Introduction
Acute lymphoblastic leukemia (ALL) is a neoplasm of
precursor cells committed to the B-cell and T-cell lineages
involving the bone marrow and blood (1). ALL occasionally presents with primary
involvement of nodal sites. Osteoporosis and spontaneous vertebral
compression fractures as presentations of ALL have been described
mainly in pediatric patients, but rarely in adult patients with ALL
(2,3).
Philadelphia chromosome-positive (Ph+) ALL accounts for
25–30% of adult ALL and its incidence increases with age in adults
>40 years old. Prior to the era of tyrosine kinase inhibitor
(TKI) treatment, Ph+ ALL was considered to be a
high-risk subgroup among the ALL population. Current intensive
chemotherapy regimes have improved the complete remission (CR)
rate, however, long-term overall survival (OS) has not improved. In
the TKI era, dramatic changes in therapeutic efficacy have
occurred, with markedly improved molecular CR and improved OS rates
(4). These changes raise questions as
to whether the conventional intensive combined chemotherapy
treatment regime should remain the front-line treatment strategy
for patients with Ph+ ALL.
The present study reported the case of a
Ph+ ALL patient who was treated with imatinib-based
individual therapy, which included conventional standard-dose
chemotherapy, rather than intensive combined regimens. Imatinib is
a first generation TKI, which specifically targets the adenosine
triphosphate binding site of the BCR/ABL kinase domain. The current
case was characterized by initial presentation of general
osteoporosis and vertebral compression fracture.
Case report
A 56-year-old previously healthy male was originally
referred to the West China Hospital (Sichuan University, Chengdu)
in June 2012. The patient presented with a 6-month history of
progressive lower back and bilateral rib pain. Initially, the
patient experienced sudden onset of sharp lower back pain, and a
spine X-ray in the local community clinic indicated slipped disc at
L2-3, L3-4 and L4-5 levels with a normal complete blood count and
white cell differentiation. Acute pain was usually resolved
spontaneously, but pain recurrence was frequent. After 3 months,
the perceived intensity of pain became more aggressive, followed by
emergence of fatigue and dizziness. A repeated complete blood count
indicated a hemoglobin level of 6.5 g/dl [normal range (NR),
13.0–17.5 g/dl] and platelet count of 15,000/µl. A computed
tomography (CT) scan revealed large absorption and compression
fracture of T8 and L3. Red blood cell transfusion was performed in
the local clinic prior to admission to the West China Hospital.
Upon physical examination, pallor was observed most prominently at
the face, eyelids, lips and palms. No lymphadenopathy or
hepatosplenomegaly were identified. The findings of a neurologic
examination, including strength testing, were normal, and other
examinations were unremarkable.
A complete blood count revealed the following:
hemoglobin level, 9.3 g/dl; white blood cell count, 4,780/µl (NR,
3,500–9,500/µl) with 3% blast cells; platelet count, 21,000/µl (NR,
100,000–300,000/µl); lactate dehydrogenase, 1,082 IU/l (NR, 72–182
IU/l); alkaline phosphatase, 6.72 µg/l (NR, 11.4–24.6 µg/l); Type I
collagen carboxyl terminal peptide, 0.125 ng/ml (NR, 0.3–0.58
ng/ml); calcium level, 2.16 mmol/l (NR, 2.1–2.7 mmol/l); and
inorganic phosphorus level, 0.74 mmol/l (NR, 0.81–1.45 mmol/l).
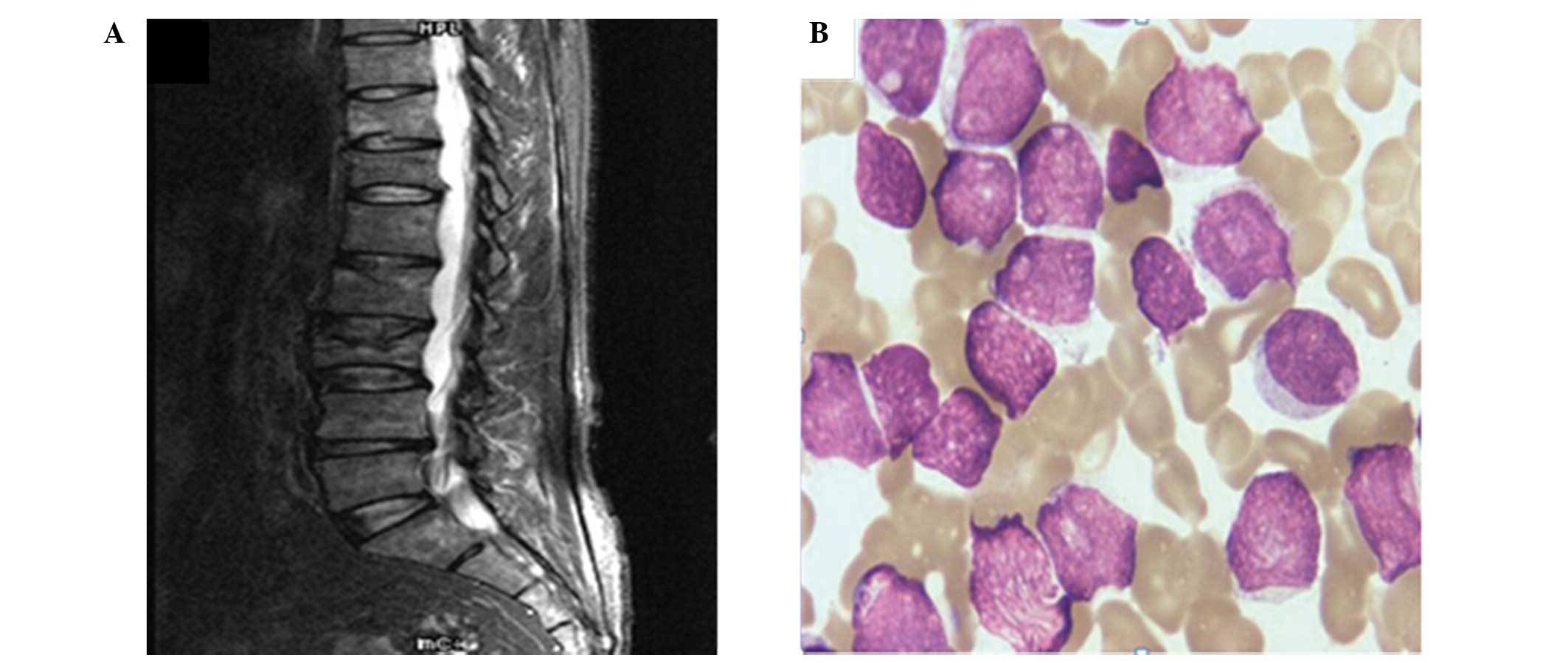
Magnetic resonance imaging (MRI) scans revealed an impaired
physiological curve of thoracolumbar spine, clusters of iso/hypo
mixed signals and pathological fracture in T8 and L3 with
inhomogeneous contrast enhancement (Fig.
1A). A chest CT image demonstrated bone destruction of the
sternum, thoracic vertebra, bilateral rib and scapular. In
addition, an X-ray showed sheet and irregular low-density lesions
on the cranial and maxillofacial bone, pelvis and bilateral
proximal femur. Based on this data, a diagnosis of multiple myeloma
(MM) was initially made. However, immunofixation electrophoresis
(IFE), serum protein electrophoresis (SPE) and urine light chain
detection indicated no evidence of monoclonal protein. Furthermore,
a bone marrow smear identified active hyperplasia and a blast cell
count of 90.0% (Fig. 1B), while the
patient was peroxidase-negative. The immunophenotype of the
leukemic blasts was analyzed by flow cytometry (FCM) and was as
high as the typical phenotype of common-B-cell ALL patients, with
CD10+, CD19+, CD13+,
CD20+, cCD79a+, CD34+ and
HLA-DR+. Cytogenetic analysis revealed
46,XY,t(9;22)(q34;q11) [20/20], while a molecular biology
examination demonstrated that the patient was
BCR/ABL-positive, as determined by a reverse
transcription-polymerase chain reaction method (5). Therefore, a diagnosis of Ph+
ALL was ultimately determined according to the Morphology,
Immunology, Cytogenetics, Molecular Biology criteria (6).
In addition to administration of 90 mg pamidronate
disodium (Bonin; Shenzhen Neptunus Bioengineering Co., Ltd.,
Shenzhen, China) to reduce bone pain, the patient was treated with
the IVD regimen (imatinib, 400 mg/day; vindesine, 4 mg/day, on days
1, 8, 15 and 22; dexamethasone, 10 mg/m2/day, on days
1–5, 8–12, 15–19 and 22–26). Hematological CR with rapid resolution
of pain was observed 2 weeks after the termination of inductive
treatment, which was confirmed by a bone marrow smear and FCM.
Meanwhile, the patient continued extramural treatment with imatinib
at 400 mg/day. Due to the lack of a matched donor, bone marrow
transplantation was not considered for this case. During subsequent
consolidation therapy, the CAM (cyclophosphamide, 750
mg/m2, days 1 and 8; cytarabine, 100 mg/m2,
days 1–3 and 8–10; 6-mercaptopurine, 60 mg/m2, days
1–7), ML (methotrexate, 3.0 g/m2, day 1; L-asparaginase,
6,000 IU/m2, days 2 and 3) and MA (mitoxantrone, 8.0
g/m2, days 1–3; cytarabine, 1.5 g/m2, days
1–3) chemotherapies were applied for ~1 month, respectively. Prior
to each consolidation regimen, a lumbar puncture combined with an
intrathecal injection was performed using 10 mg methotrexate, 30 mg
cytarabine and 5 mg dexamethasone. However, following intensive
post-remission chemotherapy, the patient experienced bone marrow
suppression with a significantly reduced total leukocyte count of
170/µl (normal range, 4,000–10,000/µl) and was affected by severe
pneumonia. A 3-week course of intravenous antibiotics was
administrated and the hemopoietic function of the bone marrow
returned to the normal levels 1 month later. Simultaneously,
molecular CR was confirmed by bone marrow examination, revealing
suppression of BCR/ABL chimeric gene expression.
Subsequently, the patient was subjected to consolidation treatment
with the IVD regimen (imatinib, 400 mg/day; vindesine, 4 mg/day,
days 1 and 11; dexamethasone, 10 mg/m2/day, days 1–5 and
days 11–15) combined with interferon-α 2b (3 million units, twice
per week) to maintain remission for 9 courses on a periodic basis
(every 1 month).
Until November 2013, the patient remained
asymptomatic, while sustained hematological and molecular genetic
normality were achieved for ~16 months after CR. In addition, a
chest CT image revealed that the bone cortex margin of the left rib
was irregular and bone density was uneven in osseous thorax without
evident osteolytic destruction. However, in November 2013, the
patient was lost to follow-up.
The present was approved by the ethics committees of
West China Hospital, Sichuan University (Chengdu, China) and
written informed consent was obtained from the patient.
Discussion
Symptoms of ALL at diagnosis usually include
nonspecific manifestation of bone marrow failure, such as fever,
weakness or bleeding (7). Although
the presence of osteopenia/osteoporosis may be observed in both the
initial and progressive phases of patients with ALL, bone pain and
spontaneous bone fractures, which mainly involve the proximal limbs
due to leukemic involvement along with adjacent joint pain or
swelling, as the sole prodrome for ALL are rarely observed
(8). As previously described,
vertebral body compression fractures associated with bone pain
occurred in <10% of children with ALL as the initial
presentation (9). However, to the
best of our knowledge, generalized osteopenia and vertebral
complications presented as the only symptoms prior to the diagnosis
of ALL has not been previously reported in an adult ALL patient.
Based on a search of the PubMed database, to the best of our
knowledge only 3 adolescent and 1 child cases have been described
in the English literature to date (9–12). These
cases presented with exclusive back pain and were then verified to
exhibit marked osteoporosis and spontaneous vertebral compression
fractures; ultimately, they were diagnosed with ALL after 3–4
months. These cases included an 8-year-old girl in Italy (10) and a 13-year-old boy in Turkey
(9), as well as a 9-year-old boy and
a 2-year-old boy in Bangladesh (11,12).
Compression fractures of the vertebrae are known to be caused by
osteoporosis (which is the most common cause), trauma to the back
and tumors that develop in the bone or spread to the bone from
other sites (13). A tumor that
develops in the spine, including MM, is more common in older people
(14). In the 56-year-old patient of
the present study, a working diagnosis of MM was preliminarily
established; thereby, IFE and SPE were applied to exclude MM.
Notably, bone destruction is not currently the determined predictor
for ALL prognosis; however, bone pain and fractures may still be
major evidence to support a diagnosis of ALL and greatly influence
the quality of life prior to establishing the ultimate diagnosis of
ALL (15). Thus, ALL must be
considered in the differential diagnosis, and close follow-up and
bone marrow examination are important when a patient presents with
unexplained marked osteopenia, bone pain and multiple fractures,
particularly prior to establishing a definite diagnosis of
idiopathic juvenile osteoporosis for children and MM for adults. In
order to suppress bone resorption and reduce bone turnover, the
case was treated with pamidronate disodium with no significant
side-effects in the entire therapeutic course.
However, the role of imatinib in Ph+ ALL
treatment has attracted increased attention due to the success of
TKI treatment of chronic myeloid leukemia. Prior to the discovery
of TKIs, Ph+ ALL was considered to be the high-risk
group with the poorest outcome among all subtypes of ALL (16). Although a CR rate of 50–60% could be
achieved by routine chemotherapy, short-term remission and high
relapse rate resulted in a poor 5-year survival rate of <10% for
adults and OS of only 20% (17). More
intensive chemotherapy regimens were only able to improve the CR
rate, rather than the long-term OS. However, with the use of TKIs
in chemotherapy, the therapeutic efficacy markedly increased,
reaching up to 95% in patients undergoing CR and 70% in those
undergoing molecular CR, as well as resulted in improved 3-year OS
of 55% (18,19). For older patients (age, ≥65 years)
with Ph+ ALL, imatinib alone or in combination with
reduced intensive chemotherapy is reported to be the first-line
inductive regimen based on the National Comprehensive Cancer
Network Guidelines since 2012 (4). In
addition, the final results of the EWALL-Ph-01 Study were presented
in 2012 at the American Society of Hematology meeting and
recommended the used of the third generation TKI, dasatinib, and
low intensity chemotherapy as the first-line treatment in patients
with de novo Ph+ ALL aged ≥55 years (20).
The 56-year-old male patient of the present study
successfully completed the IVD regimen and quickly achieved CR 2
weeks after initiation of the treatment. Whether optimal long-term
efficacy may be maintained using an imatinib-based regimen,
allogeneic hematopoietic stem cell transplantation (Allo-HSCT) or
TKI + Allo-HSCT requires further investigation. Certain authors
have recommended that TKI-based regimens may have a similar or even
superior effect compared with Allo-HSCT, particularly in patients
who may be at a high risk of transplantation-associated mortality,
such as the elderly (21). Due to the
lack of a matched donor, the current case accepted regular
intensification and continued extramural imatinib as a
consolidation approach. In addition, clinical studies are currently
ongoing to evaluate the best procedure and duration of maintenance
in patients who previously benefited from TKI-based front-line
treatment. Until recently, maintenance therapy with imatinib or
interferon demonstrated impressive effectiveness for the treatment
of patients with Ph+ ALL (22).
In conclusion, based on the aforementioned evidence,
the present case was subjected to interim maintenance treatment
using imatinib and IFN to achieve persistent molecular CR for ~16
months. The emergence of TKIs has greatly contributed to marked
improvement in the outcome of patients with Ph+ ALL;
thus, there has been much debate regarding whether regimens
including TKI could challenge the efficacy of conventional
high-dose chemotherapy for such cases. To a certain extent, the
present study suggests that the administration of TKIs may provide
certain additional benefits for patients with Ph+ ALL
compared with high-dose chemotherapy. For example, a common side
effect of high-dose chemotherapy is poor tolerance, however, TKIs
appear to be associated with improved tolerance. Further evidence
and studies from additional multicenter, prospective, randomized
clinical trials are required to clarify the role of
reduced-intensity chemotherapy combined with imatinib for the
treatment of adults with Ph+ ALL.
Acknowledgements
The authors would like to thank the patients and the
staff who participated in the present study. This study was funded
by a grant from the National Natural Science Foundation of China
for Young Scholars (no. 81302148).
References
|
1
|
Hunger SP and Mullighan CG: Redefining ALL
classification: Toward detecting high-risk ALL and implementing
precision medicine. Blood. 125:3977–3987. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
TeWinkel ML, Peiters R, Wind EJ, Bessems
JH and van den Heuvel-Eibrink MM: Management and treatment of
osteonecrosis in children and adolescents with acute lymphoblastic
leukemia. Haematologica. 99:430–436. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nair M, Kusumakumary P and Nair PS: Rare
presentation of pediatric acute promyelocytic leukemia as multiple
lytic bone lesions: Case report and review of the literature. J
Cancer Res Ther. 10:381–383. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chalandon Y, Thomas X, Hayette S, Cayuela
JM, Abbal C, Huguet F, Raffoux E, Leguay T, Rousselot P, Lepretre
S, et al: Group for Research on Adult Acute Lymphoblastic Leukemia
(GRAALL): Randomized study of reduced-intensity chemotherapy
combined with imatinib in adults with Ph-positive acute
lymphoblastic leukemia. Blood. 125:3711–3719. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ross DM, Branford S, Moore S and Hughes
TP: Limited clinical value of regular bone marrow cytogenetic
analysis in imatinib-treated chronic phase CML patients monitored
by RQ-PCR for BCR-ABL. Leukemia. 20:664–670. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Matutes E, Pickl WF, Van't Veer M, Morilla
R, Swansbury J, Strobl H, Attarbaschi A, Hopfinger G, Ashley S,
Bene MC, et al: Mixed-phenotype acute leukemia: Clinical and
laboratory features and outcome in 100 patients defined according
to the WHO 2008 classification. Blood. 117:3163–3171. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Faderl S, O'Brien S, Pui CH, Stock W,
Wetzler M, Hoelzer D and Kantarjian HM: Adult acute lymphoblastic
leukemia: Concepts and strategies. Cancer. 116:1165–1176. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Leblicq C, Laverdière C, Decarie JC,
Delisle JF, Isler MH, Moghrabi A, Chabot G and Alos N:
Effectiveness of pamidronate as treatment of symptomatic
osteonecrosis occurring in children treated for acute lymphoblastic
leukemia. Pediatr Blood Cancer. 60:741–747. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Atas A, Cakmak A, Soran M, Soker M and
Varma M: Severe osteoporosis and high level TSH in a child before
the diagnosis of acute lymphoblastic leukemia. J Pediat Hematol
Oncol. 31:588–591. 2009. View Article : Google Scholar
|
|
10
|
Bertuna G, Famà P, Lo Nigro L,
Russo-Mancuso G and Di Cataldo A: Marked osteoporosis and
spontaneous vertebral fractures in children: Don't forget, it could
be leukemia. Med Pediatr Oncol. 41:450–451. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hafiz MG and Islam A: Extensive skeletal
lesions in childhood acute lymphoblastic leukemia. Mymensingh Med
J. 18:88–94. 2009.PubMed/NCBI
|
|
12
|
Hafiz MG, Islam A and Siddique R: Back
pain and vertebral compression: An unusual presentation of
childhood acute lymphoblastic leukemia. Mymensingh Med J.
19:130–136. 2010.PubMed/NCBI
|
|
13
|
Kim DH and Vaccaro AR: Osteoporotic
compression fractures of the spine; current options and
considerations for treatment. Spine J. 6:479–487. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kaloostian PE, Zadnik PL, Etame AB,
Vrionis FD, Gokaslan ZL and Sciubba DM: Surgical management of
primary and metastatic spinal tumors. Cancer Control. 21:133–139.
2014.PubMed/NCBI
|
|
15
|
Shah NR, Landi DB, Kreissman SG, Kulbachi
E and Moran C: Presentation and outcomes for children with bone
marrow necrosis and acute lymphoblastic leukemia: A literature
review. J Pediatr Hematol Oncol. 33:e316–e319. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Usuki K, Tojo A, Maeda Y, Kobayashi Y,
Matsuda A, Ohyashiki K, Nakaseko C, Kawaguchi T, Tanaka H, Miyamura
K, et al: Efficacy and safety of nilotinib in Japanese patients
with imatinib-resistant or -intolerant Ph+ CML or
relapsed/refractory Ph+ ALL: A 36-month analysis of a phase I and
II study. Int J Hematol. 95:409–419. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Thomas DA, Faderl S, Cortes J, O'Brien S,
Giles FJ, Kornblau SM, Garcia-Manero G, Keating MJ, Andreeff M,
Jeha S, et al: Treatment of Philadelphia chromosome-positive acute
lymphocytic leukemia with hyper-CVAD and imatinib mesylate. Blood.
103:4396–4407. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mizuta S, Matsuo K, Maeda T, Yujiri T,
Hatta Y, Kimura Y, Ueda Y, Kanamori H, Usui N, Akiyama H, et al:
Prognostic factors influencing clinical outcome of allogeneic
hematopoietic stem cell transplantation following imatinib-based
therapy in BCR-ABL-positive ALL. Blood Cancer J. 2:e722012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ohno R: Changing paradigm of the treatment
of Philadelphia chromosome-positive acute lymphoblastic leukemia.
Curr Hematol Malig R. 5:213–221. 2010. View Article : Google Scholar
|
|
20
|
Philippe R, Marie M, Françoise H, et al:
Dasatinib (Sprycel®) and Low Intensity Chemotherapy for First-Line
Treatment in Patients with De Novo Philadelphia Positive ALL Aged
55 and Over: Final Results of the EWALL-Ph-01 Study. Blood (ASH
Annual Meeting Abstracts). 120:6122012.
|
|
21
|
Mizuta S, Matsuo K, Yagasaki F, et al:
Pre-transplant imatinib-based therapy improves the outcome of
allogeneic hematopoietic stem cell transplantation for
BCR-ABL-positive acute lymphoblastic leukemia. Leukemia. 25:41–47.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Heike P, Sylvia W, Binckebanck A, et al:
Imatinib (IM) and interferon-alpha (IFN-α) maintenance therapy is
associated with long-term DFS in a subset of elderly patients with
Philadelphia-positive acute lymphoblastic leukemia (Ph ALL). Blood
(ASH Annual Meeting Abstracts). 120:15032012.
|