Introduction
Lung cancer is the most notable health problem among
men and women worldwide, accounting for an estimated 1.6 million
novel cases per year and ~13% of total cancer cases diagnosed in
2008 (1,2). At present, lung cancer remains the
leading cause of cancer-associated mortality in men and the second
leading cause of cancer-associated mortality in women (1). Although lung cancer consists of several
histological types, almost 80% of patients are diagnosed with
non-small cell lung cancer (NSCLC). The current treatment options
for NSCLC are determined by the stage of the disease and include
surgery, chemotherapy and targeted therapy. However, the majority
of patients with NSCLC are diagnosed at the advanced stage of
disease, and curative surgery is not possible for these patients.
In addition, survival with early stage NSCLC is also improved by
chemotherapy following surgery (3).
Chemotherapy may be administered alone or in combination with
surgery or radiation therapy, and platinum-based anti-tumor agents,
particularly cisplatin, have been widely used for the treatment of
various human cancers, including NSCLC (4,5). These
agents are initially effective in lung cancer patients, but due to
the development of resistance and relapse in treated patients, the
benefit of these platinum based compounds has been limited. Thus,
the overall response rate is only 25–30%, and the median survival
time is 9–11 months for patients with NSCLC (6–8).
Molecularly, platinum-resistance is multifaceted
(9–11). One mechanism of resistance is the
reduction in the intracellular accumulation of the drug, which
occurs due to the impairment of drug intake, enhancement of outward
transport or a combination of the two in tumor cells (1). Thus, additional understanding of the
underlying mechanism of platinum resistance may aid in the
improvement of the effectiveness of platinum therapy and the
response to platinum agents. Previous studies have revealed that
copper ions and platinum drugs may share the same transport system
in the cell (12–14). A different study demonstrated that
copper ion transport proteins not only participate in the
metabolism of the copper ions, but also maintain the balance of
copper and are ultimately associated with the development of
cisplatin resistance (15). In
particular, human copper transporter 1 (hCtr1) is the major copper
influx transporter and also transports cisplatin and cisplatin
analogues into cells (13), while two
other copper transporters, consisting of copper-transporting p-type
adenosine triphosphatase 1 (ATP7A) and 2 (ATP7B), regulate the
efflux of cisplatin. A previous study has reported that hCtr1
expression is able to predict the prognosis of patients with
advanced NSCLC that received platinum-based chemotherapy (16). Thus, in the present study, the
expression of hCtr1, ATP7A and ATP7B proteins was detected in
surgically resected NSCLC tissues to determine the association
between the expression of these proteins with the histology,
chemotherapy response, tumor stage, differentiation and prognosis
in patients with resected NSCLC that received platinum-based
treatment.
Patients and methods
Patients
In total, 54 patients with a histologically
confirmed diagnosis of NSCLC were diagnosed at The First Affiliated
Hospital of Xi'an Jiaotong University (Xi'an, Shaanxi, China)
between 2005 and 2009, and these patients were enrolled in the
present study. These patients underwent surgical resection of the
tumor lesions. The patients were enrolled in the present study
according to certain criteria. Firstly, surgical resection was
required as the first-line treatment, followed by at least two
courses of platinum based doublet, but not single or triplet,
chemotherapy administered on an intent-to-treat basis. The
assessment of the response to therapy for lung cancer mainly relied
on the Response Evaluation Criteria in Solid Tumors (17). Paraffin-embedded pre-treatment tumor
tissues were required to be available for immunohistochemistry.
However, patients with a known history of cancer or without
subsequent clinical follow-up data were excluded from the present
study. Detailed clinicopathological and survival data, such as the
tumor location, stage, differentiation, treatment, radiography
review and outcome, were collected retrospectively. The current
study was approved by the Institutional Review Board of The First
Affiliated Hospital of Xi'an Jiaotong University. Each patient
provided informed consent for participation in the present
study.
Chemotherapy regimen
The chemotherapy regimens included the
administration of 75–80 mg/m2 cisplatin or carboplatin,
at a dose that was calculated to produce an area under the serum
concentration-time curve of 6.0 min/mg/ml, and a non-platinum
agent. The treatment was repeated every 3 weeks. The same
chemotherapy regimen was administered once every 3 weeks and drug
doses were tailored according to patient tolerance, as
necessary.
Assessment of treatment efficacy
The patients were monitored closely for respiratory
and system symptoms, signs and disease relapse. Auxiliary
examination was conducted using computed tomography, head magnetic
resonance imaging and bone scanning every 3 months during treatment
until the completion of chemotherapy, and every 3–6 months
subsequently, until disease progression or the initiation of
subsequent anticancer therapy was determined. The sum of the
maximal diameters of all measurable tumor lesions was recorded at
baseline and following treatment. These measurements were used to
calculate the largest percentage reduction or smallest percentage
increase in size using the baseline assessment as a reference. The
best response to therapy was categorized using Response Evaluation
Criteria In Solid Tumors 1.0 criteria (17). The pre- and post-treatment quality of
life was also assessed.
Immunohistochemistry
Immunohistochemistry was performed to analyze the
expression of the hCtr1, ATP7A and ATP7B proteins in the
pre-treatment tumor tissue specimens. Therefore, 4 µm thick tissue
sections were prepared from a representative tumor tissue paraffin
block for each patient. For immunohistochemical analysis, these
tissue sections were deparaffinized and rehydrated, and then
subjected to heat-induced antigen retrieval in citrate buffer in an
autoclave oven. Subsequently, the sections were blocked for
endogenous peroxidase activity in 3%
H2O2/phosphate buffered saline (PBS) solution
for 30 min at room temperature. The sections were then incubated
overnight at 4°C with the primary antibody against hCtr1 (dilution,
1:250; Santa Cruz Biotechnology, Inc., Dallas, TX, USA), ATP7A
(dilution, 1:100; Abcam, Cambridge, UK) or ATP7B (dilution, 1:100;
Abcam). On the next day, the sections were washed with PBS three
times, and then incubated with a biotin-labeled secondary antibody
and the adenosine triphosphate binding cassette complex (LSAB kit;
DAKO, Carpinteria, CA, USA) at 37°C for 20 min.
For the color reaction, the sections were incubated
with diaminobenzidine substrate solution as the chromogen. Finally,
the sections were briefly counterstained with hematoxylin. Positive
and negative controls were performed for all experiments. Ten
fields of view per stained section (magnification, ×200) were
reviewed and scored under light microscopy (BX43; Olympus Corp,
Tokyo, Japan) by two independent pathologists that were blinded to
the clinical characteristics and outcomes of the patients. Each
tissue section was semi-quantitatively scored on a 1–4 scale by
estimating the percentage of positive cytoplasmic or membranous
staining of tumor cells, as follows: 1, 1–25% stained cells; 2,
26–50% stained cells; 3, 51–75% stained cells; and 4, >75%
stained cells. Weakly positive cytoplasmic staining, with an
intensity score of 1, was observed in certain pneumocytes and
bronchiolar epithelial cells adjacent to tumor cells. With respect
to the intensity and frequency, a high expression level was defined
as tumor cells with diffuse cytoplasmic or membrane staining of
moderate/strong intensity (≥2), according to previous studies
(18,19).
Statistical analysis
The association between clinical characteristics and
immunostaining were evaluated using χ2 or Fisher's exact
tests. The overall survival (OS) time was calculated as the time
interval between the date of pathological diagnosis and the date of
mortality or last follow-up. The Kaplan-Meier method was used to
generate survival curves for the OS time and the log-rank test was
performed to evaluate the differences between the groups.
Univariate logistic regression analysis followed by multiple
logistic regression analyses was applied to evaluate the
association between protein expression and clinicopathological
parameters as predictors of the treatment response. The association
between potential predictors and survival time were evaluated by
Cox proportional hazards models, using univariate and multivariate
analyses. All statistical analyses were performed using Stata/SE
12.0 (StataCorp LP, College Station, TX, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
Patient characteristics and treatment
responses
The present cohort of patients with NSCLC received
platinum-based chemotherapy subsequent to the surgical removal of
tumor lesions. The longest follow-up time was 66 months. The
characteristics of patients are listed in Table I. In total, 14 patients were treated
with gemcitabine and cisplatin (26%), 12 with docetaxel and
cisplatin (22%), 15 (28%) with vinorelbine and cisplatin, 7 (13%)
with paclitaxel and cisplatin, 5 (9%) with pemetrexed and
cisplatin, and 1 (2%) with etoposide and cisplatin. The courses of
chemotherapy ranged between 2 and 4 cycles. Stable disease was
demonstrated by 4 patients, progressive disease was demonstrated by
15 patients and complete response was demonstrated by 1
patient.
 | Table I.Association of hCtr1, ATP7A and ATP7B
expression with clinicopathological characteristics of patients
with non-small cell lung cancer. |
Table I.
Association of hCtr1, ATP7A and ATP7B
expression with clinicopathological characteristics of patients
with non-small cell lung cancer.
|
|
| hCtr1 | ATP7A | ATP7B |
|---|
|
|
|
|
|
|
|---|
| Characteristics | Total, n | Overexpression | Low expression | P-value | Overexpression | Low expression | P-value | Overexpression | Low expression | P-value |
|---|
| All patients | 54 | 32 | 22 |
| 20 | 34 |
| 15 | 39 |
|
| Age |
|
|
|
|
|
|
|
|
| 0.33 |
| ≤60
years | 34 | 22 | 12 | 0.29 | 13 | 21 | 0.81 | 11 | 23 |
|
| >60
years | 20 | 10 | 10 |
| 7 | 13 |
| 4 | 16 |
|
| Gender |
|
|
|
|
|
|
|
|
| 0.18 |
| Male | 39 | 24 | 15 | 0.32 | 17 | 22 | 0.25 | 13 | 26 |
|
|
Female | 15 | 7 | 8 |
| 4 | 11 |
| 3 | 12 |
|
| Histology |
|
|
|
|
|
|
|
|
| 0.08 |
|
Adenocarcinoma | 20 | 15 | 5 | 0.29 | 7 | 13 | 0.97 | 10 | 10 |
|
|
Squamous cell carcinoma | 22 | 11 | 11 |
| 9 | 13 |
| 4 | 18 |
|
|
Adenosquamous carcinoma | 9 | 5 | 4 |
| 3 | 6 |
| 1 | 8 |
|
|
LCLC | 3 | 1 | 2 |
| 1 | 2 |
| 1 | 2 |
|
| Clinical stage |
|
|
|
|
|
|
|
|
| 0.09 |
| I | 26 | 17 | 9 | 0.48 | 8 | 18 | 0.2 | 11 | 15 |
|
| II | 11 | 6 | 5 |
| 6 | 5 |
| 1 | 10 |
|
|
III | 13 | 8 | 5 |
| 6 | 7 |
| 3 | 10 |
|
| IV | 4 | 1 | 3 |
| 0 | 4 |
| 0 | 4 |
|
| Differentiation
degree |
|
|
|
|
|
|
|
|
| 0.045 |
|
High | 8 | 5 | 3 | 0.62 | 5 | 3 | 0.21 | 0 | 8 |
|
|
Middle | 18 | 9 | 9 |
| 7 | 11 |
| 3 | 15 |
|
|
Low | 28 | 18 | 10 |
| 8 | 20 |
| 11 | 17 | . |
| Best measurable
response |
|
|
|
|
|
|
|
|
|
|
| CR | 1 | 0 | 1 | 0.029 | 0 | 1 | 0.313 | 0 | 1 | 0.53 |
| PR | 33 | 18 | 15 |
| 12 | 21 |
| 10 | 23 |
|
| SD | 4 | 2 | 2 |
| 0 | 4 |
| 0 | 4 |
|
| PD | 15 | 14 | 1 |
| 7 | 8 |
| 5 | 10 |
|
|
Other | 1 |
|
|
|
|
|
|
|
|
|
Expression of the hCtr1, ATP7A and
ATP7B proteins in NSCLC tissue specimens and association with
measurable tumor response
The expression of the hCtr1, ATP7A and ATP7B
proteins was observed in 59, 37 and 28% of NSCLC tissue specimens,
respectively (Fig. 1; Table I). The expression of ATP7B was
significantly associated with tumor cell differentiation, while the
expression of hCtr1 was significantly associated with improved
chemotherapeutic responses. In total, 18 out of 33 patients with
partial responses to chemotherapy demonstrated overexpression of
the hCtr1 protein, whereas 14 out of 15 patients with progressive
disease subsequent to chemotherapy demonstrated overexpression of
the hCtr1 protein (P<0.05). However, the expression of the ATP7A
and ATP7B proteins was not significantly associated with
clinicopathological data. The expression of these copper
transporters was not associated with patient age, histological
tumor type, clinical stage or best measurable response (Table I).
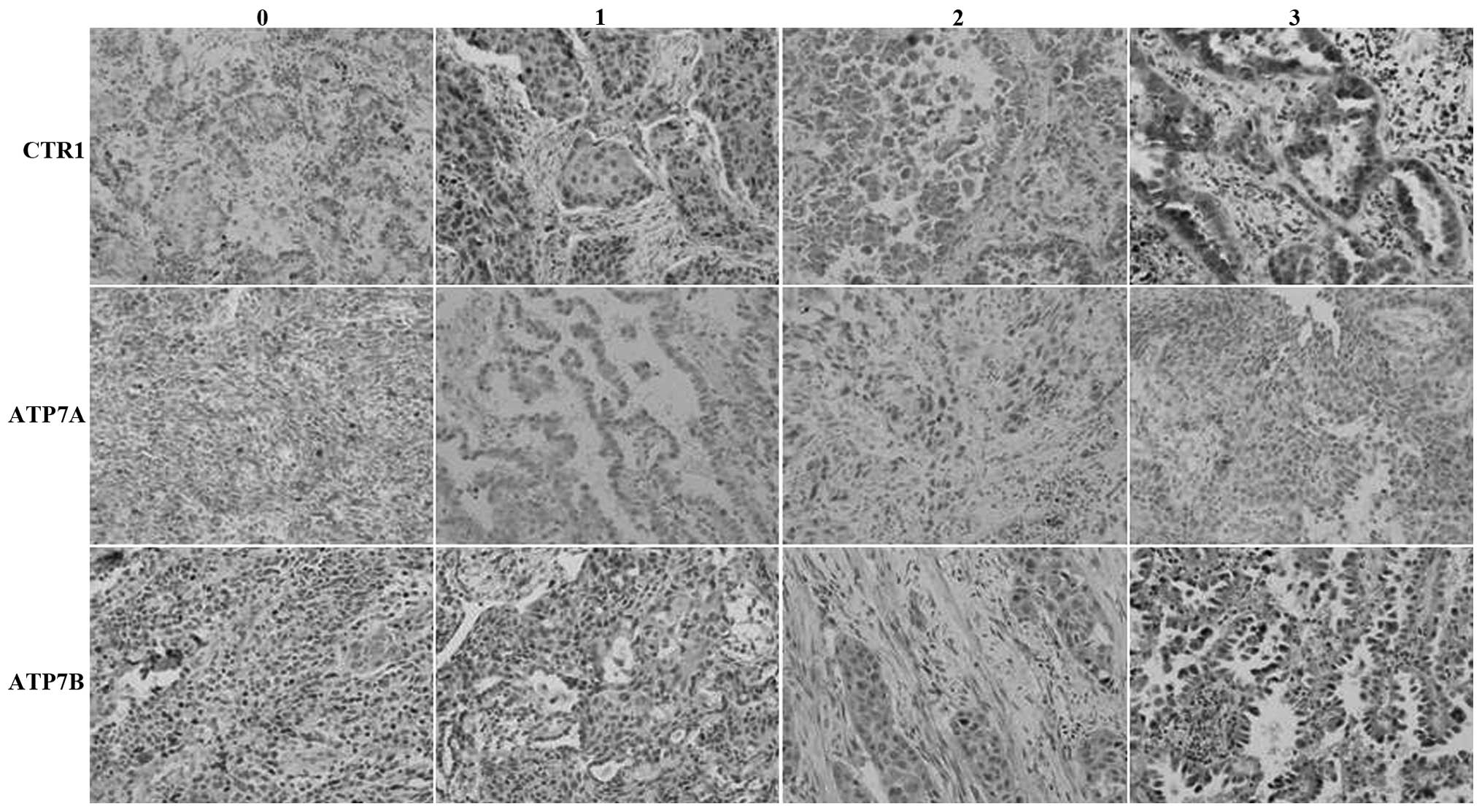 | Figure 1.Immunohistochemical analysis of the
expression of the (A) hCtr1, (B) ATP7A and (C) ATP7B proteins in
non-small cell lung cancer tissue specimens. Magnification, ×200.
0, no staining; 1, weak staining; 2, moderate staining; and 3,
strong staining. CTR1, copper transporter 1; hCtr1, human CTR1;
ATP7A, copper-transporting p-type adenosine triphosphatase 1;
ATP7B, copper-transporting p-type adenosine triphosphatase 2. |
Association between hCtr1, ATP7A and
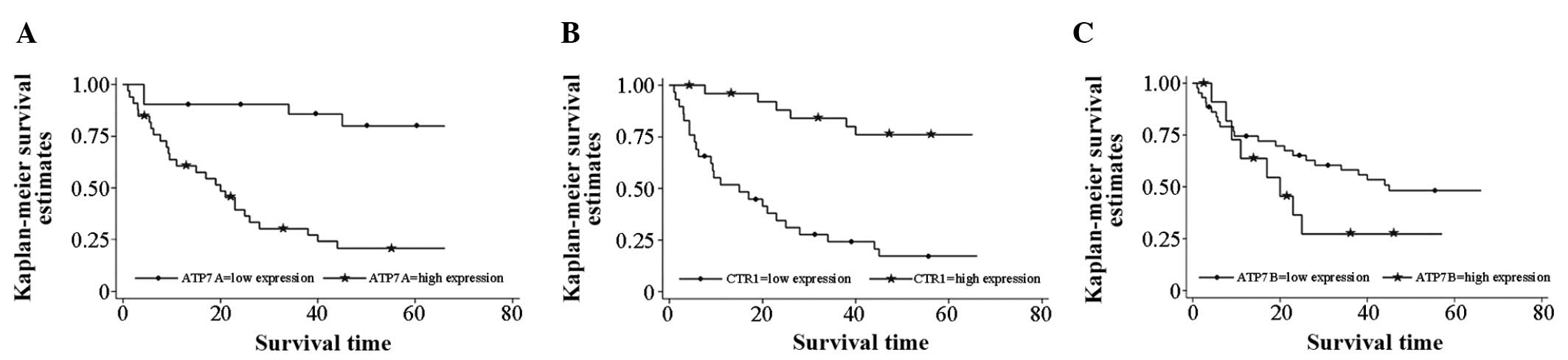
ATP7B expression and patient survival
The median survival time was found to be 45 months
in patients with low ATP7B expression in tumors, whereas it was 20
months in patients with high ATP7B expression in tumors, although
the data were not statistically significant (P=0.162). Similarly,
the median survival time was 20 months in patients with high tumor
ATP7A expression, but >66 months in patients with low tumor
ATP7A expression at the time of follow-up. The data were
statistically significant (P<0.001).
Furthermore, the median survival time was 15 months
in patients with low tumor hCtr1 expression, but >66 months in
patients with high tumor hCtr1 expression at the time of follow-up.
This difference was statistically significant (P<0.001). High
hCtr1 and low ATP7A expression were each favorable prognostic
factors subsequent to chemotherapy in patients with resected NSCLC
(Fig. 2).
In addition, the multivariate analysis data revealed
that high hCtr1 expression combined with low ATP7A expression,
tumor differentiation and patient gender were all favorable
independent predictive and prognostic factors for patients with
resected NSCLC subsequent to chemotherapy (Table II).
 | Table II.Univariate and multivariate analyses
of prognostic factors for non-small cell lung cancer patients
subsequent to treatment with platinum-based chemotherapy. |
Table II.
Univariate and multivariate analyses
of prognostic factors for non-small cell lung cancer patients
subsequent to treatment with platinum-based chemotherapy.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Variable | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| CTR1
expression |
|
|
|
|
| High
vs. low | 0.15
(0.62–0.38) | 0.000 | 0.29
(0.11–0.75) | 0.010 |
| ATP7A
expression |
|
|
|
|
| High
vs. low | 7.20
(2.48–20.87) | 0.000 | 3.68
(1.11–12.12) | 0.032 |
| ATP7B
expression |
|
|
|
|
| High
vs. low | 1.78
(0.78–4.03) | 0.168 | 0.77
(0.29–2.00) | 0.592 |
| Gender |
|
|
|
|
| Male
vs. female | 0.29
(0.10–0.85) | 0.023 | 0.28
(0.09–0.95) | 0.040 |
| Age |
|
|
|
|
| ≥60 vs.
<60 years | 0.97
(0.93–1.00) | 0.083 | 0.96
(0.92–1.01) | 0.120 |
| Tumor
differentiation |
|
|
|
|
|
Good/moderate vs. poor | 0.31
(0.19–0.49) | 0.000 | 0.44
(0.24–0.79) | 0.006 |
Discussion
In the present study, the expression of the hCtr1,
ATP7A and ATP7B proteins was analyzed in pre-treated NSCLC tissue
specimens to determine the association between the expression of
these proteins and chemosensitivity of the patients to
platinum-based treatment. Out of the 54 lung cancer patients that
underwent surgical tumor resection and first-line platinum-based
doublet chemotherapy, significant overexpression of hCtr1, ATP7A
and ATP7B was identified in 32 (59%), 20 (37%) and 15 (28%)
patients, respectively. In addition, ATP7B expression was
significantly associated with tumor differentiation, and hCtr1
expression was significantly associated with an improved
chemotherapeutic response. The multivariate analysis revealed that
high hCtr1 expression, low ATP7A expression, tumor differentiation
and patient gender were all favorable independent predictive and
prognostic factors for patients with resected NSCLC that received
chemotherapy. These data indicate that the detection of hCtr1 and
ATP7A expression may be evaluated as biomarkers to predict the
sensitivity of patients with NSCLC to platinum-based chemotherapy.
High hCtr1 and low ATP7A expression were each favorable prognostic
factors for patients with resected NSCLC subsequent to
chemotherapy.
The present results revealed that the median
survival time of patients subsequent to surgery combined with
chemotherapy was longer and was markedly increased compared with
the median survival time reported in the literature, which reports
a median survival time of 9–11 months for lung cancer patients
(6,8).
The cause of this discrepancy has not been elucidated and may be
associated with the stage of the disease, radical resection of
tumor lesions, duration of chemotherapy administration, the
presence of metastatic tumors outside the lung and individual
differences.
Platinum-based chemotherapy is the first-line
chemotherapeutic treatment either prior to or following surgery
(20,21). However, drug resistance may be an
issue and limit the efficacy of platinum-based chemotherapy in the
clinic (22) and in the pre- and
post-operative treatment of lung cancer (9). In general, copper is an essential
element for cells, but excessive copper may be toxic or even lethal
to cells. Therefore, mammalian cells have developed sophisticated
mechanisms to maintain copper levels through intake, export and
intracellular compartmentalization or buffering of copper. Ctr1
controls copper uptake, while ATP7A and ATP7B control the copper
export from cells (23–25). Previous studies have identified that
copper ion transport proteins are closely associated with the
development of cisplatin resistance (16,26,27). These
copper transporters include Ctr1 and ATP7B and ATP7A (23,28), which
together regulate the overall intracellular cisplatin levels. It
has been reported that the intracellular cisplatin level is an
important determinant for the cytotoxic activity of cisplatin.
Thus, detection of the expression of hCtr1, ATP7B and ATP7A in
tumor lesions may provide an important insight into drug resistance
in cisplatin-based cancer chemotherapy. In the present study, NSCLC
tissue specimens were revealed to express various levels of hCtr1,
ATP7A and ATP7B proteins and that ATP7B expression was
significantly associated with tumor cell differentiation, while
hCtr1 expression was significantly associated with an improved
chemotherapeutic response. Mechanistically, it is evident that
hCtr1 expression upregulates drug levels in cells and therefore
increases drug efficacy.
In addition, the present study also demonstrated
that differential expression of these copper transporters, such as
high hCtr1 expression and low ATP7B and ATP7A expression, also
contributed to a favorable prognosis in patients with resected
NSCLC that received chemotherapy. Li et al (29) demonstrated that ATP7A expression was
associated with the histological grade of NSCLC and the response to
chemotherapy. Inoue et al (30) revealed that ATP7A mRNA expression was
useful as a marker for cisplatin chemoresistance in NSCLC.
Furthermore, Nakagawa et al (19) found that the ATP7B mRNA and protein
levels were significantly increased in human NSCLC
cisplatin-resistant xenografts compared with the levels in the
cisplatin-sensitive xenografts, indicating that ATP7B was a useful
biomarker for platinum resistance in NSCLC. However, Mangala et
al (31) revealed that ATP7B was
overexpressed in chemotherapy-sensitive ovarian carcinoma cells.
However, the association between ATP7B overexpression and an
unfavorable clinical outcome in various cancers has been reported
(32). Overall, the present and
published data indicate that the role of ATP7A and ATP7B in
clinical cisplatin resistance may be complex and depend on factors
including the patient population, tumor types, drug resistance
mechanisms, treatment modalities and methods of data analysis
(5). Thus, additional studies are
required to clarify these issues.
The present study also revealed an association
between hCtr1 expression and curative effect, which is consistent
with the findings of Choi et al (5). However, based on the cohort of patients
in the present study, hCtr1 expression may have prognostic value
for predicting the treatment efficacy of chemotherapy using a
cisplatin-based regimen combined with surgery. Ishida et al
has previously reported that the expression of hCtr1 is associated
with the clinical response in ovarian cancer (33,34). Ogane
et al (35) revealed that CTR1
expression is a prognostic factor for outcome in endometrial
cancer. There are numerous factors that contribute to cisplatin
resistance, and additional studies are required to elucidate the
molecular mechanism involved in the development of resistance to
platinum-based chemotherapy in lung cancer patients. Cumulatively,
the present results revealed that the detection of hCtr1 and ATP7A
expression may be evaluated as a biomarker for the prediction of
chemosensitivity to platinum-based chemotherapy; high hCtr1 and low
ATP7A expression were each favorable prognostic factors for
patients with resected NSCLC subsequent to chemotherapy. Surgery
combined with neoadjuvant chemotherapy may improve the survival of
NSCLC patients.
Acknowledgements
This study was supported by Shaanxi Science and
Technology Research Funds (grant no., 2011K12-15).
References
|
1
|
Hildebrandt MA, Gu J and Wu X:
Pharmacogenomics of platinum-based chemotherapy in NSCLC. Expert
Opin Drug Metab Toxicol. 5:745–755. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zornosa C, Vandergrift JL, Kalemkerian GP,
Ettinger DS, Rabin MS, Reid M, Otterson GA, Koczywas M, D'Amico TA,
Niland JC, et al: First-line systemic therapy practice patterns and
concordance with NCCN guidelines for patients diagnosed with
metastatic NSCLC treated at NCCN institutions. J Natl Compr Canc
Netw. 10:847–856. 2012.PubMed/NCBI
|
|
4
|
Ivy KD and Kaplan JH: A re-evaluation of
the role of hcTR1, the human high-affinity copper transporter, in
platinum-drug entry into human cells. Mol Pharmacol. 83:1237–1246.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Choi MK and Kim DD: Platinum transporters
and drug resistance. Arch Pharm Res. 29:1067–1073. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fisher MD and D'Orazio A: Phase II and III
trials: Comparison of four chemotherapy regimens in advanced non
small-cell lung cancer (ECOG 1594). Clin Lung Cancer. 2:21–22.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Schiller JH, Harrington D, Belani CP, et
al: Comparison of four chemotherapy regimens for advanced
non-small-cell lung cancer. N Engl J Med. 346:92–98. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fossella F, Pereira JR, von Pawel J, et
al: Randomized, multinational, phase III study of docetaxel plus
platinum combinations versus vinorelbine plus cisplatin for
advanced non-small-cell lung cancer: The tax 326 study group. J
Clin Oncol. 21:3016–3024. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu JJ, Lu J and McKeage MJ: Membrane
transporters as determinants of the pharmacology of platinum
anticancer drugs. Curr Cancer Drug Targets. 12:962–986. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mellor HR and Callaghan R: Resistance to
chemotherapy in cancer: A complex and integrated cellular response.
Pharmacology. 81:275–300. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zisowsky J, Koegel S, Leyers S, et al:
Relevance of drug uptake and efflux for cisplatin sensitivity of
tumor cells. Biochem Pharmacol. 73:298–307. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gupta A and Lutsenko S: Human copper
transporters: Mechanism, role in human diseases and therapeutic
potential. Future Med Chem. 1:1125–1142. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kuo MT, Chen HH, Song IS, Savaraj N and
Ishikawa T: The roles of copper transporters in cisplatin
resistance. Cancer Metastasis Rev. 26:71–83. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Steiger D, Fetchko M, Vardanyan A,
Atanesyan L, Steiner K, Turski ML, Thiele DJ, Georgiev O and
Schaffner W: The drosophila copper transporter ctr1C functions in
male fertility. J Biol Chem. 285:17089–17097. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liang ZD, Long Y, Tsai WB, et al:
Mechanistic basis for overcoming platinum resistance using copper
chelating agents. Mol Cancer Ther. 11:2483–2494. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen HH, Yan JJ, Chen WC, et al:
Predictive and prognostic value of human copper transporter 1
(hctr1) in patients with stage III non-small-cell lung cancer
receiving first-line platinum-based doublet chemotherapy. Lung
Cancer. 75:228–234. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Taylor PT and Haverstick D: Re: New
guidelines to evaluate the response to treatment in solid tumors
(ovarian cancer). J Natl Cancer Inst. 97:1512005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Holzer AK, Varki NM, Le QT, Gibson MA,
Naredi P and Howell SB: Expression of the human copper influx
transporter 1 in normal and malignant human tissues. J Histochem
Cytochem. 54:1041–1049. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nakagawa T, Inoue Y, Kodama H, Yamazaki H,
Kawai K, Suemizu H, Masuda R, Iwazaki M, Yamada S, Ueyama Y, et al:
Expression of copper-transporting p-type adenosine triphosphatase
(ATP7B) correlates with cisplatin resistance in human non-small
cell lung cancer xenografts. Oncol Rep. 20:265–270. 2008.PubMed/NCBI
|
|
20
|
Konstantakou EG, Voutsinas GE, Karkoulis
PK, Aravantinos G, Margaritis LH and Stravopodis DJ: Human bladder
cancer cells undergo cisplatin-induced apoptosis that is associated
with p53-dependent and p53-independent responses. Int J Oncol.
5:401–416. 2009.
|
|
21
|
Köberle B, Tomicic MT, Usanova S and Kaina
B: Cisplatin resistance: Preclinical findings and clinical
implications. Biochim Biophys Acta. 1806:172–182. 2010.PubMed/NCBI
|
|
22
|
Abada P and Howell SB: Regulation of
cisplatin cytotoxicity by cu influx transporters. Metal Based
Drugs. 2010:3175812010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lenartowicz M and Krzeptowski W: Structure
and function of ATP7A and ATP7B proteins-Cu-transporting ATPases.
Postepy Biochem. 56:317–327. 2010.PubMed/NCBI
|
|
24
|
Zhou B and Gitschier J: Hctr1: A human
gene for copper uptake identified by complementation in yeast. Proc
Natl Acad Sci USA. 94:7481–7486. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cox DW and Moore SD: Copper transporting
p-type atpases and human disease. J Bioenerg Biomembr. 34:333–338.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Howell SB, Safaei R, Larson CA and Sailor
MJ: Copper transporters and the cellular pharmacology of the
platinum-containing cancer drugs. Mol Pharmacol. 77:887–894. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang Y, Li M, Yao Q and Chen C: Roles and
mechanisms of copper transporting atpases in cancer pathogenesis.
Med Sci Monit. 15:RA1–RA5. 2009.PubMed/NCBI
|
|
28
|
Furukawa T, Komatsu M, Ikeda R, Tsujikawa
K and Akiyama S: Copper transport systems are involved in multidrug
resistance and drug transport. Curr Med Chem. 15:3268–3278. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li ZH, Qiu MZ, Zeng ZL, Luo HY, Wu WJ,
Wang F, Wang ZQ, Zhang DS, Li YH and Xu RH: Copper-transporting
p-type adenosine triphosphatase (ATP7A) is associated with
platinum-resistance in non-small cell lung cancer (NSCLC). J Transl
Med. 10:212012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Inoue Y, Matsumoto H, Yamada S, Kawai K,
Suemizu H, Gika M, Takanami I, Iwazaki M and Nakamura M:
Association of ATP7A expression and in vitro sensitivity to
cisplatin in non-small cell lung cancer. Oncol Lett. 1:837–840.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mangala LS, Zuzel V, Schmandt R, Leshane
ES, Halder JB, ArmaizPena GN, Spannuth WA, Tanaka T, Shahzad MM,
Lin YG, et al: Therapeutic targeting of ATP7B in ovarian carcinoma.
Clin Cancer Res. 15:3770–3780. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Nakayama K, Kanzaki A, Terada K, Mutoh M,
Ogawa K, Sugiyama T, Takenoshita S, Itoh K, Yaegashi N, Miyazaki K,
et al: Prognostic value of the Cu-transporting ATPase in ovarian
carcinoma patients receiving cisplatin-based chemotherapy. Clin
Cancer Res. 10:2804–2811. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yoshida H, Teramae M, Yamauchi M, Fukuda
T, Yasui T, Sumi T, Honda K and Ishiko O: Association of copper
transporter expression with platinum resistance in epithelial
ovarian cancer. Anticancer Res. 33:1409–1414. 2013.PubMed/NCBI
|
|
34
|
Ishida S, McCormick F, SmithMcCune K and
Hanahan D: Enhancing tumor-specific uptake of the anticancer drug
cisplatin with a copper chelator. Cancer Cell. 17:574–583. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ogane N, Yasuda M, Kameda Y, Yokose T,
Kato H, Itoh A, Nishino S, Hashimoto Y and Kamoshida S: Prognostic
value of organic anion transporting polypeptide 1B3 and copper
transporter 1 expression in endometrial cancer patients treated
with paclitaxel and carboplatin. Biomed Res. 34:143–151. 2013.
View Article : Google Scholar : PubMed/NCBI
|