Introduction
Approximately 90% of all malignant tumors of the
vulva are squamous cell carcinomas. Sarcomas of the vulva
constitute a variety of malignant neoplasms that account for 1–2%
of vulvar cancers (1,2); the most common are rhabdomyosarcoma,
leimyosarcoma, epithelioid sarcoma, alveolar sarcoma of the soft
tissues and liposarcoma (3).
In accordance with the 2013 World Health
Organization (WHO) histological classification of tumors (4), sarcomas are classified into soft-tissue
tumors and subclassified into malignant soft-tissue tumors.
Extraskeletal myxoid chondrosarcoma (ESMC) represent <3% of all
soft-tissue sarcomas (5).
Stout and Verner first described ESMC in 1953
(6). In 1994, ESMC was included into
the group of soft-tissue tumors with uncertain differentiation
(7).
ESMC has a male to female ratio of 2:1, with the
peak occurrence in the sixth decade of life (8). ESMC is more commonly observed in the
lower extremities, however, cases of ESMC of the orbit, shoulder
and upper extremities have been reported (7,9,10–17). The
majority of these tumors are solitary, deeply-seated, superficial,
slowly-growing nodules, measuring 5–10 cm in diameter. The lesions
are typically well-circumscribed and have a gelatinous appearance,
often with hemorrhagic foci (18).
Light microscopic examination reveals the presence
of rather monomeric, small, round or oval cells with centrally
located hyperchromic or vesicular nucleus, with evenly distributed
chromatin, scant eosinophilic cytoplasm and often no evident
nucleolus. Cells form straight or bent linear chains anastomosing
between each other and forming a lacy arcade pattern. Perivascular
condensation of the tumor cells, with formation of pseudorosettes,
also occurs. Tumor structures are located in the prominent
basophilic myxoid slightly vascular matrix. The tumor is divided
into small lobules by narrow fibrous septa of different
thicknesses. The peripheral region of the tumor is characterized by
high cellularity, often with the evidence of rhabdoid and
epithelioid cells. Cysts, hemorrhages and geographic necroses can
occur in the tumor tissues (7,19–23).
Irrespective of ESMC localization, pre-operative
diagnosis may be difficult, and thus verification is carried out
based on the results of core-biopsy, followed by the evaluation of
immunohistochemical and histological changes in the tumor tissue
(24).
In accordance with the minimum clinical
recommendations of the European Society of Medical Oncology (2014)
(25), wide excision of the tumor
with negative margins (R0 resection) is the standard surgical
method for soft-tissue sarcomas. The treatment of patients with
localized ESMC should include primary tumor excision with a wide
surgical margin. High-dose radiation therapy has previously been
reported to elicit a response from ESMC, while adjuvant
chemotherapy has been reported to elicit only a poor response
(26). Radiation therapy is
recommended for high-grade sarcomas, moderately-differentiated
tumors, positive surgical margins and recurrent sarcoma (27–29). The
two drugs with the highest established response rates in
soft-tissue sarcoma are doxorubicin and ifosfamide, and this drug
combination has been used as the gold standard for a number of
years. However, this chemotherapy schedule has been found to be
ineffective for ESMC (30).
Interferon α-2b has been investigated in several studies, showing
promising results (31). Gemcitabine,
methotrexate and imatinib can also be administered for this type of
tumor in an adjuvant regimen (32).
In 2010, a study by Geyer and Karlin reported that there were no
studies showing the efficiency of the modern chemotherapy regimens
for ESMC (33). Adjuvant radiation
therapy for ESMC is used prior to chemotherapy in cases with
positive surgical margins and in cases where it is impossible to
perform a resection (32).
ESMC has been described as slowly growing and late
to metastasize. Due to the rarity, protracted clinical course and
prolonged survival of patients with ESMC, long-term follow-up is
recommended for the early detection of local recurrence and distant
metastases. ESMC has been reported to have a relatively good
prognosis. However, ESMC has a high potential for metastasis,
particularly to the lungs, regional lymph nodes and bones (21,34–36).
Enzinger and Shiraki considered that neither the tumor localization
nor the tumor size affected the disease prognosis, however, the
study underlined the fact that the prognosis is associated with the
histological grade (9). A more recent
study by Oliviera et al defined high cellularity, large
tumor size, presence of anaplasia or rhabdoid features, high
mitotic activity (>2 per 10 high-power fields) and proliferative
activity (Ki-67, ≥10%; Ki-67 ‘hot spot’, ≥25%) as adverse
prognostic factors (37).
Meis-Kindblom et al analyzed 117 cases with ESMC and showed
that the clinical parameters, but not the histological features,
were associated with decreased survival (35).
The overall 5-, 10-, and 15-year survival rates of
patients with ESMC are reported to be 82–90, 63–70 and 58%,
respectively. Two-thirds of patients develop recurrences and more
than half of the patients develop metastases. Local recurrences are
observed in 48% of cases (half of these are multiple local
recurrences) and metastatic recurrences are observed in 46% of
cases (26,32,35).
The present study reports a case of recurrent ESMC
of the vulva in a 32-year-old female. Written informed consent was
obtained from the patient.
Case report
In June 2011, a 32-year-old female presented to a
gynecological clinic (Lensk, Russia) with a painful lesion on the
right vulva arising after a trauma. Ultrasound examination revealed
a round hypovascular mass, measuring 53×34×37 mm, in the soft
tissues of the vulva. The patient underwent surgical excision of
the mass. The conclusive diagnosis of the surgical specimen was of
an organized hematoma.
A mass that was gradually increasingly painful was
observed at the site of the excision at 7 months post-surgery. The
patient was then referred to the Cancer Research Institute
(Siberian Branch of the Russian Academy of Medical Sciences, Tomsk,
Russia).
Gynecological examination revealed a tumor mass in
the upper and middle thirds of the right labia majora, with
involvement of the pubic area. The lesion consisted of multiple
nodules with uneven density and well-defined borders, and was
painful when palpated.
Histological analysis showed a spindle-like cell
sarcoma [G2 (4)]. Examination of the
tumor tissue revealed small monomeric cells with scant eosinophilic
cytoplasm. Tumor cells formed straight or linear chains and
exhibited anastomosis, forming an arcade pattern. The tumor
structures were located in the prominent basophilic myxoid with a
slightly vascular matrix. Hemorrhage and geographic necrosis were
also evident in the tumor tissue.
The ultrasonographic findings revealed a
dumbbell-shaped lesion measuring 27×70 mm, with a heterogeneous
structure, sharp and smooth contour.
Spiral computed tomography scan revealed a
well-defined multiple nodular lesion of soft-tissue density,
actively accumulating the contrast, which was located in the soft
tissues at the level of the symphysis pubis, on the right-hand
side. The tumor extended to the right labia majora. Surrounding
cellular tissue was thickened, but adjacent bone structures were
unchanged. There was no evidence of lymph node involvement and no
metastases were revealed.
No pathological changes were noted in the abdomen or
chest.
The patient underwent wide excision of the tumor
with reconstructive plastic surgery using local tissue flaps. The
tumor was removed through a vertical incision. Cytological
examination showed negative surgical margins. Intraoperative
radiation therapy (IORT) at a single dose of 10 Gy was delivered to
the bed of the removed tumor (Fig.
1). The wound was the closed layer by layer. There were no
complications in the post-operative period.
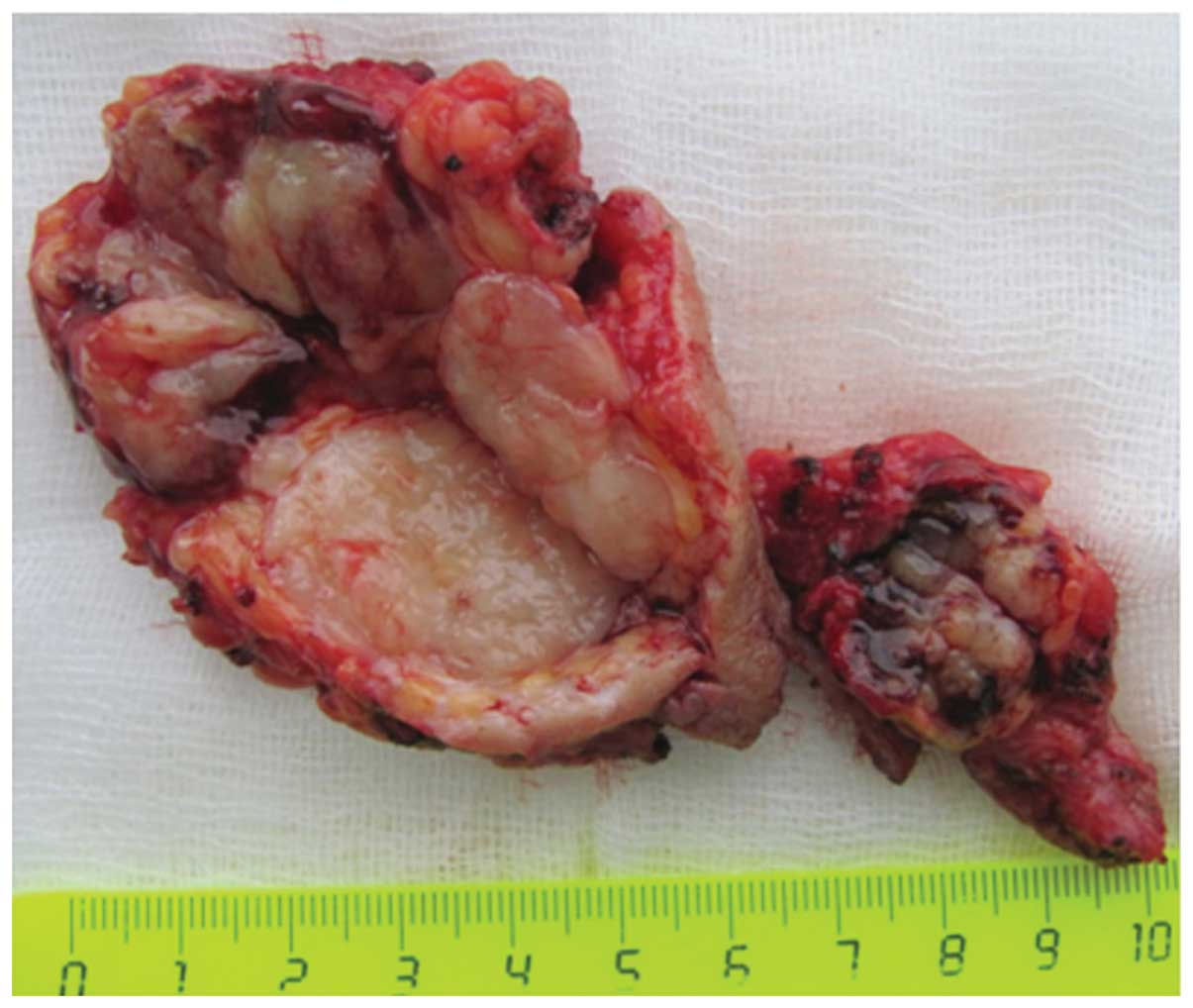
Macroscopic imaging of the surgical specimen showed
a tumor with multiple nodules, measuring 5 cm at its widest, which
was mainly of elastic consistency, with a well-defined fibrotic
capsule, and with areas containing gelatinous material and
hemorrhages (Fig. 2).
A pathological study of this case revealed that the
tumor was circumscribed by a pseudocapsule and consisted mainly of
moderately polymorphic spindle-shaped cells and to a lesser extent,
epithelioid cells, with swollen or prolate nuclei, vesicular
chromatin, with one hypertrophic hyperchromic nucleolus and with
bipolar amphophilic cytoplasmic processes. Cells formed syncytial,
clustered and lacy structures immersed in the abundant myxoid
matrix, with mild diffuse lymphoid infiltration. Fields of rhabdoid
cells and foci of necrosis were observed within the tumor. A few
atypical multinucleated giant cells were also present. Moderate
mitotic activity with the presence of pathology forms was noted.
The tumor tissue, mainly in peripheral areas, was divided into
fields by fibrous bands with mild diffuse infiltration (Fig. 3). The majority of the tumor cells
expressed neuron-specific enolase (monoclonal anti-human mouse
antibody; clone BBS/NC/VI-H14; 1:600; Dako, Glostrup, Denmark)
(Fig. 4). S-100 (monoclonal
anti-human rabbit antibody; 1:600; Dako) was weakly expressed in
the nuclei of certain cells. No expression was detected for
pan-cytokeratin (monoclonal anti-human mouse antibody; 5/6/8/18;
сlones 5D3 and LP34; 1:100; Leica Biosystems, Wetzlar, Germany),
muscle actin (monoclonal anti-human mouse antibody; clone 1A4;
1:100; Dako), cluster of differentiation 34 (monoclonal anti-human
mouse antibody; clone QBEnd 10; 1:100; Dako), desmin (monoclonal
anti-human mouse antibody; clone DE-R-11; 1:100; Dako), MyoD1
(monoclonal anti-human mouse antibody; clone 5.8A; 1:140; Dako) and
synaptophysin (monoclonal anti-human mouse antibody; clone SY38;
1:200; Dako). The proliferative activity of the tumor was high,
with a Ki-67 of 26%.
The histological structure and immunophenotype of
the tumor cells demonstrated an ESMC, G2 (4). Soft-tissue sarcoma of the vulva, stage
Ib (T1bN0M0) was diagnosed.
The patient refused chemotherapy. A new lesion
appeared in the right inguinal region 5 months after the surgery
for recurrent vulvar cancer.
Magnetic resonance imaging showed heterogeneous
enhancement of enlarged lymph nodes, measuring 37×25×34 mm, in the
right inguinal region. Cytological examination revealed sarcoma
cells. Due to disease progression, a bilateral inguinal-femoral
lymph node dissection was performed. Metastatic involvement of ESMC
in 1 of 13 lymph nodes was histologically confirmed. The patient
subsequently received 40 Gy external beam radiation therapy to the
inguinal region. The patient is currently alive with no evidence of
disease progression at the 7 month follow-up.
Discussion
ESMC of the vulva is an extremely rare malignant
neoplasm. Only 4 clinical cases have been reported in the
literature to date (38–41). The first clinical case was described
in 1996, where the diagnosis of ESMC was established in a
40-year-old female with a tumor in the left labium majus. Following
a wide excision of the vulvar tumor and inguinal lymphodissection,
the patient was followed up for 40 months with no evidence of
recurrence (39). The second clinical
case of ESMC occurred in a 46-year-old female and was published in
2005 (40). In two other recent
studies (2011), the patients were aged 24 and 66 years (38,41). In
the 24-year-old female, the histological diagnosis was established
only after total biopsy. The patient underwent vulvectomy with
vulvoperitoneal reconstruction. Microscopic examination of the
resected specimens revealed ESMC tumor nodules. However, no viable
tumor cells were present at the surgical margin. The duration of
recurrence-free follow-up was 2 years (41). The ESMC case of the 66-year-old
patient was notable due to a prolonged postmenopausal period (20
years), large tumor size (8×12 сm) and combination treatment
involving complete removal of the tumor and adjuvant radiation
therapy. There was no evidence of recurrence after 1 year of
follow-up (38).
There is little comparative data supporting the
superiority of specific treatment regimens due to the low incidence
of the ESMC.
In the present clinical case, ESMC was diagnosed too
late for antitumor treatment to be effective. Improvement of
optical imaging techniques, including histochemical methods, can
contribute to the early diagnosis of ESMC of the vulva (9).
Due to the absence of a unified approach to the
treatment of recurrent tumors, intraoperative radiation therapy to
the bed of the removed tumor was selected in the present study,
although there were have been no studies regarding the efficiency
of IORT for vulvar sarcomas, including ESMC.
In spite of the obscure pathogenesis and absence of
a pathogenetically proven therapeutic strategy for vulvar sarcomas,
including ESMC of the vulva, the analysis of each clinical case has
a high value and can contribute to the development of the optimal
treatment policy.
References
|
1
|
Bodurka DC and Gershenson DM: Sarcomas of
reproductive tract. Atlas Clin Oncol Soft Tissue Sarcomas.
15:213–227. 2002.
|
|
2
|
Tavassoli FA and Norris HJ: Smooth muscle
tumors of the vulva. Obstet Gynecol. 53:213–217. 1979.PubMed/NCBI
|
|
3
|
Korzhaevskaya YV, Kuznetsov VV and Gritsay
AH: Sarcomas of the vulva. Sib Onkol Zh. 2:10–14. 2008.(In
Russian).
|
|
4
|
Lucas DR and Stenman G: Tumours of
uncertain differentiationWorld Health Organization Classification
of Tumours. Pathology and Genetics of Tumours of Soft Tissue and
Bone. Fletcher CDM, Bridge JA, Hogendoorn PCW and Mertens F: 4th.
IARC Press; Lyon: pp. 223–224. 2013
|
|
5
|
Tsuneyoshi M, Enjoji M, Iwasaki H and
Shinohara N: Extraskeletal myxoid chondrosarcoma - a
clinicopathologic and electron microscopic study. Acta Pathol Jpn.
31:439–447. 1981.PubMed/NCBI
|
|
6
|
Stout AP and Verner EW: Chondrosarcoma of
extraskeletal soft tissues. Cancer. 6:581–590. 1953. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lucas DR and Heim S: Tumours of uncertain
differentiationPathology and Genetics of Tumours of Soft Tissue and
Bone. Fletcher CDM, Unni KK and Mertens F: IARC Press; Lyon: pp.
184–224. 2002
|
|
8
|
Hachitanda Y, Tsuneyoshi M, Daimaru Y,
Enjoji M, Nakagawara A, Ikeda K and Sueishi K: Extraskeletal myxoid
chondrosarcoma in young children. Cancer. 61:2521–2526. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Enzinger FM and Shiraki M: Extraskeletal
myxoid chondrosarcoma. An analysis of 34 cases. Hum Pathol.
3:421–435. 1972. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fukuda T, Ishikawa H, Ohnishi Y, Tachikawa
S, Onizuka S and Sakashita I: Extraskeletal myxoid chondrosarcoma
arising from the retroperitoneum. Am J Clin Pathol. 85:514–519.
1986.PubMed/NCBI
|
|
11
|
Gaudier F, Khurana JS, Dewan S and Shen T:
Fine-needle aspiration cytology of intra-abdominal wall
extraskeletal myxoid chondrosarcoma: a case report and review of
the literature. Arch Pathol Lab Med. 127:1211–1213. 2003.PubMed/NCBI
|
|
12
|
Goetz SP, Robinson RA and Landas SK:
Extraskeletal myxoid chondrosarcoma of the pleura. Report of a case
clinically simulating mesothelioma. Am J Clin Pathol. 97:498–502.
1992.PubMed/NCBI
|
|
13
|
Khouja N, Ben Amor S, Jemel H, Kchir N,
Boussen H and Khaldi M: Mesenchymal extraskeletal chondrosarcoma of
the orbit. Report of a case and review of the literature. Surg
Neurol. 52:50–53. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kilpatrick SE, Inwards CY, Fletcher CD,
Smith MA and Gitelis S: Myxoid chondrosarcoma (chordoid sarcoma) of
bone: a report of two cases and review of the literature. Cancer.
79:1903–1910. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Okamoto S, Hisaoka M, Ishida T, Imamura T,
Kanda H, Shimajiri S and Hashimoto H: Extraskeletal myxoid
chondrosarcoma: A clinicopathologic, immunohistochemical, and
molecular analysis of 18 cases. Hum Pathol. 32:1116–1124. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sato K, Kubota T, Yoshida K and Murata H:
Intracranial extraskeletal myxoid chondrosarcoma with special
reference to lamellar inclusions in the rough endoplasmic
reticulum. Acta Neuropathol. 86:525–528. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sato N, Minase T, Yoshida Y, Narimatsu E,
Muroya K, Asaishi K and Kikuchi K: An ultrastructural study of
extraskeletal mesenchymal chondrosarcoma. Acta Pathol Jpn.
34:1355–1363. 1984.PubMed/NCBI
|
|
18
|
Hisaoka M and Hashimoto H: Extraskeletal
myxoid chondrosarcoma: updated clinicopathological and molecular
genetic characteristics. Pathol Int. 55:453–463. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Goldblum JR: The series foundations in
diagnostic pathologyBone and Soft Tissue Pathology. Folpe AL and
Inwards CY: Saunders Elsevier; Philadelphia: pp. 4622010
|
|
20
|
Mavrogenis AF, Patapis P, Papaparaskeva
KT, Galanis EC and Papagelopoulos PJ: Extraskeletal myxoid
chondrosarcoma of the perineum. Orthopedics. 32:2162009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Murphey MD, Walker EA, Wilson AJ,
Kransdorf MJ, Temple HT and Gannon FH: From the archives of the
AFIP: imaging of primary chondrosarcoma: radiologic-pathologic
correlation. Radiographics. 23:1245–1278. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Weiss SW and Goldblum JR: Cartilaginous
soft tissue tumorsEnzinger and Weiss's Soft Tissue Tumors. 5th.
Mosby Elsevier; China: pp. 1017–1038. 2008
|
|
23
|
Horn LC, Werschnik C, Bilek K and Emmert
C: Diagnosis and clinical management in malignant Müllerian tumors
of the fallopian tube. A report of four cases and review of recent
literature. Arch Gynecol Obstet. 258:47–53. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ananthamurthy A, Nisheena R, Rao B and
Correa M: Extraskeletal myxoid chondrosarcoma: Diagnosis of a rare
soft tissue tumor based on fine needle aspiration cytology. J
Cytol. 26:36–38. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
ESMO/European Sarcoma Network Working
Group, . Soft tissue and visceral sarcomas: ESMO Clinical Practice
Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 25
Suppl 3:S102–S112. 2014. View Article : Google Scholar
|
|
26
|
McGrory JE, Rock MG, Nascimento AG and
Oliveira AM: Extraskeletal myxoid chondrosarcoma. Clin Orthop Relat
Res. 382:185–190. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mccarter MD, Jaques DP and Brennan MF:
Randomized clinical trials in soft tissue sarcoma. Surg Oncol Clin
N Am. 11:11–22. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Reed NS, Mangioni C, Malmström H, et al:
European Organisation for Research and Treatment of Cancer
Gynaecological Cancer Group: Phase III randomised study to evaluate
the role of adjuvant pelvic radiotherapy in the treatment of
uterine sarcomas stages I and II: An European Organisation for
Research and Treatment of Cancer Gynaecological Cancer Group Study
(protocol 55874). Eur J Cancer. 44:808–818. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wylie JP, O'Sullivan B, Catton C and
Gutierrez E: Contemporary radiotherapy for soft tissue sarcoma.
Semin Surg Oncol. 17:33–46. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Patel SR, Burgess MA, Papadopoulos NE,
Linke KA and Benjamin RS: Extraskeletal myxoid chondrosarcoma.
Long-term experience with chemotherapy. Am J Clin Oncol.
18:161–163. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Rubinger M, Plenderleith IH, Lertzman M
and Worth AJ: Metastatic extraskeletal myxoid chondrosarcoma.
Successful therapy with interferon alfa-2b. Chest. 108:281–282.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Drilon AD, Popat S, Bhuchar G, et al:
Extraskeletal myxoid chondrosarcoma: a retrospective review from 2
referral centers emphasizing long-term outcomes with surgery and
chemotherapy. Cancer. 113:3364–3371. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Geyer HL and Karlin N: Extraskeletal
myxoid chondrosarcoma of the heart and review of current
literature. Curr Oncol. 17:58–62. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gracia I, Majó J, Peiró A and Doncel A:
Extraskeletal bone sarcomas. The Internet Journal of Orthopedic
Surgery. 3:2005.
|
|
35
|
MeisKindblom JM, Bergh P, Gunterberg B and
Kindblom LG: Extraskeletal myxoid chondrosarcoma: A reappraisal of
its morphologic spectrum and prognostic factors based on 117 cases.
Am J Surg Pathol. 23:636–650. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Saleh G, Evans HL, Ro JY and Ayala AG:
Extraskeletal myxoid chondrosarcoma. A clinicopathologic study of
ten patients with long-term follow-up. Cancer. 70:2827–2830. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Oliveira AM, Sebo TJ, McGrory JE, Gaffey
TA, Rock MG and Nascimento AG: Extraskeletal myxoid chondrosarcoma:
A clinicopathologic, immunohistochemical, and ploidy analysis of 23
cases. Mod Pathol. 13:900–908. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Khan AS, Bakhshi GD, Shaikh A, Khan AA,
Khan AA and Chitale A: Extraskeletal Chondrosarcoma of Labium
Majus. Case Reports in Pathology. ID:4295622011.
|
|
39
|
Lin J, Yip KM, Maffulli N and Chow LT:
Extraskeletal mesenchymal chondrosarcoma of the labium majus.
Gynecol Oncol. 60:492–493. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Santacruz MR, Proctor L, Thomas DB and
Gehrig PA: Extraskeletal myxoid chondrosarcoma: A report of a
gynecologic case. Gynecol Oncol. 98:498–501. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Sawada M, Tochigi N, Sasajima Y, Hasegawa
T, Kasamatsu T and Kitawaki J: Primary extraskeletal myxoid
chondrosarcoma of the vulva. J Obstet Gynaecol Res. 37:1706–1710.
2011. View Article : Google Scholar : PubMed/NCBI
|