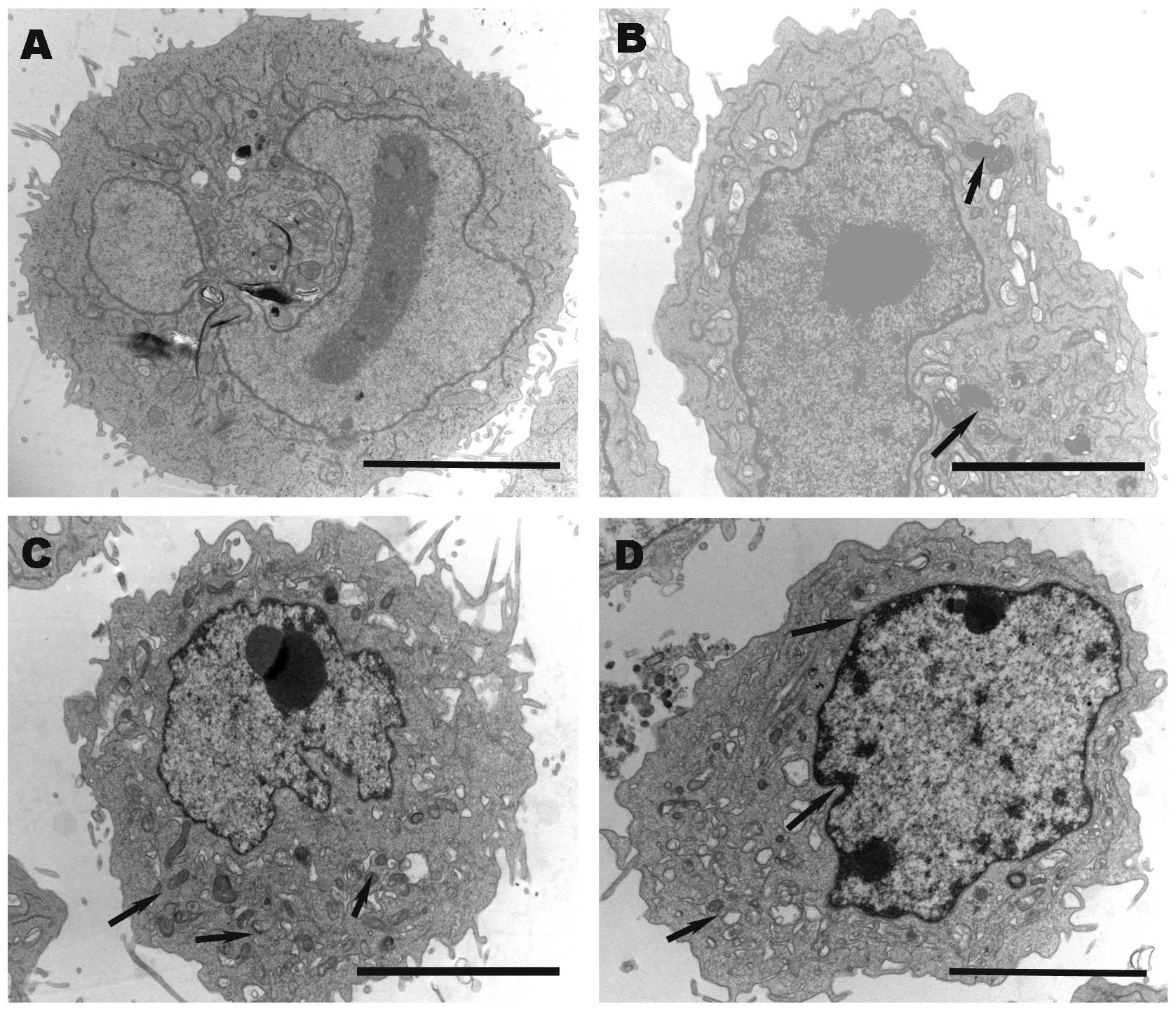
The morphological effect of EETEB treatment on
SGC-7901 cells was also evaluated using TEM. As demonstrated in
Fig. 4A, untreated control cells did
not display any morphological changes. However, EETEB treatment
resulted in a decrease in the number of microvilli, shrinkage of
the cytoplasmic organoid volume (as evidenced by the promotion of
electron density), shrinkage of the nucleolus and margination of
the heterochromatin (Fig. 4B-D).
EETEB induces the loss of
mitochondrial membrane potential in SGC-7901 cells
Changes in the mitochondrial membrane potential were
examined using FACS analysis with JC-1 staining. The
membrane-permeable JC-1 dye exhibits potential-dependent
accumulation in the mitochondria, indicated by a fluorescence
emission shift from green (wavelength, ~525 nm) to red (wavelength,
~590 nm) (20). Therefore, a collapse
in the mitochondrial membrane potential during apoptosis can be
indicated by a decrease in the ratio of red/green fluorescence
intensity. As demonstrated in Fig. 5,
following treatment with 0, 0.75, 1.0 and 1.5 mg/ml EETEB, the JC-1
red/green fluorescence ratio in SGC-7901 cells was 22.67±0.56,
51.10±1.25, 71.48±1.18 and 97.13±3.21%, respectively. This
indicated that EETEB treatment induced the loss of mitochondrial
membrane potential in SGC-7901 cells in a dose-dependent
manner.
EETEB regulates the expression of
Bcl-2 family members in SGC-7901 cells
The underlying mechanism mediating the pro-apoptotic
activity of EETEB was investigated by performing RT-PCR to detect
the mRNA expression levels and western blotting to determine the
protein expression levels of Bcl-2 and Bax in SGC-7901 cells. As
shown in Fig. 6, EETEB treatment
markedly reduced the mRNA (Fig. 6A)
and protein (Fig. 6B) expression
levels of anti-apoptotic Bcl-2. By contrast, the expression of
pro-apoptotic Bax appeared to be markedly increased following EETEB
treatment, indicating that EETEB promoted SGC-7901 cell apoptosis
by increasing the pro-apoptotic Bax/Bcl-2 ratio.
Discussion
An abnormal increase in cell proliferation or a
reduction in cell apoptosis are two important characteristics of
cancer cells (21). However, numerous
currently used anticancer agents contain compounds that directly or
indirectly damage healthy cells as well as cancer cells, limiting
their long-term use and reducing their therapeutic efficacy
(22,23). These problems highlight the
requirement for the development of novel therapeutic cancer agents.
Traditional Chinese herbal medicine compounds, which exhibit
relatively less side-effects, have been used in clinical settings
to treat various types of disease, including GC. TEB has been used
previously in numerous Traditional Chinese medicine herbal formulas
for the treatment of cancer, thyreoitis and lymphadenitis without
any toxic effects (24–27). Therefore, we hypothesized that TEB is
not cytotoxic to healthy cells and thus healthy gastric cells were
not used for comparison in the present study.
To the best of our knowledge, the present study is
the first to report that EETEB appears to reduce viability and
inhibit growth in human gastric carcinoma (SGC-7901) cells in a
dose-dependent manner. Furthermore, the current study demonstrated
that EETEB may induce SGC-7901 cell apoptosis. Thus far, the Bcl-2
family has been identified to mediate an apoptotic signaling
pathway, the mitochondrial pathway (28). Bax and Bcl-2 are members of the Bcl-2
family of proteins regulates apoptosis (29). Bax is pro-apoptotic, and Bcl-2 is
considered to be anti-apoptotic (30). Therefore, the expression of Bcl-2 or
Bax may determine whether cancer cells progress towards apoptosis
(9). In the present study, a marked
change in the mRNA and protein expression patterns of Bcl-2 and Bax
was observed following EETEB treatment. Furthermore, flow cytometry
was performed, identifying that EETEB treatment caused a
significant increase in cell apoptosis in a dose-dependent manner.
Therefore, EETEB is hypothesized to result in cell apoptosis
through a process that involves the opening of the mitochondrial
permeability transition pore complex (31). In addition, the present study
demonstrated that EETEB caused the loss of mitochondrial
transmembrane potential, a factor that is considered to be a major
determinant in cellular commitment to cellular apoptosis or death
(32). Finally, TEM was used to
observe the morphology of SGC-7901 cells treated with the EETEB.
The results indicated that the number of cell microvilli and the
cytoplasmic volume decreased, the electron density was promoted,
the nucleolus decreased in size and the heterochromatin was
marginated. Thus, the morphological changes detected in the
SGC-7901 cells demonstrated apoptosis.
In conclusion, the present the results of the
present study indicated that EETEB treatment may effectively kill
the human GC cells, SGC-7901. Furthermore, the current data
demonstrates, for the first time, that EETEB inhibits proliferation
and induces apoptosis via the mitochondrial signaling pathway.
However, it is important to note that the present study only
examined the in vitro antitumor effects of EETEB. Therefore,
the in vivo antitumor effects of EETEB, as well as potential
molecular mechanisms, require further investigation.
Acknowledgements
The present study was supported by the Research
Foundation of the Education Bureau of Fujian Province of China
(grant no. JA11132).
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sakuramoto S, Sasako M, Yamaguchi T,
Kinoshita T, Fujii M, Nashimoto A, Furukawa H, Nakajima T, Ohashi
Y, Imamura H, et al: ACTS-GC Group: Adjuvant chemotherapy for
gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med.
357:1810–1820. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Power DG, Kelsen DP and Shah MA: Advanced
gastric cancer-slow but steady progress. Cancer Treat Rev.
36:384–392. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Park HR, Tomida A, Sato S, Tsukumo Y, Yun
J, Yamori T, Hayakawa Y, Tsuruo T and Shin-ya K: Effect on tumor
cells of blocking survival response to glucose deprivation. J Natl
Cancer Inst. 96:1300–1310. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
The State Pharmacopoeia Commission:
Pharmacopoeia of People's Republic of China. Chin Med Sci Technol
Press; Beijing: pp. 312010
|
|
6
|
Xia WB, Xue Z, Li S, Wang SJ, Yang YC, He
DX, Ran GL, Kong LZ and Shi JG: Chemical constituents from tuber of
Cremastra appendiculata. Zhongguo Zhong Yao Za Zhi. 30:1827–1830.
2005.PubMed/NCBI
|
|
7
|
Feng D, Ma Y, Liu J, Xu L, Zhang Y, Qu J,
Liu Y and Qu X: Cbl-b enhances sensitivity to 5-fluorouracil via
GFR-and mitochondria-mediated pathways in gastric cancercells. Int
J Mol Sci. 14:24399–24411. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cory S, Huang DC and Adams JM: The Bcl-2
family: roles in cell survival and oncogenesis. Oncogene.
22:8590–8607. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Luo X, Budihardjo I, Zou H, Slaughter C
and Wang X: Bid, a Bcl-2 interacting protein, mediates cytochrome c
release from mitochondria in response to activation of cell surface
death receptors. Cell. 94:481–490. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mathai JP, Germain M and Shore GC:
BH3-only BIK regulates BAX, BAK-dependent release of
Ca2+ from endoplasmic reticulum stores and mitochondrial
apoptosis during stress-induced cell death. J Biol Chem.
280:23829–23836. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Martinou JC and Youle RJ: Mitochondria in
apoptosis: Bcl-2 family members and mitochondrial dynamics. Dev
Cell. 21:92–101. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yuan J: Molecular control of life and
death. Curr Opin Cell Biol. 7:211–214. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Saraste A and Pulkki K: Morphologic and
biochemical hallmarks of apoptosis. Cardiovasc Res. 45:528–537.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Boatright KM and Salvesen GS: Mechanisms
of caspase activation. Curr Opin Cell Biol. 15:725–731. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Vaux DL and Korsmeyer SJ: Cell death in
development. Cell. 96:245–254. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Antonsson B, Montessuit S, Lauper S, Eskes
R and Martinou JC: Bax oligomerization is required for
channel-forming activity in liposomes and to trigger cytochrome C
release from mitochondria. Biochem J. 345:271–278. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
O'Neill J, Manion M, Schwartz P and
Hockenbery DM: Promises and challenges of targeting Bcl-2
anti-apoptotic proteins for cancer therapy. Biochim Biophys Acta.
1705:43–51. 2004.PubMed/NCBI
|
|
18
|
Orrenius S: Mitochondrial regulation of
apoptotic cell death. Toxicol Lett. 149:19–23. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lin R, Li Z, Zeng J, Lin J and Peng J:
Effect of Pseudobulbus Cremastrae seu Pleiones (EEPCP) on human
gastric carcinoma SGC-7901 cells. Fujian Zhong Yi Yao Da Xue Xue
Bao. 22:22–24. 2012.(In Chinese).
|
|
20
|
Yokosuka T, Goto H, Fujii H, et al: Flow
cytometric chemosensitivity assay using JC 1, a sensor of
mitochondrial transmembrane potential, in acute leukemia. Cancer
Chemother Pharmacol. 72:1335–1342. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Adams JM and Cory S: The Bcl-2 apoptotic
switch in cancer development and therapy. Oncogene. 26:1324–1337.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Boos G and Stopper H: Genotoxicity of
several clinically used topoisomerase II inhibitors. Toxicol Lett.
116:7–16. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gordaliza M: Natural products as leads to
anticancer drugs. Clin Transl Oncol. 9:767–776. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen LW, Lin J, Chen W and Zhang W: Effect
of Chinese herbal medicine on patients with primary hepatic
carcinoma in III stage during perioperational period: a report of
42 cases. Zhongguo Zhong Xi Yi Jie He Za Zhi. 25:832–834. 2005.(In
Chinese). PubMed/NCBI
|
|
25
|
Chen L, Zheng C and Du J: Study on
antitumor mechanism of Qingre Xiaozheng drink by molecular docking
method. Clin Pharmacol Ther (Chin). 12:324–328. 2007.
|
|
26
|
Cao Z, Lin W, Huang Z, Chen X, Zhao J,
Zheng L, Ye H, Liu Z, Liao L and Du J: Ethyl acetate extraction
from a Chinese herbal formula, Jiedu Xiaozheng Yin, inhibits the
proliferation of hepatocellular carcinoma cells via induction of
G0/G1 phase arrest in vivo and in vitro. Int J Oncol. 42:202–210.
2013.PubMed/NCBI
|
|
27
|
Cao Z, Lin W, Huang Z, Chen X, Zhao J,
Zheng L, Ye H, Liu Z, Liao L and Du J: Jiedu Xiaozheng Yin, a
Chinese herbal formula, inhibits tumor angiogenesis via
downregulation of VEGF-A and VEGFR-2 expression in vivo and in
vitro. Oncol Rep. 29:1080–1086. 2013.PubMed/NCBI
|
|
28
|
Hossini AM and Eberle J: Apoptosis
induction by Bcl-2 proteins independent of the BH3 domain. Biochem
Pharmacol. 76:1612–1619. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gabriel B, Sureau F, Casselyn M, Teissié J
and Petit PX: Retroactive pathway involving mitochondria in
electroloaded cytochrome c-induced apoptosis. Protective properties
of Bcl-2 and Bcl-XL. Exp Cell Res. 289:195–210. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
LangRollin I, Maniati M, Jabado O,
Vekrellis K, Papantonis S, Rideout HJ and Stefanis L: Apoptosis and
the conformational change of Bax induced by proteasomal inhibition
of PC12 cells are inhibited by Bcl-xL and Bcl-2. Apoptosis.
10:809–820. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bouaziz C, Martel C, Sharaf el dein O,
Abid-Essefi S, Brenner C, Lemaire C and Bacha H: Fusarial
toxin-induced toxicity in cultured cells and in isolated
mitochondria involves PTPC-dependent activation of the
mitochondrial pathway of apoptosis. Toxicol Sci. 110:363–375. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bras M, Queenan B and Susin SA: Programmed
cell death via mitochondria: Different modes of dying. Biochemistry
(Mosc). 70:231–239. 2005. View Article : Google Scholar : PubMed/NCBI
|