Introduction
Calyceal diverticula are rare outpouchings of the
upper collecting system lying within the renal parenchyma, and are
found in 0.21–0.6% of intravenous urograms that are performed on
adults (1–4). The majority of patients with calyceal
diverticula are asymptomatic, and they are diagnosed when imaging
is performed for other reasons (2).
Calyceal diverticula often contain stones, and the treatments for
urolithiasis should be performed in patients with chronic pain,
recurrent urinary tract infection, gross hematuria or decline in
renal function (1,2). Upper tract urothelial carcinoma is a
relatively uncommon disease, accounting for 5% of all urothelial
carcinomas with an annual incidence of 2 cases per 100,000
individuals (5). Due to the low
incidence of the disease, mortality rate remains unknown and
limited clinical evidence is available regarding treatment
(5). Carcinoma within a calyceal
diverticulum is highly rare (6–8). The
present study reports a case of invasive urothelial carcinoma
within a calyceal diverticulum associated with renal stones. It was
difficult to make a definitive pre-operative diagnosis owing to
atypical imaging findings of the renal mass. Written informed
consent was obatined from the patient.
Case report
In November 2013, a 70-year-old male with diabetes
mellitus and hypertension underwent a routine health checkup by a
local general practitioner, and a slightly high value of serum
carbohydrate antigen (CA)19-9 level (36 U/ml; normal range, <35
U/ml) was found. A left renal mass was detected by abdominal
computed tomography (CT) and the patient was accordingly referred
to the Department of Intergrative Cancer Therapy and Urology,
Graduate School of medical Science, Kanazawa University (Kanazawa,
Japan) in January 2014.
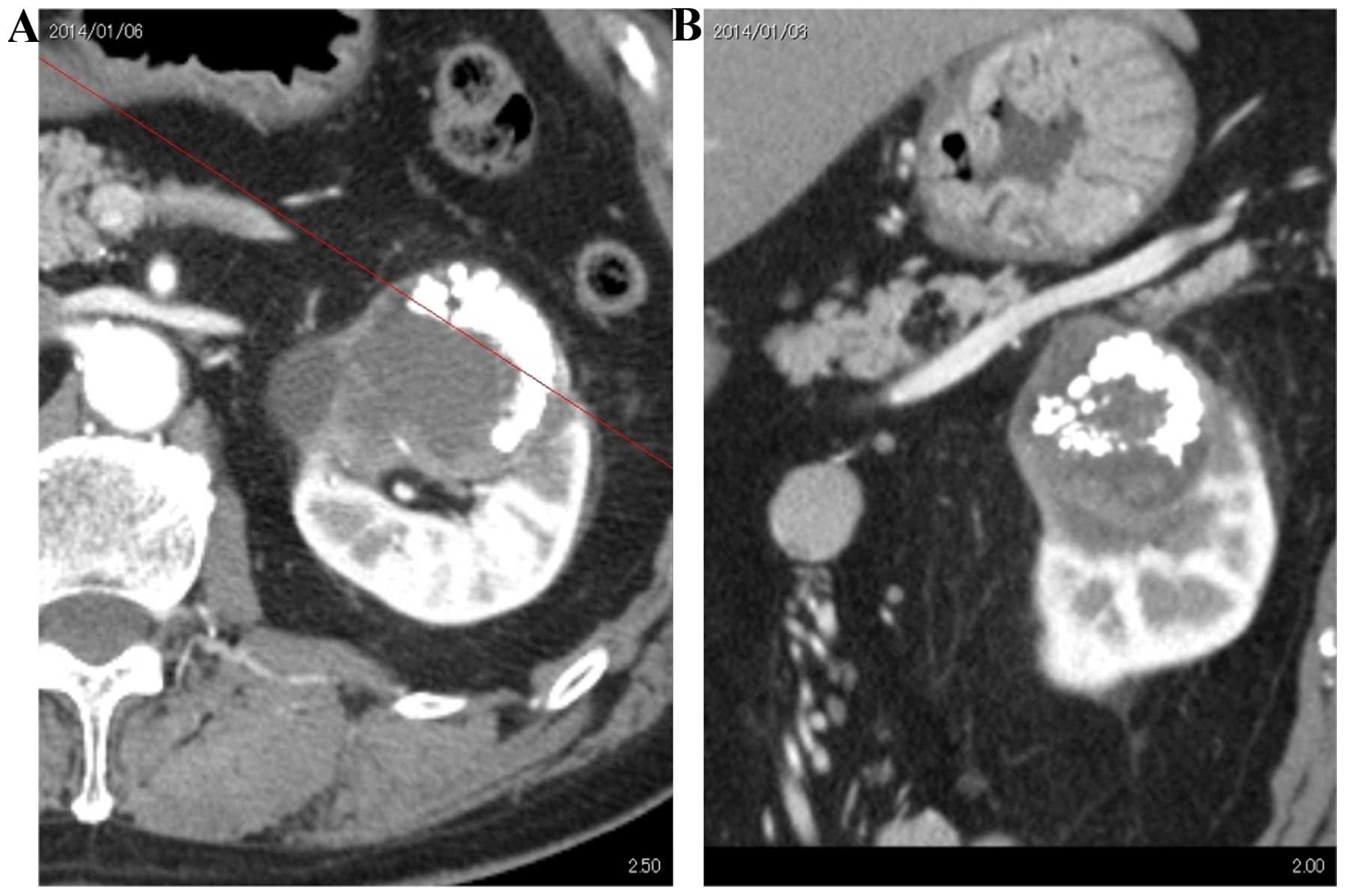
Abdominal CT and magnetic resonance imaging revealed
a hypovascular renal tumor, 7 cm in diameter, with calcification
(Fig. 1). Retrograde pyelography
showed multiple calcifications outside the renal calyx, and a small
amount of contrast media accumulated around the calcifications
(Fig. 2). Urine cytology from the
left pelvis revealed atypical cells. Although no pre-operative
definitive diagnosis could be made, these findings could have
indicated a renal tumor within a calyceal diverticulum.
A left laparoscopic radical nephroureterectomy by
retroperitoneal approach was performed. A massive tumor,
8.0×5.0×4.5 cm in size, was resected and surgical specimens
revealed multiple calcifications that were identified as renal
stones consisting of 97% calcium oxalate and 3% calcium phosphate
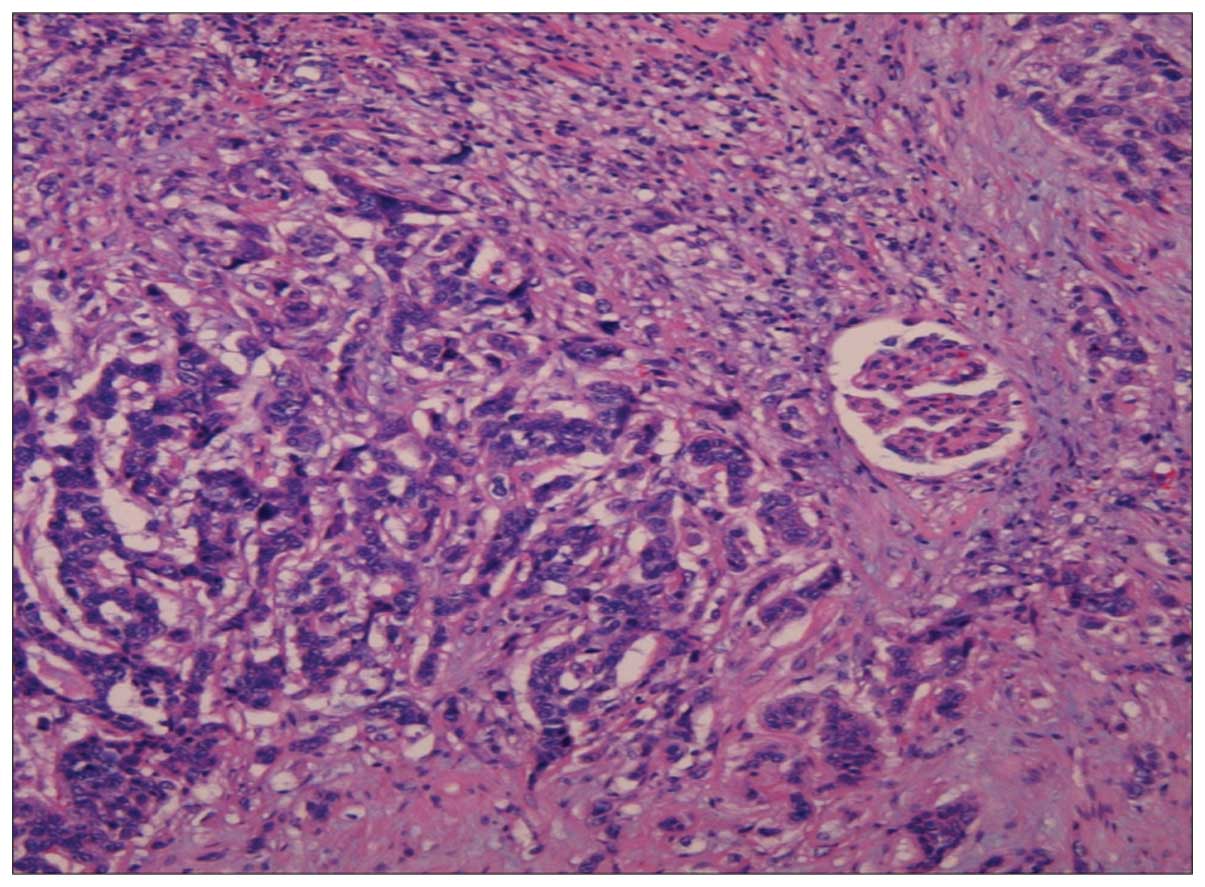
(Fig. 3). Microscopically, the tumor
was composed of high-grade invasive urothelial carcinoma and
squamous differentiated tumor cells extending into the renal cortex
(Fig. 4). The definitive pathological
diagnosis was of pT3N0M0 urothelial carcinoma based on the 7th
American Joint Committee on Cancer/Union for International Cancer
Control tumor-node-metastasis (AJCC/UICC TNM) staging system
(9). Administration of tegafur-uracil
(300 mg, daily) was begun as adjuvant chemotherapy at two weeks
post-surgery and was continued for six months. The serum CA19-9
level normalized after surgery. No recurrent findings were observed
during the follow-up period of 12 months.
Discussion
Urothelial carcinoma within calyceal diverticula is
extremely rare, and formation of a definitive diagnosis prior to
surgery is difficult (6–8). Zuckerman et al reported a case of
a calyceal diverticular urothelial (transitional cell) carcinoma
found during percutaneous nephrolithotripsy (6), however, in other cases (7,8), including
the present case, the diagnosis was confirmed following radical
nephroureterectomy or simple nephrectomy. Cases of urothelial
carcinoma associated with renal stones have been reported, however,
pre-operative imaging findings of urothelial carcinoma associated
with renal stones can complicate the pre-operative diagnosis
further (6–8). In one previous study, although
malignancy was found in ~50% of the patients who underwent
nephrectomy to treat a stone disease in a non-functioning kidney,
the lesion was observed on preoperative imaging in only 29% of
those patients (10). As the
mechanism resulting in stone-related urothelial malignancies,
chronic irritation and infection may play a significant role in the
development of renal pelvis/ureter or bladder cancer (11). There have been several studies on the
correlation between squamous cell carcinoma and stones in the
urinary tract, and the pathological findings in the present case
showed high-grade invasive urothelial carcinoma and squamous
differentiated tumor cells. Although the origins of urothelial
malignancies associated with stones remain unclear, chronic
irritation and infection due to stones may accelerate the
differentiation of urothelial carcinoma. As stones can be found in
up to 50% of calyceal diverticula (2), a possible diagnosis of urothelial
malignancy should be considered in patients with calyceal
diverticula.
Based on the 7th AJCC/UICC TNM staging system
(9), the present case was diagnosed
as pT3 invasive renal pelvic cancer. An increasing level of
attention has recently been focused on using a renal pelvic pT3
subclassification to distinguish between microscopic infiltration
of the renal parenchyma (pT3a) and macroscopic infiltration or
invasion of the peripelvic adipose tissue (pT3b) (12,13). A
previous study demonstrated that the 10-year recurrence-free (pT3a,
58% vs. pT3b, 38%; P<0.001) and cancer-specific (pT3a, 60% vs.
pT3b, 39%; P=0.002) survival rates were decreased in patients with
pT3b disease (13). Another
subclassification has also been proposed: pT3a, in which urothelial
carcinoma of the renal pelvis (UCRP) extends only into the renal
medulla, and pT3b, in which UCRP extension into the renal cortex is
present and/or in which UCRP exhibits peripelvic fat invasion
(14). In the study using this second
subclassification, the five-year cancer-specific survival rates
were 84.6 and 37.3% for pT3a and pT3b, respectively (14). The present case was diagnosed as pT3b
using each of the subclassification systems, and careful follow-up
has been indicated.
Deep invasion in urothelial carcinoma within a
calyceal diverticulum is an notable clinical problem. In the study
by Zuckerman et al, the definite pathological diagnosis in
the subsequent laparoscopic radical nephroureterectomy was
high-grade transitional cell carcinoma invading the parenchyma
(6). Although there is no consensus
on the cause of calyceal diverticula, one possible cause is derived
from dysfunction within the sphincters surrounding the calyces that
facilitate synchronized filling and emptying (2). Such calyceal achalasia results in
chronic inefficient emptying, progressive dilatation proximal to
the sphincter and subsequent formation of a diverticulum (2). In this situation, possible thinness or
loss of the sphincter surrounding the calyceal diverticula mucosa
may result in tumor invasion across the sphincter muscle layer.
Close examination is indicated in urothelial malignancies within
calyceal diverticula in the clinical setting.
In conclusion, this study presented the case of a 70
year old male with invasive urothelial carcinoma within a calyceal
diverticulum, that was associated with renal stones. Urothelial
carcinoma in calyceal diverticula is a rare condition, and the
pre-operative definite diagnosis in the present case was difficult.
The current study revealed that this disease exhibits a high
potential for invasion of the renal parenchyma. Thus, we
hypothesize that invasive urothelial carcinoma should be considered
as one of the differential diagnoses in patients with renal masses
that are associated with renal stones, which exhibit atypical
imaging findings.
References
|
1
|
TimmonsJW Jr, Malek RS, Hattery RR and
Deweerd JH: Calyceal diverticulum. J Urol. 114:6–9. 1975.PubMed/NCBI
|
|
2
|
Wainganker N, Hayek S, Smith AD and Okeke
Z: Calyceal diverticula: A comprehensive review. Rev Urol.
16:29–43. 2014.PubMed/NCBI
|
|
3
|
Wulfsohn MA: Pyelocaliceal diverticula. J
Urol. 123:1–8. 1980.PubMed/NCBI
|
|
4
|
Michel W, Funke PJ, Tunn UW and Senge T:
Pyelocalyceal diverticula. Int Urol Nephrol. 17:225–230. 1985.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Oya M and Kikuchi E: Committee for
Establishment of Clinical Practice Guideline for Management of
Upper Tract Urothelial Carcinoma; Japanese Urological Association:
Evidenced-based clinical practice guideline for upper tract
urothelial carcinoma (summary - Japanese Urological Association,
2014 edition). Int J Urol. 22:3–13. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zuckerman JM, Passman C and Assimos DG:
Transitional cell carcinoma within a calyceal diverticulum
associated with stone disease. Rev Urol. 12:52–55. 2010.PubMed/NCBI
|
|
7
|
Adachi T, Ezaki K and Funai K: A case of
transitional cell carcinoma in a pyelocaliceal diverticulum in
Japanese. Hinyoukika Kiyo. 35:1383–1386. 1989.
|
|
8
|
Yoshimura K, Yoshida H, Kawase N and Taki
Y: A case of transitional cell carcinoma and milk of calcium in a
pyelocalyceal diverticulum in Japanese. Hinyoukika Kiyo.
44:649–652. 1998.
|
|
9
|
Sobin L, Gospondarowicz M and Wittekind C:
Urological tumours, renal pelvis and ureterTNM Classification of
Malignant Tumours. 7th. Wiley-Blackwell; Hoboken, NJ: pp. 258–261.
2009
|
|
10
|
Yeh CC, Lin TH, Wu HC, Chang CH, Chen CC
and Chen WC: A high association of upper urinary tract transitional
carcinoma with nonfunctioning kidney caused by stone disease in
Taiwan. Urol Int. 79:19–23. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chow WH, Lindblad P, Gridley G, Nyrén O,
McLaughlin JK, Linet MS, Pennello GA, Adami HO and Fraumeni JF Jr:
Risk of urinary tract cancers following kidney or ureter stones. J
Natl Cancer Inst. 89:1453–1457. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rouprêt M, Babjuk M, Compérat E, Zigeuner
R, Sylvester R, Burger M, Cowan N, Böhle A, Van Rhijn BW, Kaasinen
E, et al: European guidelines on upper tract urothelial carcinomas;
2013 update. Eur Urol. 63:1059–1071. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shariat SF, Zigeuner R, Rink M, Margulis
V, Hansen J, Kikuchi E, Kassouf W, Raman JD, Remzi M, Koppie TM, et
al: Subclassification of pT3 urothelial carcinoma of the renal
pelvicalyceal system is associated with recurrence-free and
cancer-specific survival: Proposal for a revision of the current
TNM classification. Eur Urol. 62:224–231. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sassa N, Tsuzuki T, Fukatsu A, Majima T,
Kimura T, Nishikimi T, Yoshino Y, Hattori R and Gotoh M: Is pT3
urothelial carcinoma of the renal pelvis a homogeneous disease
entity? Proposal for a new subcategory of the pT3 classification.
Histopathology. 61:620–628. 2012.PubMed/NCBI
|