Introduction
The ability of sex hormones to affect lymphocyte
development is well-known (1–3), and particularly the ability of estrogen
to inhibit postnatal thymocyte development (3–6). Extended
exposure to sex steroid hormones, for instance during estrogen
therapy and pregnancy, results in thymic atrophy and loss of
cellularity in humans and animals (3). The estrogen-triggered thymic atrophy may
result in sexual dimorphism in the immune response and
downregulation of autoimmune responses (7–9). A number
of studies have reached the conclusion that estrogen induces thymic
atrophy, and certain studies have demonstrated that estrogen may
affect the development of thymomas and thymic carcinomas (9–11).
However, verification of such findings is not possible due to
limited data, and the expression and distribution of estrogen
receptors in thymomas and thymic carcinomas remain controversial
(10–12). In addition, the biological effect of
estrogen is unclear. Estrogen has been demonstrated to exhibit
pleiotropic effects by binding to intracellular receptors,
including estrogen receptors (ER)α and β. Previous meta-analyses
observed that ERα was not overexpressed in thymic tumors (including
thymomas and thymic carcinomas) compared with in benign thymic
tissues, whereas ERβ was overexpressed, which may indicate binding
to ERβ and complex estrogen physiological effects (12,13).
ERβ has at least five variant isoforms (ERβ1–5),
which have been described in numerous human organs or tissues,
including breast and prostate cancer (13–15).
Notably, no evident structural differences exist among these
variants, for example they all lack certain key parts, such as
helix 12, however, regarding their function and distribution,
certain differences have been identified; ERβ2 is mainly located in
the nucleus whereas ERβ5 is mainly found in the cytoplasm in cancer
tissues (13,14).
Therefore, the present study aimed to investigate
the expression and distribution of ERβ5 in thymomas and thymic
carcinomas, and further analyze the correlation between ERβ5
expression and prognostic factors.
Patients and methods
Patient tissue specimens and
reagents
Specimens from 103 thymoma and thymic carcinoma
patients, who had undergone thymectomy between 1999 and 2010, were
obtained from the Basic Medical College of Xinxiang Medical
University (Xinxiang, China). The study was approved by the Ethics
Committee of the Basic Medical College of Xinxiang Medical
University, and all patients provided informed consent for the use
of their samples. All patient characteristics (including gender and
age) and tumor clinical data were collected. The patient sample
included 68 male and 35 females, with a mean age of 50 years
(range, 41–65 years). According to the histological criteria of the
WHO classification (16), the thymoma
tumor subtypes were as follows: 21 cases of type A; 23 cases of
type AB; 12 cases of type B1; 15 cases of type B2 and 9 cases of
type B3 thymomas. In addition, 23 cases of stage I–IV thymic
carcinomas were identified, according to Masaoka staging (17). The normal thymi of 26 children were
used as controls (mean age, 11 years; age range, 8–15 years),
representing the ERβ5 expression levels in normal tissues. Survival
data, including the overall survival (OS) and progression-free
survival (PFS) rates, were recorded. OS was defined as the time
(months) between the primary surgical treatment and mortality
associated with the thymic tumor. PFS was defined as the interval
(months) between the primary surgical treatment and the initial
locoregional or distant recurrence.
Monoclonal mouse anti-human ERβ5 antibody (clone
5/25; product code, MCA4676T) was obtained from Bio-Rad
Laboratories, Inc. (Hercules, CA, USA). FITC-conjugated goat
anti-human IgG antibody was purchased from Santa Cruz
Biotechnology, Inc., (Dallas, TX, USA). Horseradish
peroxidase-conjugated goat anti-mouse IgG was obtained from Cell
Signaling Technology, Inc. (Danvers, MA, USA). The thymic carcinoma
cell line, TC1889, and the thymoma cell line, T1682, were
established, characterized and verified as previously described
(18).
Immune microarray and evaluation of
immunoreactivity
Tissue microarrays (TMAs) were constructed from the
paraffin-embedded blocks of the 129 thymic specimens, using a
tissue array device (cat. no. BNSW-001_TY9184; Shanghai Outdo
Biotech Co., Ltd., Shanghai, China). Representative tumor areas
were marked in each paraffin-embedded specimen and at least two
areas were sampled. The diameter of the tissue cylinders was 0.6
mm, made using tissue chip drilling apparatus (Shanghai Outdo
Biotech Co., Ltd.). For ERβ5 staining, the monoclonal ERβ5 antibody
(clone 5/25) was used at a dilution of 1:50, which was demonstrated
to be highly specific in immunohistochemical assays performed in
this study. Antigen retrieval was performed in 0.01 mol/l sodium
citrate buffer (pH 6.0) in the microwave for 15 min. To establish
the negative controls, the same procedure was followed, without the
primary antibody. A previously described scoring system was adopted
(19), the tissue microarrays were
digitized and cytoplasmic ERβ5 (cERβ5) or nuclear ERβ5 (nERβ5)
immunoreactivity was scored between - and +++ (−, no staining; +,
weak staining; ++, moderate staining; +++, strong staining). The
percentage of tumor cells displaying staining for cERβ5 or nERβ5
was determined and calculated as the average of six high power
fields per specimen. The cases were scored independently by three
specialists and discordant results were re-evaluated to reach
consensus.
Indirect immunofluorescence assay
(IIFA)
TC1889 and T1682 cells were cultured in RPMI-media
containing HEPES supplemented with 10% fetal calf serum and 1%
penicillin/streptomycin (Sigma-Aldrich) in an atmosphere containing
5% CO2 at 37°C. For IIFA, the anti-ERβ5 antibody (clone
5/25) was incubated at a dilution of 1:500 at 4°C overnight.
Fluorescein isothiocyanate-conjugated goat anti-human
immunoglobulin G (Santa Cruz Biotechnology, Inc., Santa Cruz, CA,
USA) was used as the secondary antibody and incubated at a dilution
of 1:100 for 1 h at room temperature. Cells were washed with
phosphate-buffered saline (J-H Biotechnology Ltd., Shanghai, China)
and mounted using 10 mg/ml DAPI (Sigma-Aldrich) in aqueous mountant
(Dako North America, Inc., Carpinteria, CA, USA). A fluorescence
microscope (Leica DM1000; Leica Microsystems GmbH, Wetzlar,
Germany) was used for examination of the samples.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted using the RNeasy mini kit
(Qiagen, Crawley, UK) with additional purification by
centrifugation at 12,000 × g for 15 min through QIAshredder spin
columns (Qiagen). The total RNA concentration and purity were
calculated using the Nanodrop system (Labtech International Ltd.,
Lewes, UK). Subsequently, cDNA synthesis was performed using the
SYBR® ExScript RT-PCR kit (Takara Biotechnology Co., Ltd., Dalian,
China) according to the manufacturer's instructions. Quantitative
PCR was performed using the SYBR® Premix Ex Taq™ II kit (Takara
Biotechnology Co., Ltd.) with a LightCycler system (Roche
Diagnostics, Basel, Switzerland). The primers used were as follows:
ERβ5 forward, 5′-CGGAAGCTGGCTCACTTGCT-3′, and reverse,
5′-CTTCACCCTCCGTGGAGCAC-3′; and β-actin forward,
5′-GTGGGGCGCCCAGGCACCAC-3′, and reverse,
5′-CTCCTTAATGTCACGCACGATTT-3′. The reaction volume was 50 µl and
comprised the following final quantities/concentrations: 1,000 ng
ERβ5 or 100 ng β-actin cDNA, 0.2 µM of each primer, 1 U AmpliTaq
Gold DNA polymerase (Life Technologies, Grand Island, NY, USA), 1.5
mM MgCl2 and 200 µM deoxynucleotide triphosphate. The
cycling conditions included a denaturation step at 94°C for 10 min,
45 cycles (for ERβ5) or 25 cycles (for β-actin; RNA input) at 94°C
for 45 sec, 55°C for 60 sec and 72°C for 45 sec, and a final
extension step at 72°C for 10 min. For each primer, serial
dilutions of a standard cDNA were amplified to create a standard
curve, and values of unknown samples were estimated relative to
this standard curve in order to assess the PCR efficiency.
Threshold cycle (Ct) values were collected for β-actin and the
genes of interest during log phase of the cycle. Gene of interest
levels were normalized to β-actin for each sample [ΔCt = Ct(gene of
interest) - Ct(β-actin)]. The samples were resolved on 2% agarose
gel and transferred to a nylon transfer membrane (Hybond-N+; GE
Healthcare Life Sciences, Chalfont, UK). Samples were then analyzed
using ABI PRISM 7000 SDS Software (Applied Biosystems Life
Technologies, Foster City, CA, USA).
Statistical analysis
Mann-Whitney U test, Kruskal-Wallis test and
Spearman's rank correlation were performed using the SAS software
(version 9.12; SAS Institute Inc., Cary, NC, USA). Associations
with OS were initially analyzed by Kaplan-Meier plots (log-rank
test). P<0.05 was considered to indicate a statistically
significant difference.
Results
Expression of ERβ5 in thymic tumors
and normal thymic tissues
The results of the ERβ5 immunohistochemical assay
for the 129 cases are listed in Tables
I–III. The majority of thymic
tumors exhibited ERβ5-positive staining (99.02%; 102/103 cases),
with only one case presenting negative staining. In addition,
87.37% (90/103) of the cases were positive for cERβ5, 11.62% of the
cases were positive for nERβ5 and 15 cases were positive for nERβ5
and cERβ5 (Table I; Fig. 1 A1-A6). In particular, the thymic
carcinomas exhibited strong positive staining, while only 38.46% of
normal thymic tissues (10/26; Table
I) exhibited positive staining. Furthermore, overexpression of
cERβ5 was observed in the thymic tumors compared with normal thymic
tissues, and this difference was statistically significant
(P=0.001); by contrast, nERβ5 was not overexpressed in thymic
tumors (P=0.112; Table I). Similar
results were observed in the T1682 and TC1889 cell lines following
IIFA (Fig. 1B).
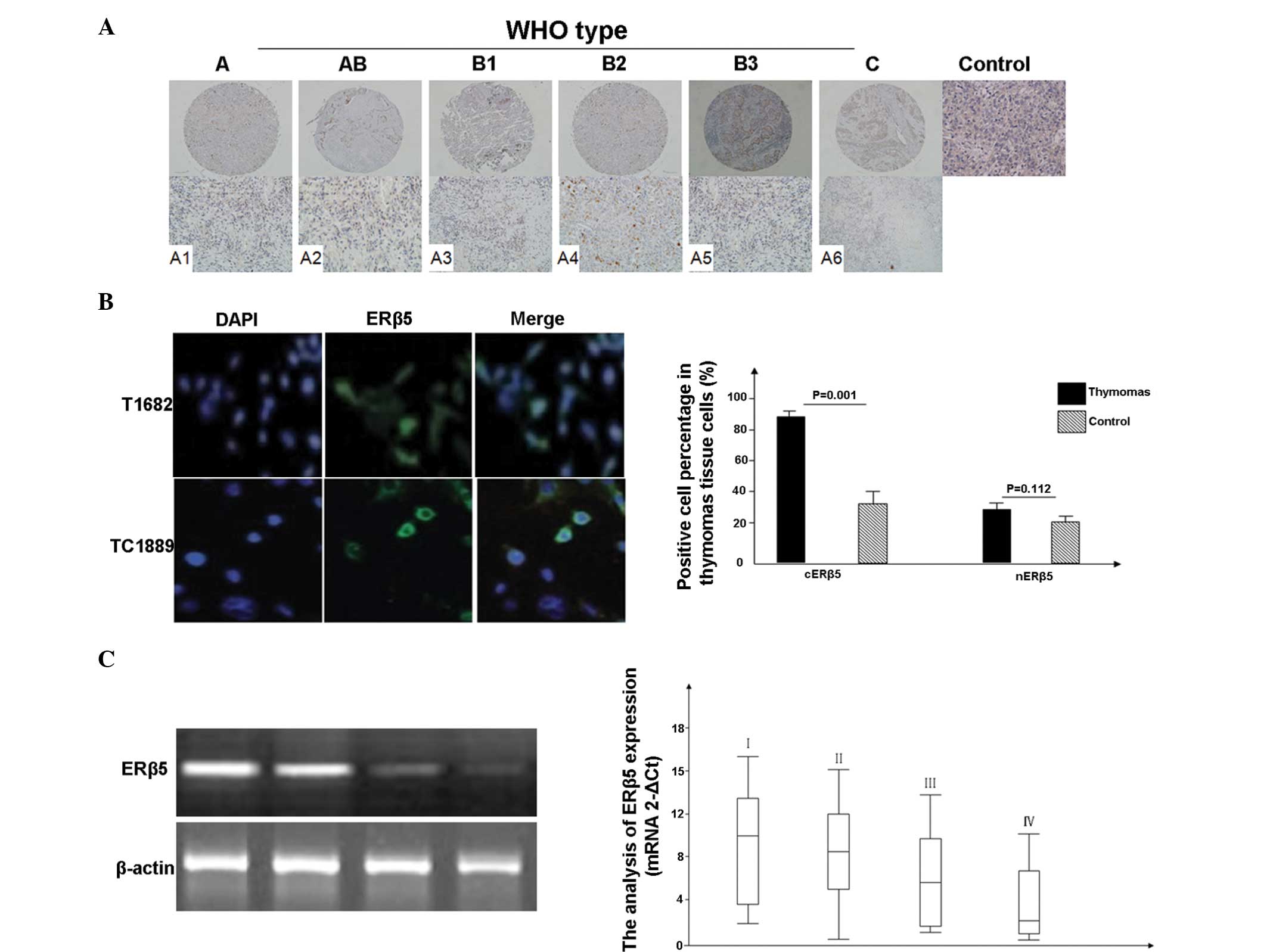 | Figure 1.Expression of ERβ5 in thymic tumors,
determined using immunohistochemical tissue microarray staining and
reverse transcription-quantitative polymerase chain reaction
(RT-qPCR). The immunopositive signal of ERβ5 protein was graded
according to the WHO staging system. (A) ERβ5 expression was mainly
detected in the cytoplasm of thymomas epithelial cells (A1-A6;
upper lane magnification, ×100; lower lane magnification, ×200).
Normal thymic tissues without primary antibody staining were used
as the control group. Overexpression of cytoplasmic ERβ5 was
observed in thymic carcinomas and thymomas, compared with the
normal thymic tissues. (B) ERβ5 expression was observed in the
T1682 and TC1889 cell lines through indirect immunofluorescence.
(C) RT-qPCR results, obtained from 49 cases of thymic tumors
(clinical stages: I, 7 cases; II, 15 cases; III, 15 cases; and IV,
12 cases). Data for benign tissue samples are not shown. The
results revealed that the mean level of ERβ5 gene expression was
lower in advanced clinical stages. ERβ5, estrogen receptor β5. |
 | Table I.Expression of nuclear and cytoplasmic
ERβ5 in thymic tumor and normal tissues. |
Table I.
Expression of nuclear and cytoplasmic
ERβ5 in thymic tumor and normal tissues.
|
| cERβ5, n |
| nERβ5, n |
|
|---|
|
|
|
|
|
|
|---|
| Groups | - | + | ++ | +++ | P-value | - | + | ++ | +++ | P-value |
|---|
| Thymic tumor | 13 | 18 | 49 | 23 | 0.001 | 76 | 20 | 6 | 1 | 0.112 |
| Normal | 18 | 5 | 1 | 2 |
| 22 | 2 | 1 | 1 |
|
| Total | 31 | 23 | 50 | 25 |
| 98 | 22 | 7 | 2 |
|
 | Table III.Correlation between cytoplasmic ERβ5
expression and tumor pathological characteristics. |
Table III.
Correlation between cytoplasmic ERβ5
expression and tumor pathological characteristics.
|
| cERβ5 |
|
|
|---|
|
|
|
|
|
|---|
| Pathological
classification | - | + | ++ | +++ |
P-valuea |
R-valueb |
|---|
| Tumor subtype |
|
|
|
| 0.024 | 0.088 |
| A | 4 | 3 | 9 | 5 |
|
|
| AB | 5 | 0 | 13 | 5 |
|
|
| B1 | 3 | 0 | 5 | 4 |
|
|
| B2 | 1 | 6 | 7 | 1 |
|
|
| B3 | 0 | 4 | 2 | 3 |
|
|
| C | 0 | 5 | 13 | 5 |
|
|
| Clinical stage |
|
|
|
| 0.003 | −0.376 |
| I | 0 | 0 | 2 | 8 |
|
|
| II | 3 | 5 | 25 | 9 |
|
|
|
III | 5 | 10 | 16 | 3 |
|
|
| IV | 5 | 3 | 5 | 4 |
|
|
mRNA quantification was conducted during RT-qPCR,
and the results were compared against β-actin, which was used as
the internal reference gene. The ERβ5 expression levels ranged
between 0.468 and 13.292 (median, 5.672; data not shown). In
advanced clinical stages, the mean level of ERβ5 gene expression
was lower (Fig. 1C).
Association between cERβ5 or nERβ5
expression levels and patient characteristics
The association between ERβ5 expression and a range
of standard patient characteristics were investigated and are
listed in Table II. No positive
correlation was detected between cERβ5 expression and patient
characteristics, including gender, age and tumor sizes (P=0.245,
P=0.514 and P=0.614, respectively). Notably, although no
statistically significant association was observed between tumor
sizes and cERβ5 expression, the latter was demonstrated to be
important in the progression of thymic tumors (Table II).
 | Table II.Correlation between cytoplasmic and
nuclear ERβ5 expression and patient characteristics. |
Table II.
Correlation between cytoplasmic and
nuclear ERβ5 expression and patient characteristics.
|
| nERβ5, n |
| cERβ5, n |
|
|---|
|
|
|
|
|
|
|---|
| Characteristic | - | + | P-value | - | + | P-value |
|---|
| Gender |
|
| 0.131 |
|
| 0.245 |
| Male | 48 | 20 |
| 7 | 61 |
|
|
Female | 29 | 6 |
| 6 | 29 |
|
| Age |
|
| 0.576 |
|
| 0.514 |
| <50
years | 10 | 3 |
| 2 | 11 |
|
| ≥50
years | 67 | 23 |
| 11 | 79 |
|
| Tumor size |
|
| 0.132 |
|
| 0.614 |
| <6
cm | 27 | 13 |
| 5 | 35 |
|
| ≥6
cm | 50 | 13 |
| 8 | 55 |
|
In addition, no statistically significant
association was determined between nERβ5 expression and patient
characteristics.
Association of ERβ5 staining with
histological subtype and stage
Since cERβ5 was overexpressed in the thymic tumors,
a statistically significant correlation was observed between cERβ5
expression and thymoma subtypes (P=0.024; Table III), which presented a particularly
strong positive expression in thymic carcinomas. In addition, a
statistically significant difference was identified between cERβ5
staining and thymic tumor stages (P=0.003); however, a negative
correlation was observed (Table
III; R=-0.376).
By contrast, no statistically significant
differences were observed between nERβ5 staining and thymomas
subtypes or stages (P=0.653; data not shown).
Kaplan-Meier plot results of TMA
analysis
The TMA analysis revealed that cERβ5 staining was
significantly associated with improved OS, whereas nERβ5
immunoreactivity was not associated with OS (data not shown). In
addition, cERβ5 staining was correlated with improved PFS, which
implied that cERβ5 had a negative biological effect in the
development of thymic tumors (Fig.
2).
Discussion
Exposure to sex steroid hormones, for instance
during estrogen therapy, results in thymic atrophy and loss of
cellularity in animals (18,20,21).
Thymic atrophy induced by estrogens contributes towards certain
complicated and unclear mechanisms. A large number of studies have
reported that estrogen is involved in biological functions in human
and animal organs through estrogen receptors, including ERα and ERβ
(which has at least five isoforms, such as ERβ5) (22–24). At
present, controversial findings exist on the expression of estrogen
receptors in thymic tumors, since a number studies reported
overexpression of ERβ in these tumors, whereas others studies
obtained contradictory results (11,12,13,19).
In the present study, the expression of ERβ5 was
investigated by immunohistochemistry. Overexpression of ERβ was
identified and strong evidence was provided on the controversial
function of estrogen receptors in thymic tumors; however, the
expression of ERα was not investigated in the present study.
To the best of our knowledge, the present study is
the first to compare the protein expression levels of ERβ5 in
specimens from a cohort of patients with thymic tumors (n=103). The
immunohistochemical expression of ERβ5 was analyzed in a set of
TMAs using an ERβ5 specific antibody. The results revealed that
ERβ5 was overexpressed and predominantly located in the cytoplasm
of thymic tumors, which indicated the important role of this
receptor in the progression of thymic tumors. Furthermore, the
present study identified statistically significant differences
between cERβ5 expression and thymic tumor subtypes and stages.
Notably, a negative correlation was identified between a high
expression of cERβ5 and tumor stages (R=-0.376), indicating that
cERβ5 may inhibit thymic tumor progression, providing an insight
into the estrogen biological mechanism.
As previously reported (25–27), the
classic hormonal mechanism involves the binding of estrogens to ERs
in the nucleus, thus promoting the association with specific
estrogen response elements in the promoters of target genes. At the
same time, ERs regulate the expression of numerous genes without
directly binding to DNA, but through protein-protein interactions
with certain factors, such as phosphoinositide 3-kinase. According
to the results of the present study, ERβ5 was mainly localized in
the cytoplasm, which may indicate that estrogen activated cERβ5 by
protein-protein interaction signaling and then inhibited thymic
tumor development.
Further analysis using Kaplan-Meier plots revealed
that a high expression of cERβ5 was a significant prognostic factor
of thymic tumors. In addition, the present study indicated that
high cERβ5 expression in thymic tumors was correlated with longer
OS and PFS, which was in accordance with previous results (18,20,21,23,28,29).
However, the fact that cERβ5 was overexpressed in thymic tumors,
while having an inhibiting biological effect, suggested that cERβ5
may be involved in other functions of the thymic tumor development
and progression. The results of the present study indicated that
the underlying mechanism of estrogen may be complex in thymic tumor
development and requires further investigation.
Due to difficulties in the identification of ERβ
isoforms in human thymic tumors, as well as other factors, ERβ
variants have not been previously reported in detail. To the best
of our knowledge, in the present study, ERβ5 was identified in
thymic tumor tissues for the first time; however, it was unclear
whether other ERβ isoforms were also expressed, as reported in some
cancer tissues, including breast and prostate cancer (28–30). The
results of the present study demonstrated that estrogen exerts a
biological effect on thymic tumors through ERβ5, at least.
Furthermore, the underlying mechanism may involve protein-protein
interaction signaling in the thymic tumor cell cytoplasm, although
this remains unclear.
In conclusion, the present study suggested that, in
part, ERβ5-mediated functions may be a potential underlying
mechanism through which estrogens alter susceptibility to thymic
tumors. In order to develop more selective and specific ER
modulators for the treatment of thymic tumor patients, further
studies are required on ligand activation of ERβ5-mediated
functions in thymic tumor patients.
References
|
1
|
Ishibashi H, Suzuki T, Suzuki S, et al:
Sex steroid hormone receptors in human thymoma. J Clin Endocrinol
Metab. 88:2309–2317. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wang C, Dehghani B, Magrisso IJ, et al:
GPR30 contributes to estrogen-induced thymic atrophy. Mol
Endocrinol. 22:636–648. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pennell LM, Galligan CL and Fish EN: Sex
affects immunity. J Autoimmun. 38:J282–J291. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hirahara H, Ogawa M, Kimura M, et al:
Glucocorticoid independence of acute thymic involution induced by
lymphotoxin and estrogen. Cell Immunol. 153:401–411. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Offner H and Polanczyk M: A potential role
for estrogen in experimental autoimmune encephalomyelitis and
multiple sclerosis. Ann NY Acad Sci. 1089:343–372. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shames RS: Gender differences in the
development and function of the immune system. J Adolesc Health 30
(Suppl). 59–70. 2002. View Article : Google Scholar
|
|
7
|
Panchanathan R and Choubey D: Murine BAFF
expression is up-regulated by estrogen and interferons:
implications for sex bias in the development of autoimmunity. Mol
Immunol. 53:15–23. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Do Y, Ryu S, Nagarkatti M and Nagarkatti
PS: Role of death receptor pathway in estradiol-induced T-cell
apoptosis in vivo. Toxicol Sci. 70:63–72. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hengartner MO: The biochemistry of
apoptosis. Nature. 407:770–776. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Okasha SA, Ryu S, Do Y, McKallip RJ, et
al: Evidence for estradiol-induced apoptosis and dysregulated T
cell maturation in the thymus. Toxicology. 163:49–62. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zoller AL, Schnell FJ and Kersh GJ: Murine
pregnancy leads to reduced proliferation of maternal thymocytes and
decreased thymic emigration. Immunology. 121:207–215. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mimae T, Tsuta K, Takahashi F, et al:
Steroid receptor expression in thymomas and thymic carcinomas.
Cancer. 117:4396–4405. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Collins F, MacPherson S, Brown P, et al:
Expression of oestrogen receptors, ERα, ERβ5 and ERβ variants, in
endometrial cancers and evidence that prostaglandin F may play a
role in regulating expression of ERα. BMC Cancer. 9:3302009.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Leung YK, Mak P, Hassan S and Ho SM:
Estrogen receptor (ER)-beta isoforms: a key to understanding
ER-beta signaling. Proc Natl Acad Sci USA. 103:13162–13167. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Murphy LC, Peng B, Lewis A, et al:
Inducible upregulation of oestorgen receptor-beta1 affects
oestrogen and tamoxifen responsiveness in MCF7 human breast cancer
cells. J Mol Endocrinol. 34:553–566. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Marx A and Müller-Hermelink HK: From basic
immunobiology to the upcoming WHO-classification of tumors of the
thymus. The Second Conference on Biological and Clinical Aspects of
Thymic Epithelial Tumors and related recent developments. Pathol
Res Pract. 195:515–533. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yamakawa Y, Masaoka A, Hashimoto T, et al:
A tentative tumor-node-metastasis classification of thymoma.
Cancer. 68:1984–1987. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Breinig M, Mayer P, Harjung A, et al: Heat
shock protein 90-sheltered overexpression of insulin-like growth
factor 1 receptor contributes to malignancy of thymic epithelial
tumors. Clin Cancer Res. 17:2237–2249. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Moser B, Janik S, Schiefer AI, et al:
Expression of RAGE and HMGB1 in thymic epithelial tumors, thymic
hyperplasia and regular thymic morphology. PLoS One. 9:e941182014.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Olde B and Leeb-Lundberg LM: GPR30/GPER1:
searching for a role in estrogen physiology. Trends Endocrinol
Metab. 20:409–416. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lang TJ: Estrogen as an immunomodulator.
Clin Immunol. 113:224–230. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
SpencerSegal JL, Tsuda MC, Mattei L,
Waters EM, et al: Estradiol acts via estrogen receptors alpha and
beta on pathways important for synaptic plasticity in the mouse
hippocampal formation. Neuroscience. 202:131–146. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hartman J, Ström A and Gustafsson JA:
Current concepts and significance of estrogen receptor β in
prostate cancer. Steroids. 77:1262–1266. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cammarata PR, Flynn J, Gottipati S, et al:
Differential expression and comparative subcellular localization of
estrogen receptor beta isoforms in virally transformed and normal
cultured human lens epithelial cells. Exp Eye Res. 81:165–175.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Loven MA, Wood JR and Nardulli AM:
Interaction of estrogen receptors α and β with estrogen response
elements. Mol Cell Endocrinol. 181:151–163. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sheldahl LC, Shapiro RA, Bryant DN, et al:
Estrogen induces rapid translocation of estrogen receptor β, but
not estrogen receptor α, to the neuronal plasma membrane.
Neuroscience. 153:751–761. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mhyre AJ and Dorsa DM: Estrogen activates
rapid signaling in the brain: Role of estrogen receptor α and
estrogen receptor β in neurons and glia. Neuroscience. 138:851–858.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Scobie GA, Macpherson S, Millar MR, et al:
Human oestrogen receptors: differential expression of ER alpha and
beta and the identification of ER beta variants. Steroids.
67:985–992. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Omoto Y, Kobayashi S, Inoue S, et al:
Evaluation of oestrogen receptor β wild-type and variant protein
expression and relationship with clinicopathological factors in
breast cancers. Eur J Cancer. 38:380–386. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang X and Kilgore MW: Signal cross-talk
between estrogen receptor alpha and beta and the peroxisome
proliferator-activated receptor gamma1 in MDA-MB-231 and MCF-7
breast cancer cells. Mol Cell Endocrinol. 194:123–133. 2002.
View Article : Google Scholar : PubMed/NCBI
|
















