Introduction
Chronic myeloid leukemia (CML) is a clonal stem cell
disorder that is characterized by the acquisition of an oncogenic
BCR/ABL fusion protein and the proliferation of myeloid cells at
all stages of development (1,2). In addition to marked granulocytic
predominance, pathological and morphological findings show that the
number of megakaryocytes in the chronic phase is frequently
increased, and that these cells exhibit abnormal features (small
with abnormal lobation) (2).
Megakaryocytes are the source of platelet production, and
dysmegakaryopoiesis implies dysregulated platelet production,
involving dysmorphology (increased size, particularly giant
platelets) (3,4), abnormal counts and impaired function
(5–10).
In the chronic phase, although >50% of patients
with CML develop thrombocytosis, there are a number of patients
with normal platelet counts (CML-N) (11). In order to assess whether normal
platelet counts mask abnormal platelet production in CML, platelet
parameters, including mean platelet volume (MPV), platelet large
cell ratio (P-LCR), and platelet distribution width (PDW), were
compared among patients with CML with elevated platelets (CML-E),
CML-N and healthy controls. In addition, correlations between the
platelet count and these parameters were analyzed. Furthermore,
bone marrow smears were reviewed in order to examine the
association between platelet production and the proportion of
dysmegakaryocytes (abnormal lobation) in patients with CML. It was
hypothesized that in patients with CML, normal platelet counts do
not indicate normal platelet production, and that apparently normal
counts may mask disordered production.
Patients and methods
Subjects
A total of 65 patients with chronic-phase CML were
recruited from April 2010 to March 2014 (Anhui provincial hospital,
Hefei, China). This study was conducted according to the World
Medical Association Declaration of Helsinki and approved by the
Institutional Ethics Committee of Anhui Provincial Hospital.
Informed consent was obtained from participants and all patients
with CML were diagnosed according to the WHO 2008 guideline
(2), which included examination of
cell morphology and the BCR-ABL fusion gene. None of the patients
had been previously treated with any BCR-ABL-targeted drugs. The
patients in the present study were divided into two groups: Those
with normal platelet counts (100–300×109/l) (CML-N) and
those with elevated platelet counts (>300×109/l)
(CML-E). Thirty-three healthy adults were enrolled as healthy
controls. Patient characteristics are provided in Table I.
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
|
| Patients with
CML |
|
|---|
|
|
|
|
|---|
| Characteristic | With normal platelet
count (n=25) | With elevated
platelet count (n=40) | Healthy controls
(n=33) |
|---|
| Age, years | 42 (15–82) | 43 (16–81) | 46 (17–74) |
| Gender, M/F | 14/11 | 31/9 | 18/15 |
| WBC
(109/l) | 198.44 (129.36) | 179.37 (80.55) | 5.99 (1.31) |
| PLT
(109/l) | 188.88 (69.39) | 541.70 (222.28) | 224.88 (37.52) |
| MPV (fl) | 11.60 (1.06) | 11.22 (1.05) | 10.66 (0.78) |
| PDW (fl) | 15.54 (2.99) | 13.93 (2.72) | 13.07 (1.74) |
| P-LCR (%) | 37.79 (8.35) | 34.19 (7.91) | 30.23 (6.7) |
| BCR-ABL1 fusion
gene | Positive | Positive | NA |
Blood routine examination
Blood samples were collected in tubes containing
EDTA and examined using the fully automated hematology analyzer
XE-5000 (Sysmex, Kobe, Japan). In addition to routine measurements
of red blood cell (RBC) and white blood cell (WBC) counts, the
platelet parameters evaluated for the purpose of the current study
were: Platelet count, MPV, P-LCR and PDW. A normal platelet count
in the Chinese population is 100–300×109/l. The results
are summarized in Table I.
Assessment of
megakaryocytopoiesis
Bone marrow smears were stained with Wright's-Giemsa
(Baso Diagnostics Inc., Zhuhai, China), and observed by light
microscopy (BX41; Olympus Corporation, Tokyo, Japan). Megakaryocyte
proliferation was evaluated using the following method: For each
sample, 50–100 fields were examined under low power (×20
objective). The total number of megakaryocytes in each field was
counted, and the average number of megakaryocytes was then
calculated (number of megakaryocytes/number of fields examined).
The average number of megakaryocytes was used as an indicator by
which to assess the proliferation of megakaryocytes. When
performing the assessment of proliferation, dysmorphic
megakaryocytes were recorded and their numbers were analyzed
separately. Dysmegakaryocytes were divided in to two groups,
according to the pattern of abnormal lobulation: Hypolobation
(mononuclear) and multinucleation (two or more round separated
nuclei; Fig. 1).
Statistical analysis
Statistical analysis was performed using SPSS 12.0
software (SPSS, Inc., Chicago, IL, USA). An independent t-test was
used to compare platelet parameters and megakaryocyte proliferation
in the different groups. Pearson's correlation test was performed
to examine the association between platelet count, and MPV, P-LCR
and PDW. χ2 test was used to compare the constituent
ratio of dysmegakaryocytes within the two CML groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
Elevated MPV and P-LCR in patients
with CML
Analysis using an independent-samples t-test,
demonstrated that the MPV and P-LCR in patients with CML were
significantly higher than those of healthy controls (P<0.05),
regardless of whether the platelet count was elevated. No
significant differences were detected between the two CML groups
(P>0.05; Table II, Fig. 2A and B).
 | Table II.P-values from independent-samples
t-test analysis in the different groups. |
Table II.
P-values from independent-samples
t-test analysis in the different groups.
|
| Platelet
parameter |
|---|
|
|
|
|---|
| Groups | MPV | P-LCR | PDW |
|---|
| CML-N vs. CML-E | 0.173 | 0.109 | 0.032a |
| CML-N vs.
Controls | 0.001a | 0.001a | 0.000b |
| CML-E vs.
Controls | 0.015a | 0.028a | 0.037a |
Increased PDW in the CML-N group
Further analysis using an independent-samples
t-test, demonstrated that the PDW in the CML-N group was
significantly higher than that in the CML-E group (P<0.05),
while the latter was significantly higher than that of healthy
controls (P<0.05; Table II,
Fig. 2C).
Platelet count is not correlated with
MPV, P-LCR or PDW in patients with CML-N
Analysis using Pearson's correlation test,
demonstrated inverse correlations between platelet count and MPV,
P-LCR and PDW in the healthy control and CML-E groups, while no
correlations between these parameters were observed in the CML-N
group (Fig. 3).
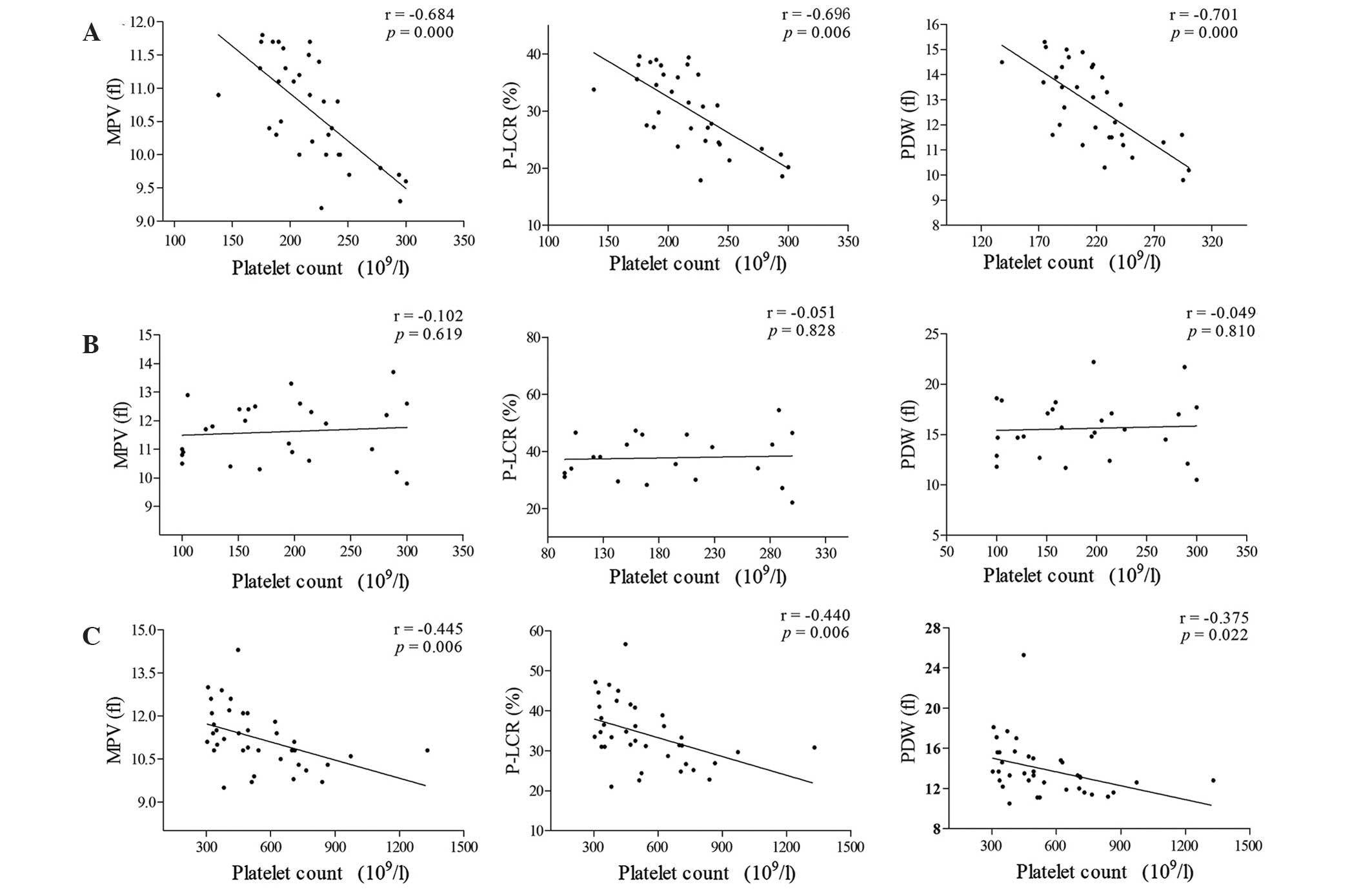 | Figure 3.Correlation between platelet counts
and other platelet parameters among healthy controls, CML-N and
CML-E groups. (A) Healthy controls. (B) CML-N group. (C) CML-E
group. Inverse correlations were observed between platelet count
and MPV, P-LCR and PDW in the CML-E group and healthy controls.
However, no significant correlations between platelet count and
MPV, P-LCR or PDW were observed in the CML-N group. CML, chronic
myeloid leukemia; CML-N, patients with CML with a normal platelet
count; CML-E, patients with CML with an elevated platelet count;
MPV, mean platelet volume; fl, femtoliters; P-LCR, platelet large
cell ratio; PDW, platelet distribution width. |
Proliferation and lobulation of
megakaryocytes is altered in patients with CML
As platelets are known to be produced by
megakaryocytes, bone marrow smears were examined, and the
proliferation and lobulation of megakaryocytes was analyzed.
Analysis using an independent-samples t-test, demonstrated that the
proliferation of megakaryocytes was higher in the CML-E group than
that in the CML-N group (P=0.032). Furthermore, the results of the
χ2 test, demonstrated a higher constituent ratio of
multinucleated megakaryocytes in the CML-N group than in the CML-E
group (12.17 vs. 4.69%; χ2=29.79; P=0.000), while no
difference in hypolobation was detected between the two CML groups
(10.08 vs. 11.88%; χ2=0.626; P=0.429).
Discussion
Patients with CML often exhibit morphological
abnormalities in megakaryocytes, which are characterized by small
and abnormal lobation features. Platelet production represents the
final stage of megakaryocyte development and dysmegakaryopoiesis
may result in platelet disorders, involving dysmorphology, abnormal
counts and impaired function (3,9,10). In hematological malignancies, such as
myelodysplastic syndrome (MDS), essential thrombocythemia, and
other myeloproliferative or myeloproliferative/myelodysplastic
neoplasms, abnormal platelet counts usually reflect altered
thrombopoiesis (12). However, normal
counts in patients may not be evidence that the process of platelet
production is unimpaired. In the present study, patient in the
chronic phase of CML were recruited and divided into two groups:
Patients with normal platelet counts (CML-N) and patients with
elevated platelets (CML-E). Although each of the CML groups
exhibited higher MPV, P-LCR and PDW than the healthy controls, PDW
was higher in the CML-N than that in the CML-E group. Furthermore,
no correlation between platelet count and MPV, P-LCR or PDW was
observed in the CML-N group. These results indicated that patients
with CML with a normal platelet count have a higher PDW, while the
normal association between platelet count and other platelet
parameters is lost. The reason for this is unclear.
In order to address this question, bone marrow
smears were examined. A difference in the proportion of dyslobated
megakaryocytes was observed between the CML groups, and a higher
proliferation of megakaryocytes was observed in the CML-E group.
Dyslobated megakaryocytes, including cells with hypolobation
(mononuclear) and multinucleation (binucleate or more round
separated nuclei) are a pathological feature of MDS (13), and hypolobated megakaryocytes are also
frequently observed in patients with CML (2). Bone marrow examination in the present
study, demonstrated that in addition to hypolobation, a number of
multinucleated megakaryocytes were also observed in patients with
CML, and the ratio of dyslobated megakaryocytes to megakaryocytes
was higher in the CML-N group than that in the CML-E group.
Ordinarily, the transition from megakaryocyte to
platelet is under rigorous control (14–17).
However, regulation of this process may be disrupted in certain
diseases, particularly in neoplasms with a clonal origin (12,18–20). It
was hypothesized that dysmegakaryocytes reflect disorders of
megakaryocyte development and result in dysregulated platelet
production, as indicated by higher MPV, P-LCR and PDW values in
patients with CML than in healthy controls. Furthermore, loss of
the correlation between platelet count and other parameters of
platelet function in the CML-N group, may indicate dysregulation of
platelet production. Patients in the CML-E group exhibited normal
megakaryocytes and platelets, which masked the abnormal platelet
production. However, a lower ratio of multinucleated megakaryocytes
to normal megakaryocytes indicated that this population was less
heterogeneous, which may be another explanation for the lower PDW
in the CML-E group compared with the CML-N group.
Platelet formation following megakaryocyte
development is a complex process and the mechanisms underlying this
process remain unclear. Further research into abnormal
megakaryocytes and thrombopoiesis may provide novel insights into
hematological malignancies.
Acknowledgements
The authors would like to thank the staff of the
Laboratory of Hematology, Second Affiliated Hospital of Anhui
Medical University (Hefei, China) and the Laboratory of Hematology,
Anhui Provincial Hospital (Hefei, China) for their technical
assistance. This work was supported by a grant from the Major
Program of Nature Science Research in Colleges and Universities in
Anhui Province (grant no. KJ2014Z017).
References
|
1
|
Tefferi A and Vardiman JW: Classification
and diagnosis of myeloproliferative neoplasms: The 2008 World
Health Organization criteria and point-of-care diagnostic
algorithms. Leukemia. 22:14–22. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Swerdlow SH, Campo E, Harris NL, et al:
WHO classification of tumours of haematopoietic and lymphoid
tissues. Fourth. IARC Press; Lyon: pp. 32–37. 2008
|
|
3
|
Kabutomori O, Kanakura Y and Iwatani Y:
Increase in platelet-large cell ratio in chronic myeloid leukemia.
Leuk Res. 25:8732001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kouides PA and Bennett JM: Morphology and
classification of myelodysplastic syndromes. Hematol Oncol Clin
North Am. 6:485–499. 1992.PubMed/NCBI
|
|
5
|
Raman BK, Van Slyck EJ, Riddle J, Sawdyk
MA, Abraham JP and Saeed SM: Platelet function and structure in
myeloproliferative disease, myelodysplastic syndrome and secondary
thrombocytosis. Am J Clin Pathol. 91:647–655. 1989.PubMed/NCBI
|
|
6
|
Rasi V and Lintula R: Platelet function in
the myelodysplastic syndromes. Scand J Haematol Suppl. 45:71–73.
1986.PubMed/NCBI
|
|
7
|
Wehmeier A, Fricke S, Scharf RE and
Schneider W: A prospective study of haemostatic parameters in
relation to the clinical course of myeloproliferative disorders.
Eur J Haematol. 45:191–197. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shibata K, Nishimura J, Yufu Y and Nawata
H: Alterations in thrombin-induced protein tyrosine phosphorylation
of platelets from patients with chronic myelogenous leukemia. Int J
Haematol. 55:189–196. 1992.
|
|
9
|
RaszejaSpecht A, Skibowska A, Kabata J and
Hellmann A: Platelet defects in chronic myeloproliferative
disorders. Acta Haematol Pol. 25:253–260. 1994.(In Polish).
PubMed/NCBI
|
|
10
|
Popov VM, Vladareanu AM, Bumbea H, Kovacs
E, Moisescu MG, Onisai M, Iordache MM and Savopol T: Assessment of
changes in membrane properties of platelets from patients with
chronic myeloid leukaemia in different stages of the disease. Blood
Coagul Fibrinolysis. 25:142–150. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
MasonJE Jr, DeVita VT and Canellos GP:
Thrombocytosis in chronic granulocytic leukemia: Incidence and
clinical significance. Blood. 44:483–487. 1974.PubMed/NCBI
|
|
12
|
Osselaer JC, Jamart J and Scheiff JM:
Platelet distribution width for differential diagnosis of
thrombocytosis. Clin Chem. 43:1072–1076. 1997.PubMed/NCBI
|
|
13
|
Liu D, Chen Z, Xue Y, Lu D, Zhou Y, Gong
J, Wu W, Liang J, Ma Q, Pan J, et al: The significance of bone
marrow cell morphology and its correlation with cytogenetic
features in the diagnosis of MDS-RA patients. Leuk Res.
33:1029–1038. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Machlus KR and Italiano JE Jr: The
incredible journey: From megakaryocyte development to platelet
formation. J Cell Biol. 201:785–796. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gao Y, Smith E, Ker E, Campbell P, Cheng
EC, Zou S, Lin S, Wang L, Halene S and Krause DS: Role of
RhoA-specific guanine exchange factors in regulation of endomitosis
in megakaryocytes. Dev Cell. 22:573–584. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kleiman NS, Freedman JE, Tracy PB, Furie
BC, Bray PF, Rao SV, Phillips DR, Storey RF, Rusconi CP, French PA,
et al: Platelets: Developmental biology, physiology and
translatable platforms for preclinical investigation and drug
development. Platelets. 19:239–251. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
White MJ, Schoenwaelder SM, Josefsson EC,
Jarman KE, Henley KJ, James C, Debrincat MA, Jackson SP, Huang DC
and Kile BT: Caspase-9 mediates the apoptotic death of
megakaryocytes and platelets, but is dispensable for their
generation and function. Blood. 119:4283–4290. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Van der Lelie J and Von dem Borne AK:
Platelet volume analysis for differential diagnosis of
thrombocytosis. J Clin Pathol. 39:129–133. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhou J, Ye Y, Zeng S, Zhou Y, Mao Z, Song
X, Ying B, Lu X, Jiang H and Wang L: Impact of JAK2 V617F mutation
on hemogram variation in patients with non-reactive elevated
platelet counts. PLoS One. 8:e578562013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mottalib MA, Sultana TA, Khalil MI, Gan
SH, Islam MS, Choudhury S and Hossain MA: Phase distribution of
chronic myeloid leukemia in Bangladesh. BMC Res Notes. 7:1422014.
View Article : Google Scholar : PubMed/NCBI
|

















