Introduction
Trichoepitheliomas (TEs) are benign cutaneous
neoplasms derived from the hair follicle. Three distinctive
variants of TE are recognized, namely, solitary TE, multiple TE and
desmoplastic TE (DTE) (1). DTE is a
rare benign adnexal tumor that is derived from basal cells in the
outer root sheath of the hair follicle. The tumor occurs at an
incidence of 1 in 5,000 skin biopsies in adults, and is usually
observed in middle-aged females, but has been reported in all age
groups and genders. DTE usually presents as an asymptomatic,
flesh-colored, solitary, annular, indurated and centrally depressed
papule or plaque (2,3). The most commonly affected areas are the
sun-exposed areas, particularly facial areas such as the cheeks,
chin and forehead; less commonly, the tumors may be localized to
the upper trunk area, the neck and the scalp (4).
The history of DTE goes back to 1904 when Hartzell
described benign cystic epithelioma, which was clinically similar
to DTE (5). In 1977, using a series
of 49 cases, Brownstein and Shapiro (6) described the microscopic features of DTE,
and histologically noted narrow strands of basaloid tumor cells,
keratinous cysts and a desmoplastic stroma. Since then, these
features have remained as a unique triad for the dermatopathology
of DTE.
DTE lesions are usually superficial and rarely reach
the lower dermis. The tendency for perineural and intraneural
invasion, such as has been found in other cutaneous malignancies,
has rarely been described in the previous literature. According to
these studies, there is no pleomorphism, mitotic figures or
apoptotic activity in the epithelium, which morphologically
resembles the main tumor. Therefore, it is clear that DTE should
also be listed with other cutaneous neoplasms showing perineural
involvement, particularly the desmoplastic malignancies, and that
care should be employed in following any aggressive treatment
approach, much like for other cutaneous malignancies, particularly
when lesions are located in cosmetically sensitive areas.
Immunohistochemical studies reveal the consistent expression of
cytokeratin (CK)20 (7) for Merkel
cells surrounded by stromal cells.
Clinical features and histopathological features may
aid in the diagnosis of DTE (8). The
most common features of DTE are that the tumor is slow-growing,
white-gray to flesh-colored, indurated, centrally depressed,
non-ulcerated and 2–18 mm in diameter (9). The tumor has an annular border, is
present as a papule or plaque, and predominately occurs on the
face. Diagnosis based only on the clinical background may be
difficult is certain cases, and for those cases, biopsy may be of
use. The lesion is usually well circumscribed, symmetrical and
confined to the papillary dermis and upper two-thirds of the
reticular dermis. The presence of narrow strands of epithelial
basaloid cells, numerous horn cysts, a dense fibrous stroma, a
foreign body-type granulomatous reaction, calcification and osteoma
may serve as diagnostic characteristics (10).
Clinically, DTE is difficult to distinguish from
other skin lesions caused by certain diseases, such as breast
cancer. In this study, three cases of DTE were presented and the
associated literature was reviewed. The aim of the study was to
improve understanding with regard to the clinical diagnosis and
treatment of DTE. Written consent was obtained from each patient or
their relatives for publication of this study.
Case reports
Case one
In January 2009, a 45-year-old male presented to the
Second Affiliated Hospital of Xi'an Jiatong University (Xi'an,
China) with an asymptomatic flesh-colored plaque below the right
edge of the outer canthus that had been apparent for seven years.
The lesion had first become apparent at 38 years old, when the
patient was injured by a piece of iron that left a small scar below
the right edge of the outer canthus. There was no pain, itching or
any associated symptoms, so no further management was provided.
However, in the last seven years, the lesion slowly and
progressively increased in size without any symptoms or known
cause.
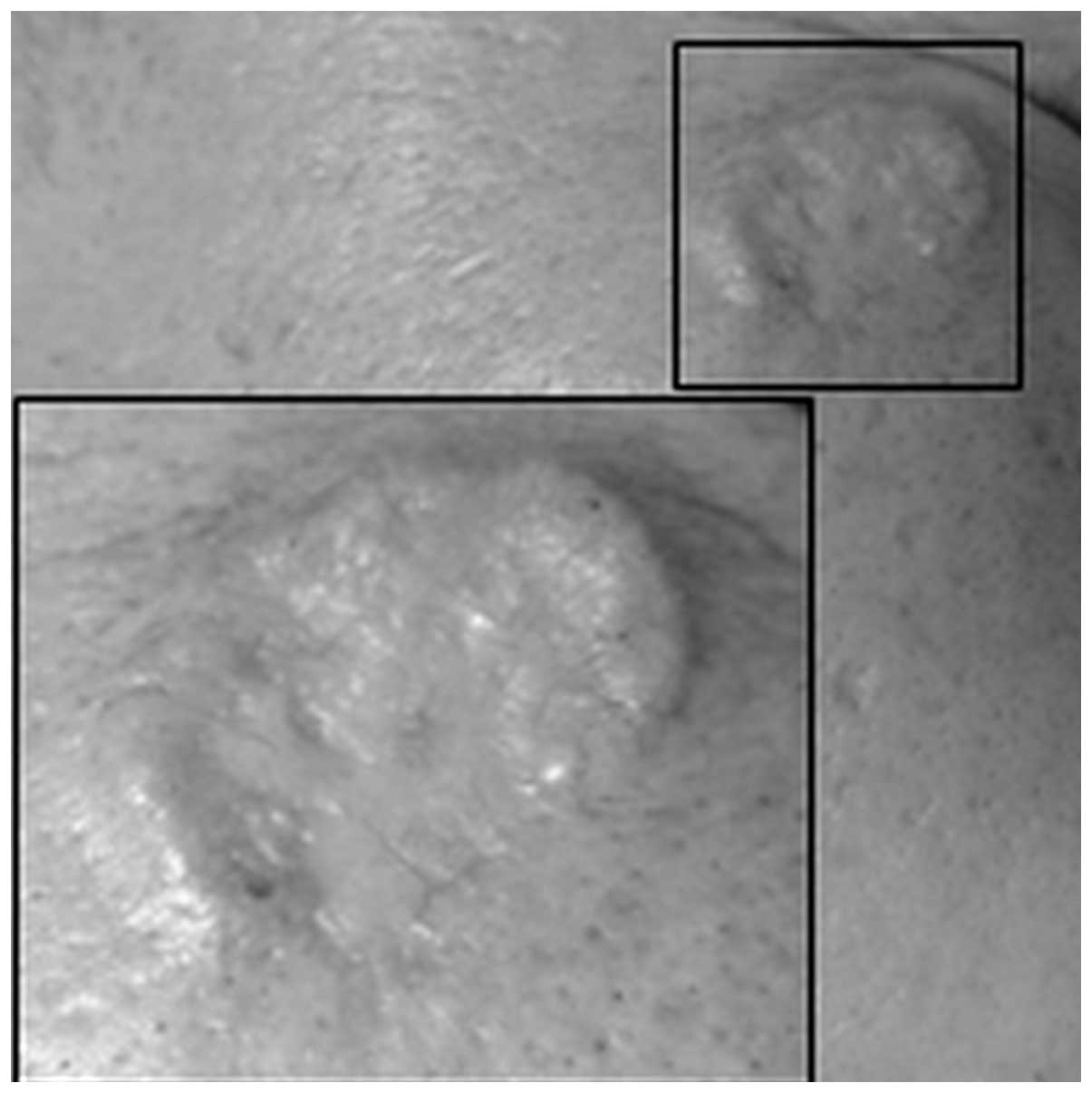
Dermatological examination of the lesion revealed it
to be flesh-colored and centrally depressed, with elevated borders
of ~15 mm in size, and located laterally over the right periorbital
region. The lesion was not ulcerated (Fig. 1). The patient's eating habits, bowel
habits and urination were normal. There was no family history of
similar disease and no associated symptoms. Upon histological
examination, nests of small strands and cords of epithelial
elements were observed within the upper and mid dermis. The chords
and nests of basaloid cells varied in size and were embedded in a
dense stroma. These aggregations were rimmed by bundles of collagen
fibers. Multiple horn cysts were also apparent in the stroma and
were lined by stratified squamous epithelium. A thin drag line
elicited by focal calcification was also observed. Mitotic figures
were not apparent. Pleomorphism and peripheral palisading were not
observed. By reviewing the histopathological findings and
correlating these with the clinical findings, a diagnosis of DTE
was established (Fig. 2). The
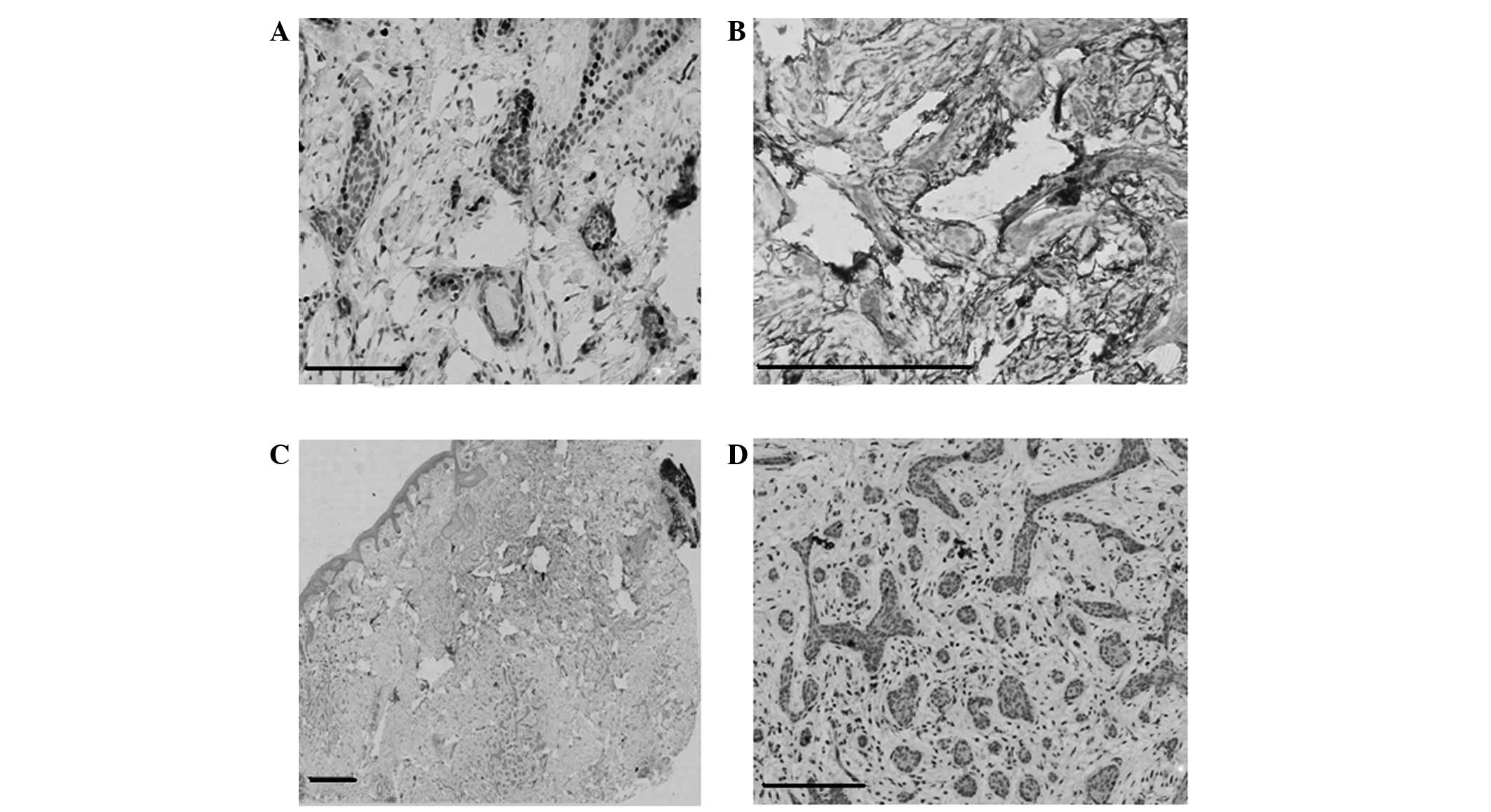
immunohistochemistry revealed CK20-positive cells (Fig. 3A) diffusely scattered within strands
of the tumor, including the wall of a horn cyst, and cluster of
differentiation (CD)34-positive cells (Fig. 3B) surrounding the tumor mass.
Immunohistochemistry for epithelial membrane antigen (EMA; Fig. 3C) and androgen receptor (AR; Fig. 3D) expression was negative. The patient
underwent surgery with complete resectioning with no
recurrence.
Case two
In October 2010, a 23-year-old female presented to
the Second Affiliated Hospital of Xi'an Jiatong University with an
asymptomatic skin lesion that had been apparent for nine years and
was slowly and progressively increasing in size. There was no
relevant medical history of any disease and no family history of a
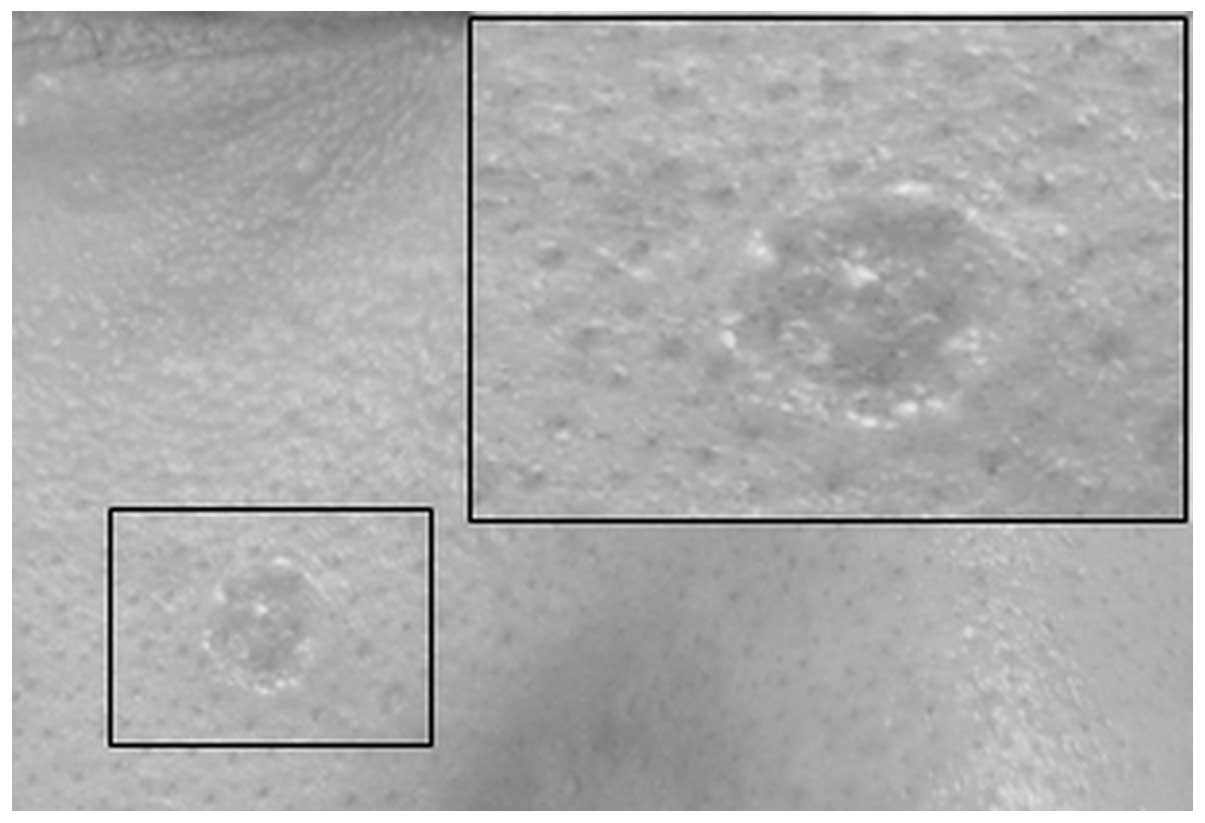
similar case. Examination of the lesion revealed a small,
flesh-colored, centrally depressed, bean-sized single lesion
located on the right cheek, with raised borders (Fig. 4). A systemic examination was
performed, but did not reveal any regional lymphadenopathy or
cutaneous abnormalities. The patient's eating habits, bowel habits
and urination were normal. A biopsy specimen obtained from the site
revealed small strands of basaloid cells in a desmoplastic stroma,
with keratinaceous cysts positioned adjacently and attached to the
basaloid cells. Following a review of the clinicohistopathological
findings, a diagnosis of DTE was established (Fig. 5).
The immunohistochemistry revealed the presence of a
few CK20-positive cells (Fig. 6A)
scattered within the strands of the tumor. CD-34-positive cells
(Fig. 6B) surrounded the tumor mass.
Immunohistochemistry for EMA (Fig.
6C) and AR (Fig. 6D) was
negative, while B-cell lymphoma (Bcl)-2 expression (Fig. 6E) was weakly positive in the basal
layer. The patient underwent surgery with complete resectioning
with no recurrence.
Case three
In November 2012, a 26-year-old female presented to
the Second Affiliated Hospital of Xi'an Jiatong University with a
hard yellowish-white plaque, which had gradually grown over three
years and formed a rectangular, 3×4-cm patch (Fig. 7) on the tip of the left brow, without
evident cause or subjective symptoms. There was no relevant medical
history of any disease and no family history of a similar case.
Systemic and laboratory examinations revealed no abnormalities.
Pathological examination was performed; the hematoxylin and eosin
staining of the biopsy specimen is shown in Fig. 8, which revealed mild atrophy of the
epidermis, with large cords identified in the shallow and middle
dermis. In addition, hyperplasia of the connective tissue was
observed at the horn cyst. The patient underwent surgery with
complete resectioning with no recurrence.
Discussion
DTE is a relatively rare, benign, cutaneous
neoplasm, whose microscopical and histological features were first
described by Brownstein and Shapiro in 1977 (6). The lesion has been reported in previous
studies throughout the literature as several different entities,
including solitary TE, epithelioma adenoides cysticum, morphea-like
epithelioma and sclerosing epithelial hamartoma (11,12). DTE
usually presents as a slow-growing, asymptomatic, solitary,
indurated plaque or papule. The lesion has a raised annular border
and depressed non-ulcerating center. DTE most commonly occurs on
sun-exposed areas, particularly the face, and is often mistaken for
a basal cell carcinoma (BCC) in older patients. Moreover, DTE is
believed to occur more on the right side of the face, with the most
common site being the cheek, followed by the nose, chin, forehead,
periorbital region and lips (2,6). Females
are more commonly affected than males, with occurrences in the age
range of 8–81 years (13).
On histopathological examination, the lesion is
usually well circumscribed, symmetrical and confined to the
papillary dermis and upper two-thirds of the reticular dermis
(9). The three cases examined within
the present study exhibited the typical clinical and
histopathological features of DTE that have been described by
previous studies (4,5,10). The
depressed, non-ulcerating, and raised angular border that is
described in previous studies (4,5,10) was a typical feature in the present
cases. The triad of histopathological characteristics first
described by Brownstein and Shapiro (6), i.e., narrow strands of basaloid tumor
cells, keratinous cysts and a desmoplastic stroma, was a consistent
presence in all three of the current cases. Another feature of DTE
is the presence of horn cysts and frequent calcification. In the
present study, multiple horn cysts and focal calcifications were
observed on all cases. There were no signs of pleomorphism, mitotic
figures or apoptotic activity in the epithelium. Immunological
markers were studied in two cases. Each of the cases showed CK20
expression, however, in case one, numerous cells expressed strong
CK20 expression compared with a few cells expressing CK20 and
multiple cells weakly expressing CK20 in case two. Bcl-2 was also
mainly expressed in the basal layers. EMA expression was negative,
while CD34 expression was positive around the tumor mass.
The diagnosis of DTE may occasionally be difficult,
even when assessed by an expert, and particularly when the tumor
mimics other benign and malignant tumors. DTE may clinically and
histopathologically mimic syringoma, morpheaform BCC (MBCC),
microcystic adnexal carcinoma (MAC), conventional TE and other
tumors. While histology findings combined with clinical features
may be useful in making a definitive diagnosis of some of these
lesions (14,15), MBCC may still be misdiagnosed. Other
frequently associated conditions that may mimic similar clinical
and histological features are sebaceous hyperplasia, granuloma
annulare, scar tissue and cutaneous squamous cell carcinoma
(16). The features of DTE, as
aforementioned, may be extremely hard to distinguish when provided
with a small sample of biopsy specimen. Also, as the majority of
the biopsy specimens may be superficial, it may be hard to make a
full pathological evaluation. Certain studies have suggested that
the thin-walled epidermis and the lack of surface telangiectasias,
along with the aforementioned features, can aid in the diagnosis of
DTE (2,10). However, differentiating DTE from BCC
and MAC remains challenging. Takei et al (17) discovered that the majority of
clinically diagnosed DTE cases were actually BCC. Other lesions in
their series were sebaceous hyperplasias, hamartoma, TE,
melanocystic nevi and keratoses.
Several attempts have been made to overcome those
diagnostic challenges in ultrastructural pathology (14), molecular pathology (18), immunofluorescence (19) and immunohistochemical (17,20–23)
studies. Immunohistochemical markers that have been proposed as
criteria for the diagnosis and differentiation between DTE and MBCC
are CK20 (for Merkel cells), p53, p75, CD10, CD34, PHLDA, AR, Ki-67
and Bcl-2. Costache et al (18) investigated several immunohistological
markers, namely CK20, AR, Ki-67, CD34, p53, Bcl-2 and CD10. The
study attempted to re-evaluate the histomorphological and
immunohistochemical criteria previously proposed by Takei et
al (17), which were available
for the differentiation of DTE from MBCC, in order to figure out
which of the criteria are the most reliable for a definitive
diagnosis. The study found that CK20 and AR are the most reliable
immunohistochemical markers for the differentiation between DTE and
MBCC, supporting the data of previous studies by Abesamis-Cubillan
et al (22) and Izikson et
al (23). However, the study also
suggested that the number of Merkel cells in DTE may vary from a
large number to very few or even one. Therefore, relying on CK20
for small biopsy specimens may remain problematic, particularly in
view of the ever decreasing size of skin biopsies and the increased
use of the shave biopsy technique, which often yields only
superficial small specimens. Costache et al (20) further concluded that Ki-67 and Bcl-2
were not useful markers in the differentiation between DTE and
MBCC.
Although DTE may be diagnosed using the clinical and
morphological features alone, in certain cases, this may be
challenging for a dermatologist. DTE may resemble other benign and
malignant tumors clinically and histologically, including MBCC,
MAC, cutaneous metastatic breast cancer and syringoma (9,24).
Syringoma is a benign, adnexal neoplasm with ductal
differentiation. The condition usually presents as small, multiple,
skin-colored papules over the cheeks and lower eyelids. The lesions
are usually asymptomatic and tend to first appear at puberty. The
histopathological features of syringoma show multiple eccrine
ducts, which are lined by two layers of cuboidal epithelium and are
scattered within a fibrous stroma in the dermis. The main
histopathological differential diagnosis includes MBCC, MAC,
eccrine syringocarcinoma and DTE. Syringoma, unlike DTE, is often
observed as multiple lesions on the periorbital region. The lesions
are generally confined to the superficial dermis and consist of
tubular structures (2). All these
features are absent in DTE. Narrow strands of basaloid cells,
foreign body granulomas and calcification may rarely be observed in
syringoma, but may frequently be found in DTE (25). DTEs also tend to be solitary and lack
the ductal differentiation observed in syringomas. Additionally,
the presence of horn cysts, calcification, follicular
differentiation and long epithelial strands can distinguish DTE
from syringoma, where these features are not commonly observed.
Moreover, immunohistochemical markers may also aid in
differentiating between DTE and syringoma. The immunohistochemical
CK20 marker for Merkel cells is nearly always immunopositive for
DTE, but rarely or never for syringoma (7). DTE is also negative for carcinoembryonic
antigen (26,27) compared with syringoma, which shows a
positive reaction in the luminal cells. The most common features
that may aid in differentiating DTE from syringoma are listed in
Table I.
 | Table I.Major features for differentiating
between DTE and syringoma. |
Table I.
Major features for differentiating
between DTE and syringoma.
| Features | DTE | Syringoma |
|---|
| Narrow strands of
tumor cells | Constant | Unusual |
| Hard, annular
lesions | Typical | Rare |
| Horn cysts | Constant | Rare |
| Solitary
tumors | Common | Rare |
| Epidermal
hyperplasia | Common | Rare |
| Ductal
differentiation | Rare | Common |
| Foreign body
granuloma | Frequent | Rare |
| Calcification | Frequent | Rare |
| Periorbital
involvement | Rare | Common |
| Immunohistochemical
markers |
|
|
|
CK20 | Strong
positive | Rarely
positive |
|
Carcinoembryonic antigen | Negative | Positive |
In the majority of cases, cutaneous metastasis
occurs following the initial diagnosis of the primary cancer. In
the minority of cases, metastasis may be discovered at the same
time or prior to the diagnosis of carcinoma. Breast cancer is one
of the most common malignancies to spread to the skin (28,29). It is
estimated that 30% of breast cancers have the tendency to
metastasize. Although the most common sites of cutaneous metastatic
breast cancer are the chest and abdomen, metastasis can less
commonly be discovered on the scalp, face, neck, upper extremities,
abdomen and back. Patients may present with rapidly-growing,
asymptomatic, firm, scar-like nodules or tumors on the face, which
may mimic DTE (30). Any
rapidly-growing lesions should warrant careful consideration of the
possibility of metastasis. The most common features that may aid in
differentiating between DTE and cutaneous metastatic breast cancer
are listed in Table II.
 | Table II.Major features for differentiating
between DTE and cutaneous metastastic breast cancer. |
Table II.
Major features for differentiating
between DTE and cutaneous metastastic breast cancer.
| Features | DTE | Cutaneous
metastatic breast cancer |
|---|
| Chest
involvement | No | Common |
| Annular
lesions | Typical | No |
| Horn cysts | Common | Rare |
| Narrow strands of
basaloid cells | Constant | Rare |
| Large masses of
tumor cells | Never | Common |
| Epidermal
hyperplasia | Common | Rare |
| Keratin granulomas
and calcification | Common | Rare |
| Cellular
atypia | Never | Common |
MBCC, also known as sclerosing BCC is a rare BCC
variant that exhibits aggressive characteristics and an atypical
clinical presentation. The preponderance of BCCs are nodular or
superficial. MBCC is considered a potentially more aggressive
subtype necessitating complete surgical excision, as opposed to the
benign nature of DTE (31).
MBCC presents as solitary, yellowish or
skin-colored, pale, firm, ill-defined, waxy or scar-like, flat or
slightly depressed lesions, which resemble numerous other benign
lesions, such as DTE. The head and neck regions, particularly the
face and less so the trunk and limbs, are the most frequently
affected by MBCC (32).
MBCC may clinically and histologically mimic DTE.
Since the two tumors are each composed of follicular germinative
cells, numerous morphological characteristics are common between
them. There is significant overlap between DTE and MBCC (21). Differentiating between these two
neoplasms may be challenging for anyone. Like DTE, MBCC also
consists of infiltrating strands and islands of basaloid and
monomorphic cells embedded within a dense fibrous and sclerotic
stroma.
DTE and MBCC may be differentiated between using
five distinct clinicohistopathological findings (21): Annular lesions, horn-cysts, epidermal
hyperplasia, Keratin granulomas and calcification. The large masses
of tumor cells that are often encountered in MBCC are rarely found
in DTE (21). A number of the common
features that may aid in differentiating between DTE and MBCC are
listed in Table III.
 | Table III.Features for differentiating between
DTE and MBCC. |
Table III.
Features for differentiating between
DTE and MBCC.
| Features | DTE | MBCC |
|---|
| Symmetry | Often
symmetrical | Often
asymmetrical |
| Annular lesion |
Characteristics | Rare |
| Horn cyst | Always present | Very rare |
| Ulceration | Rare | Common |
| Depression in the
center | Common | Uncommon |
| presense of larger
aggregations | Uncommon | Common |
| Rims of collagen
bundles | Constant | Less common |
| Small strands of
epithelial elements | Frequent | Less frequent |
| Calcification | Common | Uncommon |
| Follicular,
sebaceous, infundibular differentiation | Common | Uncommon |
| Clefts between
aggregations and stroma | Rare | Often |
| Mitotic
figures | Rare | Frequent |
| Cut artefacts | Common | Uncommon |
| Granulomatous
inflammation | Frequently
observed | Infrequently
seen |
| Solar elastosis
below the lesion | Rare | Common |
| Immunohistochemical
markers |
|
|
|
CK20 | Strongly
positive | Negative |
| AR | Rare | Common |
MAC is a rare adnexal neoplasm that normally occurs
on the head and neck region, particularly the central face
(33). MAC clinically presents as a
slow-growing, firm, flesh-colored and indurated plaque or nodule,
with diffuse, ill-defined margins, occasionally with overlying
telangiectasia. MAC is large, poorly circumscribed and asymmetric,
and extends into the subcutaneous fat. The neoplasm consists
predominantly of proliferating tubular structures (34).
While DTE is a benign neoplasm with indolent
behavior, MAC can be highly aggressive, resulting in substantial
local destruction and possible metastasis. Although MAC has widely
been recognized as a discrete clinicopathological entity, confusion
with other benign adnexal tumors, particularly DTE, remains likely
(18). Superficial biopsies result in
the misdiagnosis of MAC as squamous cell carcinoma, syringoma or
DTE in up to 30% of cases. A number of the features (21) that may aid in differentiating between
DTE and MAC are listed in Table
IV.
 | Table IV.Features for differentiating between
DTE and MAC. |
Table IV.
Features for differentiating between
DTE and MAC.
| Features | DTE | MAC |
|---|
| Symmetry | Symmetrical | Asymmetrical |
| Ductal
structures | Infrequent | Frequent |
| Intramuscular,
perichondral and perineural involvement | Uncommon | Common |
| Circumscribe | Well
circumscribed | Poorly
circumscribed |
| Infilteration | Confined to the
papillary dermis and the upper two-thirds of the reticular
dermis | Extending beyond
the reticular dermis |
Immunohistochemical markers have also been studied
to aid in differentiating between DTE and MAC. These markers
include CK20, CK7, CK15, CD10 and BerEP4 (20). However, none of the proposed
immunohistochemical markers are believed to be totally reliable.
Debate remains over the reliability of the markers (23,35). As a
consequence, further studies are required in order to find a
definitive diagnostic marker able to differentiate between MAC and
DTE.
Several approaches, including laser surgery,
dermabrasion, topical 5% imiquimod (14), curettage and electrodesiccation, and
radiosurgical ablation, have been attempted with some success, but
the chances of recurrence for these techniques may be higher than
that for local surgical excision (3,14,36). It is also important to consider that
the majority of biopsy specimens obtained during these methods may
only be superficial, resulting in a poor pathological evaluation.
Moreover, these techniques may not permit histological margin
analysis and may not be appropriate for high-risk tumors resembling
DTE. Local surgical excision is the treatment of choice for DTE and
is considered as a first-line treatment for the majority of benign
tumors. Although complete remission with minimal recurrence can be
achieved with this technique, post-surgical complications,
including scarring and hypopigmentation, remain the main problem,
particularly for the cosmetically sensitive areas such as the face,
where minimizing the occurrence of any complications is extremely
important. The study by Mamelak et al (2) recommends Mohs micrographic surgery for
treating DTE, in order to prevent recurrence and local invasion. A
review of the literature shows that certain studies agree on the
fact that aggressiveness and local invasion for DTE is extremely
rare (37,38). Moreover, Mohs micrographic surgery is
relatively expensive compared with other alternative or surgical
modalities (39). For classical cases
with definitive benign results, DTE can be cost-effectively managed
by closed monitoring only, with regular follow-ups or local
excision if required. However, in cases with atypical clinical and
histological features and where there is concern about the tumor
arising in cosmetically sensitive areas like the face, in which the
sparing of normal surrounding tissue is important, Mohs surgery may
be beneficial (38).
From the results of the present study and the
previous literature, it may be concluded that DTE is a particularly
rare benign adnexal tumor. The treatment of choice is local
excision, but a ‘wait and watch’ policy can be used as a management
technique in those cases where the clinical features are typical to
DTE. For a tumor as rare as DTE, the data for recurrence is not
reliable; therefore, the specific recurrence rate cannot be
reliably calculated. The tumor has been shown to share a number of
clinicohistopathological similarities with MBCC and MAC. Although
histopathological and immunohistochemical markers may aid in the
differentiation of other malignant tumors, specific diagnostic
techniques for the differentiation of this tumor are still lacking.
While the majority of cases may be left untreated, the diagnosis
and differentiation of DTE remains essential, as the treatment and
prognosis of other tumors mimicking DTE is different. Overall, the
low incidence of DTE limits the histopathological and
immunohistochemical observations, and the treatment studies that
may be performed.
References
|
1
|
D'Souza M, Garg BR, Ratnakar C and Agrawal
K: Multiple trichoepitheliomas with rare features. J Dermatol.
21:582–585. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mamelak AJ, Goldberg LH, Katz TM, Graves
JJ, Arnon O and Kimyai-Asadi A: Desmoplastic trichoepithelioma. J
Am Acad Dermatol. 62:102–106. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Moynihan GD, Skrokov RA, Huh J, Pardes JB
and Septon R: Desmoplastic trichoepithelioma. J Am Acad Dermatol.
64:438–439. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Brichta RF and Feldman BD: Multiple
flesh-colored facial papules. Multiple trichoepitheliomas. Arch
Dermatol. 126:953–956. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lazorik FC and Wood MG: Multiple
desmoplastic trichoepitheliomas. Arch Dermatol. 118:361–362. 1982.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Brownstein MH and Shapiro L: Desmoplastic
trichoepithelioma. Cancer. 40:2979–2986. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Katona TM, Perkins SM and Billings SD:
Does the panel of cytokeratin 20 and androgen receptor antibodies
differentiate desmoplastic trichoepithelioma from
morpheaform/infiltrative basal cell carcinoma? J Cutan Pathol.
35:174–179. 2008.PubMed/NCBI
|
|
8
|
López-Navarro N, Alcaide A, Gallego E,
Herrera-Acosta E, Castillo R and Herrera E: Dermatoscopy in the
diagnosis of combined desmoplastic trichoepithelioma and naevus.
Clin Exp Dermatol. 34:e395–e396. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Khelifa E, Masouyé I, Kaya G and Le Gal
FA: Dermoscopy of desmoplastic trichoepithelioma reveals other
criteria to distinguish it from basal cell carcinoma. Dermatology.
226:101–104. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ardigo M, Zieff J, Scope A, et al:
Dermoscopic and reflectance confocal microscope findings of
trichoepithelioma. Dermatology. 215:354–358. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Steigleder GK: Solitary trichoepithelioma.
Hautarzt. 23:323–324. 1972.(In German). PubMed/NCBI
|
|
12
|
Verma KC and Chaudhry SD: Epithelioma
adenoides cysticum (trichoepithelioma). Indian J Med Sci.
27:627–629. 1973.PubMed/NCBI
|
|
13
|
Abbas O, Richards JE and Mahalingam M:
Fibroblast-activation protein: a single marker that confidently
differentiates morpheaform/infiltrative basal cell carcinoma from
desmoplastic trichoepithelioma. Mod Pathol. 23:1535–1543. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yamamoto O, Hamada T, Doi Y, Sasaguri Y
and Hashimoto H: Immunohistochemical and ultrastructural
observations of desmoplastic trichoepithelioma with a special
reference to a morphological comparison with normal apocrine
acrosyringeum. J Cutan Pathol. 29:15–26. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mitcov M, Scrivener Y and Cribier B:
Desmoplastic trichoepithelioma: a clinicopathological study,
including a comparison with morpheiform basal cell carcinoma. Ann
Dermatol Venereol. 136:501–507. 2009.(In French). View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sumithra S, Jayaraman M and Yesudian P:
Desmoplastic trichoepithelioma and multiple epidermal cysts. Int J
Dermatol. 32:747–748. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Takei Y, Fukushiro S and Ackerman AB:
Criteria for histologic differentiation of desmoplastic
trichoepithelioma (sclerosing epithelial hamartoma) from
morphea-like basal-cell carcinoma. Am J Dermatopathol. 7:207–221.
1985. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tse JY, Nguyen AT, Le LP and Hoang MP:
Microcystic adnexal carcinoma versus desmoplastic
trichoepithelioma: a comparative study. Am J Dermatopathol.
35:50–55. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mizutani Y, Iwamoto I, Kanoh H, Seishima M
and Nagata K: Expression of drebrin, an actin binding protein, in
basal cell carcinoma, trichoblastoma and trichoepithelioma. Histol
Histopathol. 29:757–766. 2014.PubMed/NCBI
|
|
20
|
Costache M, Bresch M and Böer A:
Desmoplastic trichoepithelioma versus morphoeic basal cell
carcinoma: a critical reappraisal of histomorphological and
immunohistochemical criteria for differentiation. Histopathology.
52:865–876. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sellheyer K, Nelson P, Kutzner H and Patel
RM: The immunohistochemical differential diagnosis of microcystic
adnexal carcinoma, desmoplastic trichoepithelioma and morpheaform
basal cell carcinoma using BerEP4 and stem cell markers. J Cutan
Pathol. 40:363–370. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
AbesamisCubillan E, El-Shabrawi-Caelen L
and LeBoit PE: Merked cells and sclerosing epithelial neoplasms. Am
J Dermatopathol. 22:311–315. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Izikson L, Bhan A and Zembowicz A:
Androgen receptor expression helps to differentiate basal cell
carcinoma from benign trichoblastic tumors. Am J Dermatopathol.
27:91–95. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Vorechovský I, Undén AB, Sandstedt B,
Toftgård R and Ståhle-Bäckdahl M: Trichoepitheliomas contain
somatic mutations in the overexpressed PTCH gene: support for a
gatekeeper mechanism in skin tumorigenesis. Cancer Res.
57:4677–4681. 1997.PubMed/NCBI
|
|
25
|
Ahmed M: Cutaneous metastases from breast
carcinoma. BMJ Case Rep. 2011:bcr06201143982011.PubMed/NCBI
|
|
26
|
Wick MR, Cooper PH, Swanson PE, Kaye VN
and Sun TT: Microcystic adnexal carcinoma. An immunohistochemical
comparison with other cutaneous appendage tumors. Arch Dermatol.
126:189–194. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hoang MP, Dresser KA, Kapur P, High WA and
Mahalingam M: Microcystic adnexal carcinoma: an immunohistochemical
reappraisal. Mod Pathol. 21:178–185. 2008.PubMed/NCBI
|
|
28
|
Prabhu S, Pai SB, Handattu S, Kudur MH and
Vasanth V: Cutaneous metastases from carcinoma breast: the common
and the rare. Indian J Dermatol Venereol Leprol. 75:499–502. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Moore S: Cutaneous metastatic breast
cancer. Clin J Oncol Nurs. 6:255–260. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lai YL, Chang HH, Huang MJ, et al:
Combined effect of topical arsenic trioxide and radiation therapy
on skin-infiltrating lesions of breast cancer-a pilot study.
Anticancer Drugs. 14:825–828. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Richman T and Penneys NS: Analysis of
morpheaform basal cell carcinoma. J Cutan Pathol. 15:359–362. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bozikov K and Taggart I: Metastatic basal
cell carcinoma: is infiltrative/morpheaform subtype a risk factor?
Eur J Dermatol. 16:691–692. 2006.PubMed/NCBI
|
|
33
|
Pugh TJ, Lee NY, Pacheco T and Raben D:
Microcystic adnexal carcinoma of the face treated with radiation
therapy: a case report and review of the literature. Head Neck.
34:1045–1050. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Fischer S, Breuninger H, Metzler G and
Hoffmann J: Microcystic adnexal carcinoma: an often misdiagnosed,
locally aggressive growing skin tumor. J Craniofac Surg. 16:53–58.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Merritt BG, Snow SN and Longley BJ:
Desmoplastic trichoepithelioma, infiltrative/morpheaform BCC, and
microcystic adnexal carcinoma: differentiation by
immunohistochemistry and determining the need for Mohs micrographic
surgery. Cutis. 85:254–258. 2010.PubMed/NCBI
|
|
36
|
Jedrych J and McNiff JM: Expression of p75
neurotrophin receptor in desmoplastic trichoepithelioma,
infiltrative basal cell carcinoma, and microcystic adnexal
carcinoma. Am J DermatoPathol. 35:308–315. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Karikal A, Shetty P, Karikal A and Shetty
SR: Multiple trichoepitheliomas: A rare occurrence. South Asian J
Cancer. 2:542013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yiltok SJ, Echejoh GO, Mohammad AM, Ituen
AM, Igoche MI and Dades OT: Multiple familial trichoepithelioma: a
case report and review of literature. Niger J Clin Pract.
13:230–232. 2010.PubMed/NCBI
|
|
39
|
Clayton AS and Stasko T: Treatment of
nonmelanoma skin cancer in organ transplant recipients: review of
responses to a survey. J Am Acad Dermatol. 49:413–416. 2003.
View Article : Google Scholar : PubMed/NCBI
|