Introduction
Pancreatic cancer is the 13th most common type of
cancer, and the 8th leading cause of cancer-related mortality,
accounting for 6.9% of all cancer-related mortalities, worldwide
(1). The initial symptoms of
pancreatic cancer are often nonspecific, such as nausea, fatigue,
jaundice, weight loss, light-colored stools, dark urine and pain in
the back or stomach area (2).
Pancreatic cancer may be treated with surgery, radiotherapy or
chemotherapy (3). Chemotherapy and
radiation therapy are important adjuvant or neoadjuvant therapies,
particularly for patients with unresectable disease (4). Pancreatic cancer has an extremely poor
prognosis; the median survival time for all patients is 4–6 months,
and the overall five-year survival rate is 7.2% (1). Emerging evidence suggests that the
serine-protease urokinase-type plasminogen activator (uPA) and its
receptor (uPAR) are significant in pancreatic cancer invasion and
metastasis (5–7). Overexpression of uPAR in pancreatic
cancer has been determined to be a strong and independent predictor
of short overall survival (6). uPAR
is recognized as a novel marker of cancer invasion and metastasis,
and is a promising candidate as a molecular target for cancer
therapy (8,9). The ability to visualize and quantify
uPAR expression non-invasively in vivo is required for the
potential clinical application of anticancer therapy based on the
uPA/uPAR system (10,11).
Therefore, in the present study, a high-affinity
9-mer peptide antagonist of uPA-uPAR (AE105) was selected to
develop a technetium-99m (99mTc)-labeled tracer for
non-invasive single-photon emission computed tomography (SPECT)
assessment of uPAR expression in pancreatic cancer.
99mTc-Hynic-PEG-AE105 was prepared, together with a
non-binding version (99mTc-Hynic-PEG-AE105mut) as a
control, and the quantitative association between tracer uptake and
uPAR expression was investigated in pancreatic tumor tissues.
Materials and methods
Reagents
All commercially available chemical reagents were
used without further purification. The peptide antagonist
HYNIC-PEG-AE105 and a non-binding variant of HYNIC-PEG-AE105
(HYNIC-PEG-AE105mut) were synthesized (purity >95%) by Shanghai
Apeptide Co., Ltd. (Shanghai, China). The sequences of
HYNIC-PEG-AE105 and HYNIC-PEG-AE105mut were D-Cha-F-s-r-Y-L-W-S and
D-Cha-F-s-r-Y-L-E-S, respectively.
99mTc-O4− was obtained from
Beijing Atom HighTech Co., Ltd. (Beijing, China).
SnCL2•H2O (purity >99.99%) was
purchased from Gracia Chengdu Chemical Technology Co., Ltd.
(Chengdu, China). Tricine (purity >99%) was purchased from
Sigma-Aldrich (St. Louis, MO, USA).
Labeling of peptides
99mTc peptide labeling was performed at
room temperature using Tricine as a co-ligand and
SnCL2 as the reducing agent. Tricine and
99mTc-O4− (specific activity, 370
MBq/ml) in 100 µl SnCL2•H2O was
diluted in 80 µl Hynic-PEG-AE105 dissolved in HEPES (1 mg/ml; pH
5.5) and incubated at room temperature. The labeling was optimized
by changing the reaction time (0, 5, 10, 20, 30 and 60 min), dosage
of SnCL2 (40, 60, 80, 100, 120 and 150 µg),
dosage of Tricine (20, 40, 60, 80 and 100 mg), dosage of
Hynic-PEG-AE105 (40, 80, 160, 240 and 320 µg) and dosage of
99mTc-O4− (111, 185, 370 and 555
MBq). The reaction was stopped by adding an excess of 1.0 mol/l
glycine. The labeling rate of 99mTc-Hynic-PEG-AE105 was
detected by thin layer chromatography, as described previously
(12). Each experiment was repeated 3
times. Hynic-PEG-AE105mut was labeled under the same conditions.
The optimal conditions for the 99mTc labeling of
Hynic-PEG-AE105 and Hynic-PEG-AE105mut were as follows: 60 mg
Tricine and 1 ml 99mTc-O4− (~10
mCi) in 80 µl SnCL2•H2O (1 mg/ml)
were diluted in 160 µl Hynic-PEG-AE105 (1 mg/ml), followed by
incubation at room temperature for 10 min.
Purification of
99mTc-labeled peptides
99mTc-labeled peptide was subsequently
purified using Sep-Pak Light C18 cartridges (Waters Corporation,
Milford, MA, USA), as described previously (13), and diluted with 8 volumes of water for
injection. To determine the specific radioactivity of the labeled
peptides, radioactivity was measured by a dose calibrator (CRC-25R;
Capintec Inc., Ramsey, NJ, USA) following the manufacturer's
instructions.
Pancreatic cancer xenografts in nude
mice
The animal experiments were approved by the
Institutional Animal Care and Use Committee (IACUC) of Dalian
Medical University (Dalian, Liaoning, China). Sodium pentobarbital
anesthesia was used to minimize animal suffering. Male nude mice
(4–5 weeks old) were obtained from Dalian Medical University Animal
Center, and kept under pathogen-free conditions in accordance with
the guidelines of the IACUC of Dalian Medical University. For the
xenograft tumor growth assay, BxPC-3 cells were obtained from the
Chinese Academy of Sciences (Shanghai, China) and the cultured
cells (1×106 cells) were injected subcutaneously into
the right flank of the mice, which were anesthetized with 2% sodium
pentobarbital (dose, 45 mg/kg weight). At 2 weeks post-inoculation,
the tumor size was measured every 3–4 days until the tumors grew to
a diameter of 10 mm or until the tumor burden exceeded 10% of their
body weight, at which time the mice were enrolled in SPECT
studies.
Biodistribution studies
In brief, the nude mice bearing BxPC-3 xenografts
were injected into the tail vein with 18.5 MBq of
99mTc-Hynic-PEG-AE105 or
99mTc-Hynic-PEG-AE105mut. The mice were euthanized at
0.5, 1, 2, 4 or 8 h post-injection. Blood, tumor and major organs
were collected (wet-weight) and the radioactivity was measured
using a γ-counter (Perkin Elmer Inc., Waltham, MA, USA) (n=5
mice/group). Tumor/non-tumor (T/NT) ratios were calculated based on
the radioscans by outlining regions of equal areas of tumor tissues
and the corresponding non-tumor tissues.
SPECT imaging
Prior to being sacrificed, all the mice underwent
SPECT imaging (Millennium VG; GE Healthcare, Milwaukee, WI, USA),
at 2, 4 and 6 h post-injection, respectively. The mice were laid in
the center of the field of view. A low-energy high-resolution
parallel holes collimator was used. SPECT images were obtained with
a zoom factor of 3.0 for 5 min, and were digitally stored in a
128×128 matrix and analyzed using a GE Integra workstation (GE
Healthcare).
Immunohistochemistry (IHC)
IHC was performed using a standard
streptavidin-biotin-peroxidase complex method. In brief,
non-specific binding was blocked with 10% normal rabbit serum for 20
min. Tumor sections were deparaffinized and rehydrated. Endogenous
peroxidase activity was blocked with 0.3% hydrogen peroxide for 20
min. For antigen retrieval, the sections were microwave-treated in
10 mM citrate buffer (pH 6.0) for 10 min. The sections were
incubated with rabbit uPAR polyclonal antibody (1:500 dilution;
Santa Cruz Biotechnology Inc., Dallas, TX, USA) overnight, then
incubated with a biotinylated goat anti-rabbit immunoglobulin G
antibody (1:2,000 dilution; Sigma-Aldrich, St. Louis, MO, USA) for
30 min and subsequently reacted with a streptavidin-peroxidase
conjugate and 3–3′-diaminobenzidine (Sigma-Aldrich). The sections
were counter-stained using Meyer's haematoxylin. Negative controls
were performed by replacing the primary antibody with rabbit serum.
The sections were observed under a light microscope and five fields
(×400 magnification) of each section were randomly selected for
analysis. The staining density was calculated based on absorbance
using the Image-pro Plus 6.0 image analysis system (Media
Cybernetics Inc., Rockville, MD, USA).
Statistical analysis
Statistical analysis was performed with SPSS
software (version 10.0; SPSS Inc., Chicago, IL, USA). Data are
presented as the mean ± standard error of the mean and were
assessed by a two-tailed Student's t-test. P<0.05 was used to
indicate a statistically significant difference. The correlation
between tracer and uPAR expression was analyzed using Pearson's
χ2 test.
Results
Radiolabeling of peptides
The efficiency of the 99mTc labeling of
Hynic-PEG-AE105 and inactive Hynic-PEG-AE105 was 94.64±0.72 and
92.03±0.81%, respectively. The optimal conditions for the
99mTc labeling of Hynic-PEG-AE105 and Hynic-PEG-AE105mut
were as follows: 60 mg Tricine and 1 ml
99mTc-O4− (~10 mCi) in 80 µl
SnCL2•H2O (1 mg/ml) were diluted
in 160 µl Hynic-PEG-AE105 (1 mg/ml), followed by incubation at room
temperature for 10 min. The radiochemical purity of
99mTc-Hynic-PEG-AE105 and
99mTc-Hynic-PEG-AE105mut was 97.72±1.73 and 96.70±1.32%,
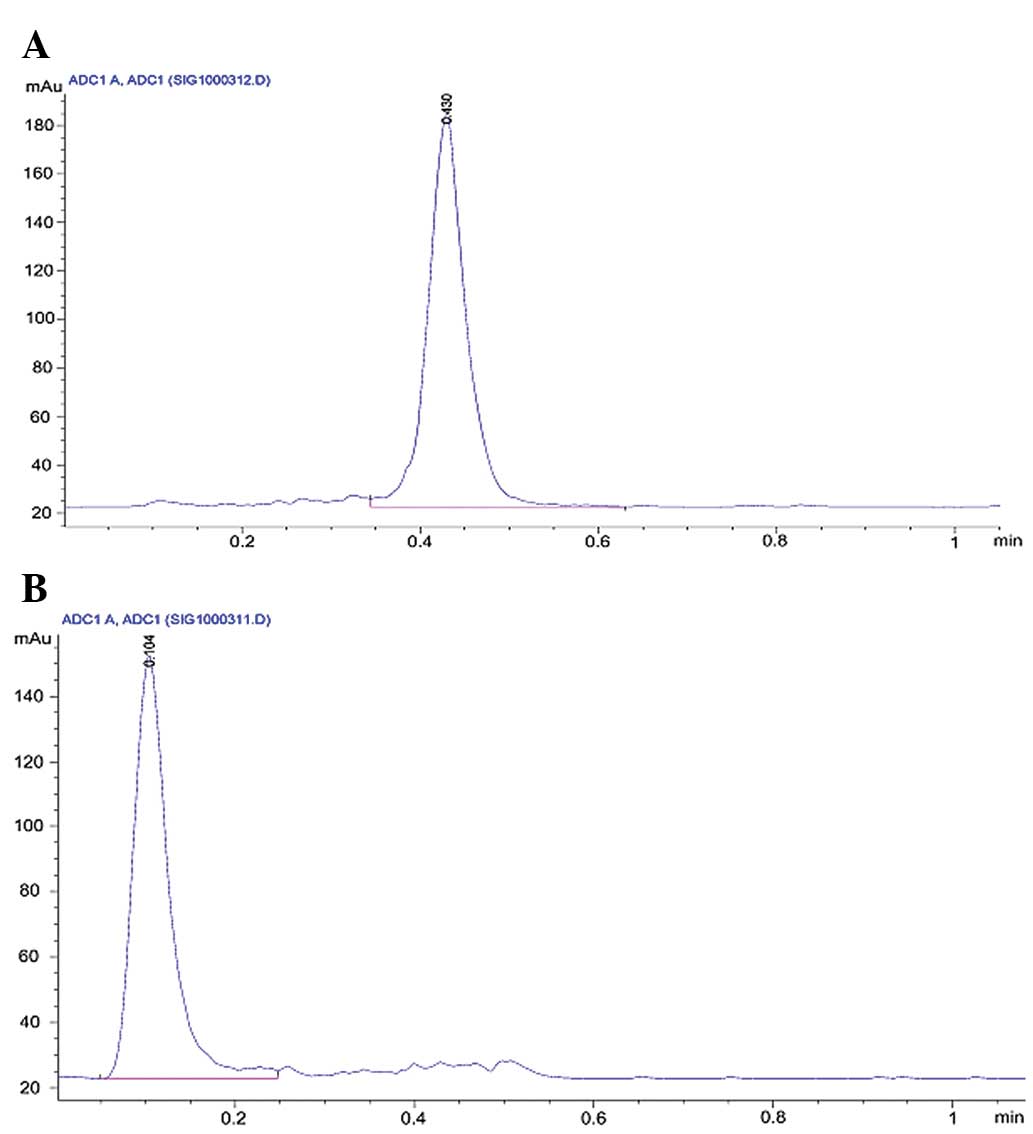
respectively, following Sep-Pak purification (Fig. 1). No significant degradation of any
99mTc-labeled peptides was observed in physiological
saline following incubation for 8 h (Table I).
 | Table I.Radioactivity of
99mTc-labeled peptides following incubation in
saline. |
Table I.
Radioactivity of
99mTc-labeled peptides following incubation in
saline.
| Incubation
time | Hynic-PEG-AE105,
% | Hynic-PEG-AE105mut,
% |
|---|
| 2 h |
96.14±1.26 |
96.38±1.15 |
| 4 h |
95.22±0.91 |
95.27±1.43 |
| 6 h |
94.93±1.12 |
94.26±0.96 |
| 8 h |
94.15±1.44 |
93.66±0.83 |
Biodistribution and specificity of
99mTc-Hynic-PEG-AE105
Next, the study investigated the in vivo
pharmacokinetics of 99mTc-Hynic-PEG-AE105 and
99mTc-Hynic-PEG-AE105 in pancreatic cancer BxPC-3
cell-bearing animals. A fast clearance rate of radiolabeled
peptides from the blood and all organs investigated following
resection was found (Tables II and
III). The two radiolabeled peptides
were distributed to the various organs of the body and cleared
rapidly from the blood, primarily via the hepatic-intestinal route
and kidneys. The tumor uptake of 99mTc-Hynic-PEG-AE105
was significantly higher than the normal pancreatic tissue uptake
at 4 h and 6 h post-injection (P<0.01), whereas the uptake in
the blood was 2.87±0.13 (4 h)/2.73±0.35 (6 h), and the uptake in
the muscle was 0.53±0.21 (4 h)/0.49±0.08 (4 h), thus generating a
tumor-to-blood and tumor-to-muscle ratio of 1.09±0.12 (4
h)/1.11±0.20 (6 h) and 6.29±1.59 (4 h)/6.26±1.20 (6 h),
respectively. The tumor uptake of the control peptide,
99mTc-Hynic-PEG-AE105mut, at 4 or 6 h was significantly
reduced to 1.65±0.53 (4 h) and 1.41±0.38 (6 h) (P<0.01),
respectively, indicating the specificity of
99mTc-Hynic-PEG-AE105 to human uPAR.
 | Table II.Biodistribution and specificity of
99mTc-Hynic-PEG-AE105. |
Table II.
Biodistribution and specificity of
99mTc-Hynic-PEG-AE105.
|
| Radioactivity
(%ID/g) |
|---|
|
|
|
|---|
| Location | 0.5 h | 1 h | 2 h | 4 h | 6 h | 8 h |
|---|
| Blood |
5.38±0.25 |
3.74±0.43 |
2.31±0.53 |
2.87±0.13 |
2.73±0.35 |
2.44±0.22 |
| Tumor |
4.65±0.41 |
3.96±0.26 |
2.72±0.45 |
3.12±0.27 |
2.98±0.15 |
2.15±0.29 |
| Heart |
3.43±0.51 |
1.87±0.53 |
1.37±0.20 |
1.56±0.44 |
1.71±0.48 |
1.03±0.13 |
| Liver |
4.86±0.30 |
3.86±0.61 |
3.19±0.29 |
2.99±0.65 |
3.31±0.93 |
2.09±0.20 |
| Spleen |
4.09±1.07 |
1.41±0.70 |
0.95±0.10 |
1.61±0.74 |
1.53±0.45 |
0.93±0.06 |
| Pancreas |
3.74±0.47 |
1.30±0.20 |
1.12±0.72 |
1.45±0.73 |
1.30±0.41 |
0.52±0.09 |
| Lung |
4.63±0.18 |
3.46±1.70 |
1.78±0.36 |
2.06±0.23 |
1.97±0.38 |
1.39±0.36 |
| Kidney |
5.72±0.65 |
4.35±0.28 |
2.52±0.17 |
3.21±0.21 |
3.32±0.21 |
2.50±0.37 |
| Stomach |
2.83±0.27 |
1.46±0.42 |
0.65±0.12 |
1.23±0.42 |
1.00±0.35 |
0.54±0.02 |
| Intestine |
2.67±1.19 |
1.18±0.65 |
0.89±0.33 |
1.28±0.88 |
0.79±0.30 |
0.46±0.03 |
| Bone |
2.32±0.36 |
1.60±1.10 |
0.56±0.12 |
1.12±0.36 |
1.34±0.47 |
0.67±0.07 |
| Muscle |
1.60±0.34 |
0.55±0.12 |
0.40±0.07 |
0.53±0.21 |
0.49±0.08 |
0.30±0.01 |
| Brain |
0.35±0.07 |
0.35±0.31 |
0.11±0.01 |
0.14±0.05 |
0.13±0.04 |
0.08±0.01 |
 | Table III.Biodistribution and specificity of
99mTc-Hynic-PEG-AE105mut. |
Table III.
Biodistribution and specificity of
99mTc-Hynic-PEG-AE105mut.
|
| Radioactivity
(%ID/g) |
|---|
|
|
|
|---|
| Location | 0.5 h | 1 h | 2 h | 4 h | 6 h | 8 h |
|---|
| Blood |
4.16±0.79 |
2.82±0.68 |
3.20±0.20 |
1.56±0.47 |
1.53±0.23 |
1.33±0.22 |
| Tumor |
3.53±0.42 |
2.53±0.87 |
3.21±0.29 |
1.65±0.53 |
1.41±0.38 |
1.21±0.20 |
| Heart |
2.54±0.28 |
2.11±0.67 |
2.46±0.31 |
1.25±0.46 |
1.21±0.28 |
1.01±0.28 |
| Liver |
3.92±0.33 |
2.92±1.31 |
3.52±0.63 |
3.26±1.33 |
1.90±1.81 |
1.65±0.87 |
| Spleen |
2.71±0.05 |
2.04±0.90 |
2.96±0.80 |
2.15±1.29 |
0.96±0.69 |
0.82±0.20 |
| Pancreas |
1.74±0.09 |
1.50±0.38 |
1.55±0.18 |
0.75±0.08 |
0.88±0.49 |
0.73±0.26 |
| Lung |
1.82±0.12 |
1.62±0.11 |
1.57±0.21 |
1.37±0.51 |
1.12±0.17 |
1.00±0.18 |
| Kidney |
4.41±0.59 |
3.07±0.66 |
3.47±0.33 |
2.41±0.41 |
2.28±0.26 |
2.03±0.40 |
| Stomach |
1.57±0.26 |
1.37±0.19 |
1.82±0.55 |
0.70±0.19 |
0.47±0.18 |
0.42±0.13 |
| Intestine |
2.45±0.27 |
2.36±0.31 |
2.60±1.04 |
0.56±0.10 |
0.66±0.27 |
0.61±0.20 |
| Bone |
1.79±0.34 |
1.59±0.45 |
1.70±0.33 |
0.69±0.19 |
0.56±0.05 |
0.56±0.21 |
| Muscle |
0.89±0.05 |
0.72±0.20 |
0.80±0.19 |
0.54±0.21 |
0.36±0.10 |
0.31±0.10 |
| Brain |
0.34±0.05 |
0.18±0.15 |
0.24±0.03 |
0.08±0.03 |
0.08±0.02 |
0.07±0.01 |
SPECT study
The BxPC-3 tumor-bearing mice were SPECT-scanned at
2, 4 and 6 h post-intravenous injection of
99mTc-Hynic-PEG-AE or
99mTc-Hynic-PEG-AE105mut. The representative images for
each group of mice at 2, 4 and 6 h post-injection are shown in
Fig. 2. The tumor was clearly visible
as early as 2 h post-injection of 99mTc-Hynic-PEG-AE105,
and the uptake kept increasing and reached a plateau at 6 h
post-injection. By contrast, in the mice injected with
99mTc-Hynic-PEG-AE105mut, the tumor was not clear at 2,
4 and 6 h post-injection. Using quantitative region of interest
analysis, a significantly higher radioactive uptake ratio (T/NT)
was found for 99mTc-Hynic-PEG-AE105 than for control
peptide 99mTc-Hynic-PEG-AE105mut at 4 h (3.37±0.11 vs.
1.36±0.18; P<0.001) and 6 h (3.64±0.25 vs. 1.28±0.20;
P<0.001).
uPAR expression is correlated with the
tumor uptake of 99mTc-Hynic-PEG-AE105
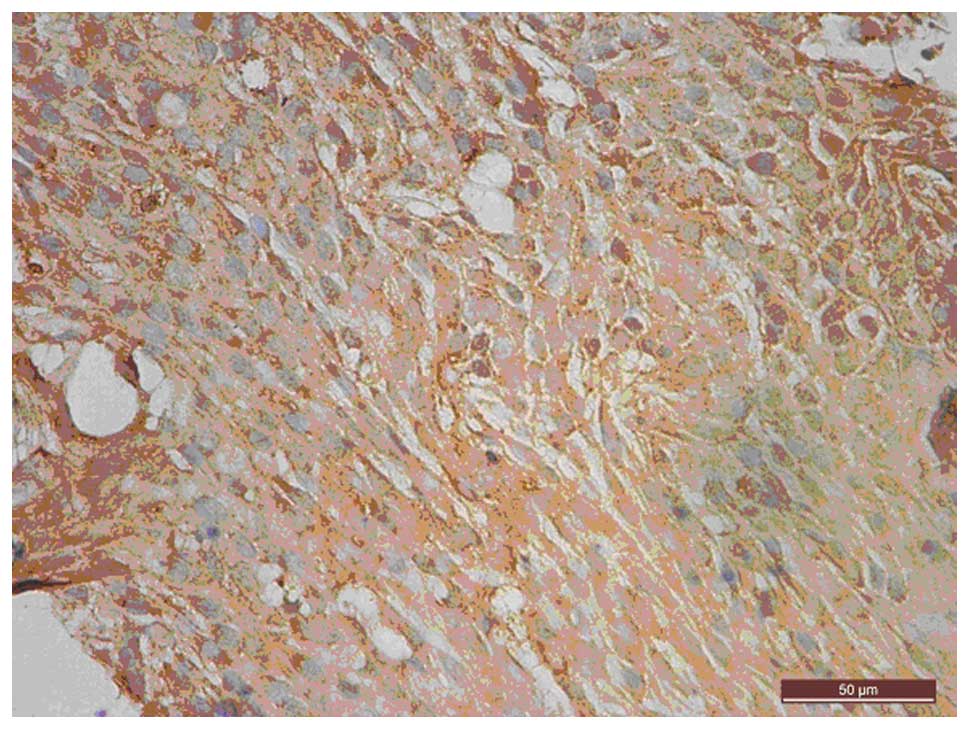
IHC showed that uPAR was mainly stained in the
cytoplasm and on the membrane surface of the BxPC-3 cells (Fig. 3). Semi-quantification of uPAR staining
showed that uPAR expression was not significantly different between
the experimental and control groups (0.481±0.024 vs. 0.574±0.021;
P=0.173). By association analysis, a significant correlation was
found between the tumor uptake of 99mTc-Hynic-PEG-AE105
and uPAR expression at 4 to 6 h post-injection (r=0.791, P=0.006;
Fig. 4).
Discussion
In the present study, 99mTc-labeled
Hynic-PEG-AE105 was introduced as a SPECT tracer for imaging of
uPAR expression for the first time.
99mTc-Hynic-PEG-AE105 exhibited high affinity and
specificity to uPAR in vivo, and uPAR expression was
significantly correlated with the uptake of
99mTc-Hynic-PEG-AE105 in the pancreatic cancer xenograft
mouse model.
Despite its relatively low incidence, pancreatic
cancer ranks fourth in the number of cancer mortalities each year
(14). Overall, <5% of individuals
will survive 5 years beyond their diagnosis (15). Therefore, novel and improved therapy
options are required. Recent studies demonstrated that
RNAi-mediated uPAR-knockdown was able to retard the invasive
ability and angiogenic potential of cancer cells in vitro
and in vivo (5,8,9). These
results suggest that the targeting of uPAR has significant
therapeutic potential for the treatment of pancreatic cancer. For
the potential clinical application of anticancer therapy based on
the uPA/uPAR system, we sought to develop a non-invasive imaging
method for the detection of pancreatic cancer based on uPAR
expression.
Previous studies investigated the use of a
high-affinity 9-mer peptide antagonist of the uPA-uPAR (AE105) for
positron emission tomography (PET) imaging of uPAR expression, and
showed that copper-64 (64Cu)-labeled DOTA-AE105
exhibited specific and high-affinity binding to uPAR in
vitro and in vivo (16–18).
However, the clinical application of this protocol is restricted
due to the limited availability of 64Cu and the high
cost of PET imaging. 99mTc is a nuclear isomer of
99Tc that is detectable within the body using medical
equipment such as γ-ray cameras, which emit readily detectable CXL
keV γ rays (the same wavelength as emitted by conventional X-ray
equipment), and the half-life for γ emission is only 6 h (19). Safe and fast scanning procedures are a
result of the relatively short physical half-life of
99Tc and its biological half-life of 1 day in terms of
human activity and metabolism (20).
Therefore, 99mTc-labeled peptides have been used for
in vivo targeting of tumors, including pancreatic cancer
(21–23).
In the present study, 99mTc-labeled
peptide was employed for SPECT imaging of uPAR in pancreatic
cancer. First, the conditions for the 99mTc labeling of
Hynic-PEG-AE105 and Hynic-PEG-AE105 were optimized. It was found
that under the conditions optimized, the radiochemical purity of
99mTc-Hynic-PEG-AE105 was 97.72±1.73% following Sep-Pak
purification. Similarly, the radiochemical purity of
99mTc-Hynic-PEG-AE105mut was 96.70±1.32%.
uPAR is widely expressed in pancreatic cancer cells
such as Panc-1, MIA PaCa-2 and BxPC-3 (24). Preliminary experiments in the present
study showed that among these cell lines, the expression level of
uPAR is the highest in the BxPC-3 cell line (data not shown), thus
BxPC-3 cells were chosen for further analysis. To analyze the
biodistribution and specificity of 99mTc-Hynic-PEG-AE105
in vivo, a nude mouse xenografted with BxPC-3 cells was used
as the animal model. It was found that the distribution of
radioactivity in the tumor tissue was significantly higher than
that in the normal tissue. Taken together, these data demonstrate
the specificity of 99mTc-Hynic-PEG-AE105 for pancreatic
cancer cells that highly express uPAR. SPECT imaging of nude mice
further confirms the sensitivity and specificity of
99mTc-Hynic-PEG-AE105. The tumor xenograft was clearly
visible as early as 2 h post-injection of
99mTc-Hynic-PEG-AE105, and the uptake kept increasing
and reached a plateau at 6 h post-injection. In addition, a
significant correlation was found between the tumor uptake of
99mTc-Hynic-PEG-AE105 and uPAR expression in the
xenografted tumors, thus providing a strong argument for the
specificity of 99mTc-Hynic-PEG-AE105.
However, the blood clearance rate of
99mTc-AE105 is not fast enough, leading to continued
retention of the tracer in the blood, liver and kidneys, and
interference in detecting the tumor lesion. Therefore, further
studies are required to speed up the rate of the blood clearance of
99mTc-AE105 and improve the imaging of the
target/background ratio.
In summary, the present study reported the
radiosynthesis of 99mTc-Hynic-PEG-AE105 and achieved a
high yield of 99mTc labeling of Hynic-PEG-AE105.
Significantly, it was demonstrated that the distribution of
99mTc-Hynic-PEG-AE105 in the xenografted tumor tissue
was correlated with the level of uPAR expression in pancreatic
cancer. 99mTc-Hynic-PEG-AE105 is a promising agent for
the non-invasive determination of uPAR expression in pancreatic
cancer.
Acknowledgements
This study was funded by the National Natural
Science Foundation of China (grant no. 81071173).
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
LortetTieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ryan DP, Hong TS and Bardeesy N:
Pancreatic adenocarcinoma. N Engl J Med. 371:2140–2141. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Parikh PY and Lillemoe KD: Surgical
management of pancreatic cancer - distal pancreatectomy. Semin
Oncol. 42:110–122. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rombouts SJ, Vogel JA, van Santvoort HC,
van Lienden KP, van Hillegersberg R, Busch OR, Besselink MG and
Molenaar IQ: Systematic review of innovative ablative therapies for
the treatment of locally advanced pancreatic cancer. Br J Surg.
102:182–193. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gorantla B, Asuthkar S, Rao JS, Patel J
and Gondi CS: Suppression of the uPAR-uPA system retards
angiogenesis, invasion and in vivo tumor development in pancreatic
cancer cells. Mol Cancer Res. 9:377–389. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sorio C, Mafficini A, Furlan F, Barbi S,
Bonora A, Brocco G, Blasi F, Talamini G, Bassi C and Scarpa A:
Elevated urinary levels of urokinase-type plasminogen activator
receptor (uPAR) in pancreatic ductal adenocarcinoma identify a
clinically high-risk group. BMC Cancer. 11:4482011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Khoi PN, Xia Y, Lian S, Kim HD, Kim do H,
Joo YE, Chay KO, Kim KK and Jung YD: Cadmium induces urokinase-type
plasminogen activator receptor expression and the cell invasiveness
of human gastric cancer cells via the ERK-1/2, NF-κB and AP-1
signaling pathways. Int J Oncol. 45:1760–1768. 2014.PubMed/NCBI
|
|
8
|
Kotipatruni RR, Nalla AK, Asuthkar S,
Gondi CS, Dinh DH and Rao JS: Apoptosis induced by knockdown of
uPAR and MMP-9 is mediated by inactivation of EGFR/STAT3 signaling
in medulloblastoma. PLoS One. 7:e447982012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhuo J, Tan EH, Yan B, Tochhawng L,
Jayapal M, Koh S, Tay HK, Maciver SK, Hooi SC, SaltoTellez M, et
al: Gelsolin induces colorectal tumor cell invasion via modulation
of the urokinase-type plasminogen activator cascade. PLoS One.
7:e435942012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Knör S, Sato S, Huber T, Morgenstern A,
Bruchertseifer F, Schmitt M, Kessler H, Senekowitsch-Schmidtke R,
Magdolen V and Seidl C: Development and evaluation of peptidic
ligands targeting tumour-associated urokinase plasminogen activator
receptor (uPAR) for use in alpha-emitter therapy for disseminated
ovarian cancer. Eur J Nucl Med Mol Imaging. 35:53–64. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu D, Overbey D, Watkinson L and Giblin
MF: Synthesis and characterization of an (111) In-labeled peptide
for the in vivo localization of human cancers expressing the
urokinase-type plasminogen activator receptor (uPAR). Bioconjug
Chem. 20:888–894. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Daozhen C, Lu L, Min Y, Xinyu J and Ying
H.: Synthesis of (131)
I-labeled-[(131)I]iodo-17-allylamino-17-demethoxy geldanamycin
([(131)I]iodo-17-AAG) and its biodistribution in mice. Cancer
Biother Radiopharm. 22:607–612. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
McPherson DW and Knapp FF Jr: A rapid and
simple Sep Pak method for purification of radioiodinated IQNP, a
high affinity ligand for the muscarinic receptor. Nucl Med Biol.
26:859–863. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Michl P and Gress TM: Current concepts and
novel targets in advanced pancreatic cancer. Gut. 62:317–326. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Persson M, Madsen J, Østergaard S, Jensen
MM, Jørgensen JT, Juhl K, Lehmann C, Ploug M and Kjaer A:
Quantitative PET of human urokinase-type plasminogen activator
receptor with 64Cu-DOTA-AE105: Implications for visualizing cancer
invasion. J Nucl Med. 53:138–145. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li ZB, Niu G, Wang H, He L, Yang L, Ploug
M and Chen X: Imaging of urokinase-type plasminogen activator
receptor expression using a 64Cu-labeled linear peptide antagonist
by microPET. Clin Cancer Res. 14:4758–4766. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Persson M, Madsen J, Østergaard S, Ploug M
and Kjaer A: 68Ga-labeling and in vivo evaluation of a uPAR binding
DOTA- and NODAGA-conjugated peptide for PET imaging of invasive
cancers. Nucl Med Biol. 39:560–569. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Younes CK, Boisgard R and Tavitian B:
Labelled oligonucleotides as radiopharmaceuticals: Pitfalls,
problems and perspectives. Curr Pharm Des. 8:1451–1466. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lindberg H, Hofström C, Altai M, Honorvar
H, Wållberg H, Orlova A, Ståhl S, Gräslund T and Tolmachev V:
Evaluation of a HER2-targeting affibody molecule combining an
N-terminal HEHEHE-tag with a GGGC chelator for 99mTc-labelling at
the C terminus. Tumour Biol. 33:641–651. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nock B and Maina T: Tetraamine-coupled
peptides and resulting (99m)Tc-radioligands: An effective route for
receptor-targeted diagnostic imaging of human tumors. Curr Top Med
Chem. 12:2655–2667. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Spanu A, Farris A, Chessa F, Sanna D,
Pittalis M, Manca A and Madeddu G: Planar scintimammography and
SPECT in neoadjuvant chemo or hormonotherapy response evaluation in
locally advanced primary breast cancer. Int J Oncol. 32:1275–1283.
2008.PubMed/NCBI
|
|
23
|
Cyran CC, Paprottka PM, Eisenblätter M,
Clevert DA, Rist C, Nikolaou K, Lauber K, Wenz F, Hausmann D,
Reiser MF, et al: Visualization, imaging and new preclinical
diagnostics in radiation oncology. Radiation Oncology. 9:32014.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gorantla B, Asuthkar S, Rao JS, Patel J
and Gondi CS: Suppression of the uPAR-uPA system retards
angiogenesis, invasion and in vivo tumor development in pancreatic
cancer cells. Mol Cancer Res. 9:377–389. 2011. View Article : Google Scholar : PubMed/NCBI
|