Introduction
Glioblastoma is the most common and most aggressive
type of primary brain tumor, accounting for nearly 65% of all
primary intracranial tumors and conferring a median survival time
of ~14 months (1). The molecular
mechanisms underlying the pathology of glioblastoma, which is not
completely curable by means of chemotherapy, radiotherapy and
surgery, are yet to be fully elucidated. Therefore, the search for
candidate molecules to be employed as countermeasures against
glioblastoma remains essential. In addition, it is equally
important to identify potential target molecules responsible for
the pathogenesis of glioblastoma in order to develop novel clinical
treatment modalities.
Peptidyl-prolyl cis/trans isomerase NIMA-interacting
1 (Pin1) is a member of the parvulin family; the peptidyl-prolyl
cis/trans isomerase (PPIase) group of proteins (2). Previous data has identified that Pin1 is
broadly overexpressed in various types of tumors, including gliomas
(3). Members of the Parvulin family
have a variety of functions, including modulating the assembly,
folding, activation/inactivation and transport of essential
proteins to their intracellular targets (4). In addition, the proteins regulate
intracellular signaling, transcription, cell cycle progression and
apoptosis by altering the function and/or stability of target
proteins (5). Pin1 induces
conformational changes in its target phospho-proteins by binding
and isomerizing the peptidyl-prolyl bond in phosphorylated
Ser/Thr-Pro motifs. Consequently, it has roles in a wide range of
cellular activities, since Ser/Thr-Pro motifs are also specific
phosphorylation sites on a number of protein kinases.
Pin1 expression has been identified to be positively
correlated with the expression of vascular endothelial growth
factor (VEGF), a key molecule involved in angiogenesis. Pin1 is
also known to indirectly regulate VEGF expression through the
isomerization of the hypoxia-inducible factor 1 (HIF-1) and
activating protein-1 (AP1) transcription factors (6). Furthermore, the overexpression of Pin1
in breast cancer cell lines has been demonstrated to lead to an
upregulation in VEGF and a consequent promotion of angiogenesis
during cancer progression (6). A
previous study also revealed that the stable inhibition of Pin1
using retrovirus-mediated small interfering RNA (siRNA) suppressed
cell growth, migration ability and angiogenesis in prostate cancer
cells (7). Pin1 dependency has been
identified to be a specific feature of cancer cells, which may be
important with respect to cancer cell selective treatments that
utilize Pin1-targeted drugs (7).
Juglone (also known as 5-hydroxy-1,4-naphthoquinone), which blocks
the function of Pin1, has emerged as an efficient candidate
inhibitor molecule. Juglone has been reported to selectively
inhibit Pin1 by irreversibly modifying its sulfhydryl groups,
whilst not affecting the function of other PPIase family members
(8). Juglone has also been used in
other studies, due to its selective inhibitory function of Pin1
(9,10).
The aim of the present study was investigate the
potential role of Pin1 in glioblastoma pathology. The expression
levels of VEGF and matrix metalloproteinase 9 (MMP9) were
investigated to determine the effects of Pin1 inhibition on the
cell growth, migration and angiogenic potential of glioblastoma
cells. In addition, the effect of Pin1 inhibition on the apoptotic
response of glioblastoma cells was also investigated. We
hypothesize that Pin1 may exhibit a crucial role in the
pathogenesis of glioblastoma and thus may present a novel potential
molecular target for the treatment of glioblastoma. Furthermore,
Pin1 inhibitory molecules, including Juglone or alternative
synthetic derivatives, may present promising therapeutic agents for
the treatment of glioblastoma and other tumor types that exhibit
similar pathological features.
Materials and methods
Cell cultures
U87-MG glioblastoma cells bearing epithelial
morphology (American Type Culture Collection, Manassas, VA, USA)
were used for all experiments, as the primary aim of the present
study was to elucidate the tumorigenic properties of glioblastoma
cells. The cells were plated into high-glucose Dulbecco's modified
Eagle's medium (DMEM) supplemented with 2 mM non-essential amino
acids and 10% fetal bovine serum (FBS) and maintained at 37°C in 5%
CO2.
RNA interference (RNAi) and juglone
treatment
Pin1 siRNA and non-targeting siRNA (GE Dharmacon,
Lafayette, CO, USA) fragments were used for knockdown. The
transfections were performed using DharmaFect Transfection
Reagent-1 (GE Dharmacon) according to the manufacturer's
instructions. Separately, for juglone treatment, 0.5 mM juglone
(EMD Millipore, Billerica, MA, USA) main stock solution was
prepared by first dissolving juglone in DMSO. The solution was then
brought up to the final volume with dH2O.
MTT assay
The human glioblastoma U87-MG cells were cultured in
DMEM supplemented with 2 mM non-essential amino acids, 10% FBS and
1% penicillin/streptomycin. The cells were treated with juglone,
Pin1 siRNA or non-targeting siRNA. Cell proliferation was measured
at different time-points using the Vybrant-MTT Cell Proliferation
Assay kit (Invitrogen Life Technologies, Carlsbad, CA, USA). All
experiments were performed three times in triplicate. A one-way
analysis of variance was performed in order to determine the
statistical significance of the differences between groups.
Microscopy and apoptosis analysis
The acridine orange/ethidium bromide vital staining
technique was used to determine the ratio of living to apoptotic
cells, as previously described (11).
The cells were incubated for 3 days with Pin1 siRNA or
non-targeting siRNA in order to observe knockdown at the protein
level.
Wound-healing assay
The U87-MG glioblastoma cells were plated in 12-well
plates and cultured for 48 h. A wound was made using the tip of a
pipette, as previously described (12). After 36 h, images of the wounds were
captured by light microscopy (CH30; Olympus Corporation, Tokyo,
Japan).
Western blotting
Protein isolation was performed using the Mammalian
Cell Extraction kit (BioVision, Inc., Milpitas, CA, USA). The
protein lysates were subjected to 12% SDS-polyacrylamide gel
electrophoresis and transferred to a nitrocellulose membrane. Next,
the membrane was blocked with 2.5% skimmed milk powder and 1%
bovine serum albumin, and then probed with the following primary
antibodies at a 1:500 dilution: Pin1 mouse polyclonal
immunoglobulin G (IgG; Santa Cruz Biotechnology, Inc., Dallas, TX,
USA), β-actin mouse polyclonal IgG (Santa Cruz Biotechnology, Inc.)
and MMP9 rabbit polyclonal IgG (Abcam, Cambridge, UK), in blocking
solution. The horseradish peroxidase (HRP)-conjugated secondary
antibodies (Promega Corporation, Madison, WI, USA) were used at
1:5,000 dilutions in blocking solution. Amersham ECL Plus Western
Blotting Detection Reagents (GE Healthcare Life Sciences, Chalfont,
UK) were used to obtain a signal following the HRP/luminogen
reaction.
Results
Pin1 inhibition decreases glioblastoma
cell growth and activates apoptosis in vitro
The overexpression and inhibition of Pin1 has
previously been associated with increased VEGF (6) and decreased MMP9 activity (13), respectively. Pin1 overexpression is
also associated with the promotion of certain tumorigenic features
that are shared by various tumor types (3). The present study also observed high
levels of Pin1, VEGF and MMP9 in the glioblastoma cells (Fig. 1). Pin1 has been associated with
essential cellular events that underlie tumorigenicity. Therefore,
it was hypothesized that the knockdown of Pin1 in glioblastoma
cells would inhibit certain pathological features that are
important for tumor cell survival. The effects of Pin1 inhibition
on glioblastoma cells were investigated (Fig. 1A). The results were consistent with
those of a previous study (7), which
identified reduced growth rates following Pin1-knockdown in
prostate cancer cell lines. In the present study, there was a
slight increase in cell growth on the final day of treatment with 5
µM juglone, but not with any other concentration. An increase in
the MMP9 protein level was also observed at the same concentration
of juglone. Speculatively, inhibition at this specific
concentration may trigger a survival response that results in an
increase in growth. However, the wound-healing assay revealed that
the overall number of cells was lower following Pin1 inhibition,
which is consistent with the reduction trend observed in the growth
assay.
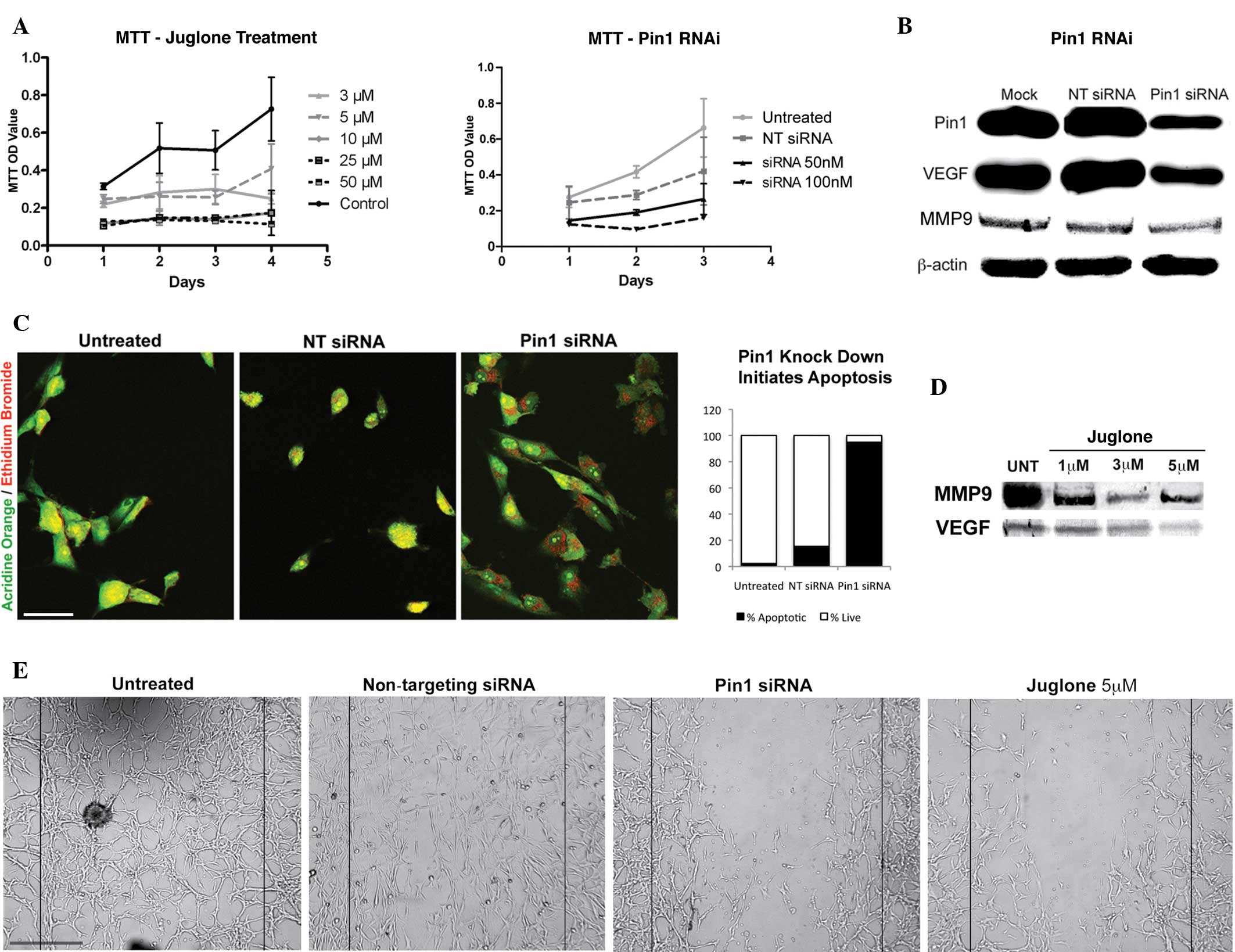 | Figure 1.(A) Graphs revealing the reduction in
proliferation caused by Pin1 siRNA and juglone in glioblastoma
cells in vitro. The difference between juglone-treated and
-untreated groups and siRNA-treated versus non-targeting siRNA or
untreated groups was significant (P<0.05). (B) Immunoblotting
revealing the reduction in the levels of Pin1, VEGF and MMP9 by
Pin1 siRNA. (C) Pin1 siRNA transfection activated apoptosis in the
glioblastoma cells, whereas the non-targeting siRNA-treated and
control cells were healthy. Acridine orange/ethidium bromide vital
staining reveals apoptotic cells with red and orange dots in the
perinuclear region, healthy cells in green and necrotic cells in
red. In total, 95% of the cells that were counted in the Pin1 siRNA
group were apoptotic (n=115) (scale bar, 200 µm). (D) A significant
reduction in VEGF and MMP9 levels observed following juglone
treatment is shown. (E) Wound healing assay revealing that Pin1
siRNA and juglone were effective at disrupting the migration
ability of glioblastoma cells (scale bar, 50 µm). Pin1,
peptidyl-prolyl cis/trans isomerase NIMA-interacting 1; siRNA,
small interfering RNA; VEGF, vascular endothelial growth factor;
MMP9, matrix metalloproteinase 9; RNAi, RNA interference; NT siRNA,
non-targeting siRNA; UNT, untreated; OD, optical density. |
The results of the apoptosis assay revealed an
increase in apoptosis following Pin1 inhibition (Fig. 1B and C). In total, >90% of the
glioblastoma cells transfected with Pin1 siRNA entered into
apoptosis, but not necrosis. By contrast, the apoptotic activity
following treatment with non-targeting siRNA was significantly
lower (Fig. 1C).
Inhibition of Pin1 decreases VEGF and
MMP9 levels
While the levels of Pin1 and VEGF were reduced in
the glioblastoma cells following siRNA-mediated Pin1-knockdown,
non-targeting siRNA had no marked effect on the levels of Pin1 and
VEGF (Fig. 1B). The present study
also demonstrated that Pin1 inhibition affected the migration and
wound-healing capacities of the glioblastoma cells. The results of
the wound-healing assay suggested that Pin1 may be an important
molecule involved in the process of tumor cell migration, and that
it may regulate MMP9 activity. Juglone treatment was also effective
in blocking the migration and wound-healing capacity of the cells.
Therefore, MMP9 protein levels were assessed following
juglone-mediated Pin1 inhibition (Fig.
1D). The results indicated a decrease in the level of MMP9,
which may explain the reduced wound-healing capacity of the cells
(Fig. 1E). A decrease in Pin1
following juglone treatment was also observed, which may be the
result of juglone-bound, non-functional Pin1 being targeted for
degradation in the cell.
Discussion
Pin1 is a novel regulator, which functions at the
center of a number of important signaling pathways that are vital
for cell growth, differentiation, division and survival (14,15). Pin1
is expressed at varying levels in different cell types and
pathological conditions, which indicates a function that is
fine-tuned to specific cell types. This makes Pin1 an attractive
target molecule, particularly in studies concerning cancer and
neurodegeneration. Furthermore, its overexpression in various human
cancers makes it an effective prognostic marker (16–18). It
has been established that multiple oncogenic signaling pathways
involved in tumorigenesis are affected by the overexpression of
Pin1 (7,17,19,20).
Therefore, based on its differential expression patterns, Pin1
inhibition has the potential to target continuously dividing
cancerous cells. Pin1-targeting agents, including juglone (8), aryl-indanyl ketones (21), D-phospho-Thr containing peptides
(22), and novel and less cytotoxic
Pin1 inhibitors, such as TME-001 (23), are potential candidate molecules that
could be further developed during the course of drug design
strategies. Notably, Pin1 has been identified to be downregulated
in neurodegeneration, and has been associated with abnormal cell
cycle re-entry in hippocampal neurons, eventually contributing to
the neurodegenerative pathology (24,25).
Therefore, Pin1 may be a potential target for cell type-specific
therapeutic strategies, as an adjuvant (10) or as a primary approach, that may have
clinical implications in cancer and neurodegeneration.
The present study revealed that the knockdown of
Pin1 altered certain tumorigenic features of glioblastoma cells
through the induction of apoptosis and a decrease in cell
proliferation, migration and wound-healing abilities. Following the
inhibition of Pin1, a significant reduction in the growth rate of
the glioblastoma cells was observed. In addition, knockdown of Pin1
significantly decreased the levels of VEGF and MMP9, which are
responsible for angiogenesis and metastasis, respectively. A number
of the currently available therapeutic agents used in cancer
treatment depend upon anti-VEGF strategies that target either VEGF
itself or its receptors (26). The
present study instead targeted the transcriptional regulation of
VEGF by inhibiting Pin1, since the molecular links between Pin1 and
VEGF expression are relatively clear. The VEGF promoter region
contains several binding sites for specific protein-1, and signal
transducer and activator of transcription-3, along with HIF-1 and
AP1 (27). AP1 is a transcriptional
complex that is activated by Pin1 (6). Pin1 has also been identified to activate
HIF-1 in an indirect manner by inactivating GSK3; a protein that
phosphorylates HIF-1 for degradation (28). Pin1-mediated transcriptional
activation of VEGF is known to depend upon AP1, since there are
three separate binding sites for AP1 on the VEGF promoter (29).
In addition to VEGF, the regulation of MMP9 was also
altered upon Pin1 inhibition in the present study. However, the
possible molecular links between Pin1 and MMP9 require further
elucidation. Previous studies have established that MMP9 expression
is associated with glioblastoma growth and invasion (30). Recently, it has been demonstrated that
the inhibition of Pin1 in colorectal carcinoma cells decreases the
level and enzymatic activity of MMP9, presumably via nuclear
factor-κB signaling (13). Similarly,
a reduction in MMP9 following Pin1 inhibition was observed in the
present study. This decrease may have lead to a reduction in the
migration and wound-healing capacity of the glioblastoma cells.
The molecular connections between Pin1 and cancer
were first suggested by a study that demonstrated the
overexpression of Pin1 in cancer tissues (18). Further studies confirmed the
widespread overexpression of Pin1 in 60 different tumor types
(3). The abundance of Pin1 in brain
tumors suggests that it may be involved in the pathogenesis of
glioblastoma. In addition, it has been demonstrated that the
overexpression of Pin1 in malignant gliomas inhibited the apoptosis
mediated by death-associated protein 6 (Daxx), an oxidative stress
responsive death-associated protein, which in turn lead to tumor
progression (29). Increasing
evidence has indicated that Daxx has a significant role in the
cellular apoptotic response induced by ultraviolet light, oxidative
stress and glucose deprivation (31–33). It
has been established that Pin1 binds to the
phospho-Ser178-Pro motif of the Daxx protein, and that
the overexpression of Pin1 results in the degradation of Daxx
through the ubiquitin-proteasome pathway (3). By contrast, a previous study revealed
that the inhibition of Pin1 in malignant gliomas increased
oxidative stress-induced cellular responses, and therefore
sensitized tumor cells to apoptosis through elevated Daxx levels
(34). The apoptotic response
observed in the present study following Pin1 inhibition may also be
associated with an alteration in this pathway, as well as the loss
of other aberrant oncogenic regulatory mechanisms induced by the
overexpression of Pin1.
At present, further elucidation of the role of Pin1
in cellular pathways in gliomas is important for the development of
effective treatment strategies. Through cell type-specific
targeting strategies, we believe that Pin1-targeted therapies will
hold promise for the treatment of glioblastoma.
Acknowledgements
This study was supported by the Brain Research
Society of Turkey/Pfizer Research Grant Award, 2009. Professor
Turker Kilic was supported by the Turkish Academy of Sciences. The
authors would like to thank Mr. Cemil Ozan Ceyhan for editing the
manuscript.
References
|
1
|
Ohgaki H and Kleihues P: Epidemiology and
etiology of gliomas. Acta Neuropathol. 109:93–108. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lu KP, Hanes SD and Hunter T: A human
peptidyl-prolyl isomerase essential for regulation of mitosis.
Nature. 380:544–547. 1996. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bao L, Kimzey A, Sauter G, Sowadski JM, Lu
KP and Wang DG: Prevalent overexpression of prolyl isomerase Pin1
in human cancers. Am J Pathol. 164:1727–1737. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Göthel SF and Marahiel MA: Peptidyl-prolyl
cis-trans isomerases, a superfamily of ubiquitous folding
catalysts. Cell Mol Life Sci. 55:423–436. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hunter T: Prolyl isomerases and nuclear
function. Cell. 92:141–143. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kim MR, Choi HS, Heo TH, Hwang SW and Kang
KW: Induction of vascular endothelial growth factor by
peptidyl-prolyl isomerase Pin1 in breast cancer cells. Biochem
Biophys Res Commun. 369:547–553. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ryo A, Uemura H, Ishiguro H, Saitoh T,
Yamaguchi A, Perrem K, Kubota Y, Lu KP and Aoki I: Stable
suppression of tumorigenicity by Pin1-targeted RNA interference in
prostate cancer. Clin Cancer Res. 11:7523–7531. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hennig L, Christner C, Kipping M,
Schelbert B, Rücknagel KP, Grabley S, Küllertz G and Fischer G:
Selective inactivation of parvulin-like peptidyl-prolyl cis/trans
isomerases by juglone. Biochemistry. 37:5953–5960. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kesavapany S, Patel V, Zheng YL, Pareek
TK, Bjelogrlic M, Albers W, Amin N, Jaffe H, Gutkind JS, Strong MJ,
Grant P and Pant HC: Inhibition of Pin1 reduces glutamate-induced
perikaryal accumulation of phosphorylated neurofilament-H in
neurons. Mol Biol Cell. 18:3645–3655. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mathur R, Chandna S, Kapoor NP and
Dwarakanath SB: Peptidyl prolyl isomerase, Pin1 is a potential
target for enhancing the therapeutic efficacy of etoposide. Curr
Cancer Drug Targets. 11:380–392. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mironova EV, Evstratova AA and Antonov SM:
A fluorescence vital assay for the recognition and quantification
of excitotoxic cell death by necrosis and apoptosis using confocal
microscopy on neurons in culture. J Neurosci Methods. 163:1–8.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ongusaha PP, Kwak JC, Zwible AJ, Macip S,
Higashiyama S, Taniguchi N, Fang L and Lee SW: HB-EGF is a potent
inducer of tumor growth and angiogenesis. Cancer Res. 64:5283–5290.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Qin L, Li M, Ren W, Zhang D, Zhang J,
Zhang Y and Cheng N: Silencing Pin1 suppresses the expression and
bioactivity of MMP-9 through NF-κB in colorectal carcinoma SW480
cells. Clin Oncol Cancer Res. 7:12–17. 2010. View Article : Google Scholar
|
|
14
|
Lu KP: Prolyl isomerase Pin1 as a
molecular target for cancer diagnostics and therapeutics. Cancer
Cell. 4:175–180. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Atkinson GP, Nozell SE, Harrison DK,
Stonecypher MS, Chen D and Benveniste EN: The prolyl isomerase Pin1
regulates the NF-kappaB signaling pathway and interleukin-8
expression in glioblastoma. Oncogene. 28:3735–3745. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ayala G, Wang D, Wulf G, Frolov A, Li R,
Sowadski J, Wheeler TM, Lu KP and Bao L: The prolyl isomerase Pin1
is a novel prognostic marker in human prostate cancer. Cancer Res.
63:6244–6251. 2003.PubMed/NCBI
|
|
17
|
Ryo A, Nakamura M, Wulf G, Liou YC and Lu
KP: Pin1 regulates turnover and subcellular localization of
beta-catenin by inhibiting its interaction with APC. Nat Cell Biol.
3:793–801. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wulf GM, Ryo A, Wulf GG, Lee SW, Niu T,
Petkova V and Lu KP: Pin1 is overexpressed in breast cancer and
cooperates with Ras signaling in increasing the transcriptional
activity of c-Jun towards cyclin D1. EMBO J. 20:3459–3472. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liou YC, Ryo A, Huang HK, Lu PJ, Bronson
R, Fujimori F, Uchida T, Hunter T and Lu KP: Loss of Pin1 function
in the mouse causes phenotypes resembling cyclin D1-null
phenotypes. Proc Natl Acad Sci USA. 99:1335–1340. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ryo A, Liou YC, Lu KP and Wulf G: Prolyl
isomerase Pin1: a catalyst for oncogenesis and a potential
therapeutic target in cancer. J Cell Sci. 116:773–783. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Daum S, Erdmann F, Fischer G, Féaux de
Lacroix B, Hessamian-Alinejad A, Houben S, Frank W and Braun M:
Aryl indanyl ketones: efficient inhibitors of the human peptidyl
prolyl cis/trans isomerase Pin1. Angew Chem Int Ed Engl.
45:7454–7458. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang Y, Daum S, Wildemann D, Zhou XZ,
Verdecia MA, Bowman ME, Lücke C, Hunter T, Lu KP, Fischer G and
Noel JP: Structural basis for high-affinity peptide inhibition of
human Pin1. ACS Chem Biol. 2:320–328. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mori T, Hidaka M, Lin YC, Yoshizawa I,
Okabe T, Egashira S, Kojima H, Nagano T, Koketsu M, Takamiya M and
Uchida T: A dual inhibitor against prolyl isomerase Pin1 and
cyclophilin discovered by a novel real-time fluorescence detection
method. Biochem Biophys Res Commun. 406:439–443. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lee TH, Pastorino L and Lu KP:
Peptidyl-prolyl cis-trans isomerase Pin1 in ageing, cancer and
Alzheimer disease. Expert Rev Mol Med. 13:e212011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Atabay KD and Karabay A: Pin1 inhibition
activates cyclin D and produces neurodegenerative pathology. J
Neurochem. 120:430–439. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sun J, Blaskovich MA, Jain RK, et al:
Blocking angiogenesis and tumorigenesis with GFA-116, a synthetic
molecule that inhibits binding of vascular endothelial growth
factor to its receptor. Cancer Res. 64:3586–3592. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Xie K, Wei D, Shi Q and Huang S:
Constitutive and inducible expression and regulation of vascular
endothelial growth factor. Cytokine Growth Factor Rev. 15:297–324.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Flügel D, Görlach A, Michiels C and
Kietzmann T: Glycogen synthase kinase 3 phosphorylates
hypoxia-inducible factor 1alpha and mediates its destabilization in
a VHL-independent manner. Mol Cell Biol. 27:3253–3265. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chabannes E, Fauconnet S, Bernardini S,
Wallerand H, Adessi G and Bittard H: Protein kinase C signalling
pathway is involved in the regulation of vascular endothelial
growth factor expression in human bladder transitional carcinoma
cells. Cell Signal. 13:585–591. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Choe G, Park JK, JoubenSteele L, Kremen
TJ, Liau LM, Vinters HV, Cloughesy TF and Mischel PS: Active matrix
metalloproteinase 9 expression is associated with primary
glioblastoma subtype. Clin Cancer Res. 8:2894–2901. 2002.PubMed/NCBI
|
|
31
|
Zhong S, Salomoni P, Ronchetti S, Guo A,
Ruggero D and Pandolfi PP: Promyelocytic leukemia protein (PML) and
Daxx participate in a novel nuclear pathway for apoptosis. J Exp
Med. 191:631–640. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Perlman R, Schiemann W, Brooks MW, Lodish
HF and Weinberg RA: TGF-beta-induced apoptosis is mediated by the
adapter protein Daxx that facilitates JNK activation. Nat Cell
Biol. 3:708–714. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
33
|
Salomoni P and Khelifi A: Daxx: death or
survival protein? Trends Cell Biol. 16:97–104. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ryo A, Hirai A, Nishi M, Liou YC, Perrem
K, Lin SC, Hirano H, Lee SW and Aoki I: A suppressive role of the
prolyl isomerase Pin1 in cellular apoptosis mediated by the
death-associated protein Daxx. J Biol Chem. 282:36671–36681. 2007.
View Article : Google Scholar : PubMed/NCBI
|















