Introduction
Bone hemangiomas are benign lesions originating from
new capillaries or cavernous vascular vessels located in the bone.
They are characterized by an increased number of normal or abnormal
blood vessels. Bone hemangiomas are initially asymptomatic,
however, as the tumor grows local pain is often experienced
(1). Degradation can occur during the
growth of hemangioma (2). These rare,
slow growing neoplasms may occur at any age, and account for ~1% of
bone tumors (3–5). Hemangiomas may occur in any bone of the
body, most commonly in the spine, craniofacial bone, skull, ribs
and long bones (6–15); however, occurrence in the scapula is
extremely rare. Hemangiomas of the long bone or flat bone commonly
have a ‘foam’ or ‘honeycomb’ appearance (16–18). Bone
hemangiomas disrupt the cortex and commonly grow expansively, which
may result in the lesions being misdiagnosed as aggressive tumors
or osteofibrous dysplasia and infectious processes (9,19). At
present, treatments include radiotherapy, surgery and vascular
embolization (1). In the present
study, a rare case of a capillary hemangioma of the scapula, which
proved difficult to diagnose, was presented.
To the best of our knowledge, this is the first case
of pure primary hemangioma of the scapula that was misdiagnosed as
an osteofibrous dysplasia (20) and
treated with surgery.
Case report
In June 2013, a 58 year-old female presented at the
First Affiliated Hospital of Nanchang University (Nanchang, China)
with intermittent/repeated left shoulder pain, which had lasted for
~1 year. The main clinical manifestations included local
tenderness, an osseous lump and limited shoulder movement with a
little pain, which was alleviated by rest. The patient was referred
to the Department of Orthopedics for further investigation of local
tenderness of the scapula. Written informed consent was obtained
from the patient for participation in the present study.
The left shoulder joint was investigated by X-ray
examination (Fig. 1A), which revealed
a mass with bone destruction of the left acromion. The patient had
a previous history of hypertension, which had been successfully
managed by ongoing antihypertensive drug treatment. Blood tests
revealed that the levels of the tumor markers, carcinoembryonic
antigen, cancer antigen125 and α-fetoprotein, were within the
normal limits. Three-dimensional reconstruction using CT revealed
swelling of the left shoulder scapula with bone destruction within
the soft tissue mass density. Furthermore, interruption and
destruction were identified in the cortex and trabecula of the
bone, as well as a slight periosteal reaction and visible lesions
with separated shadows; however, the mass boundaries were unclear
(Fig. 1B). MRI was performed to
confirm the diagnosis, revealing expansive bone destruction of the
left scapula acromion. The mass exhibited longer signal intensity
on T2-weighted sequences and equal T1-weighted sequences, while the
fat suppression sequences presented as a high signal with a small
number of uneven signals. Part of the edge appeared a little fuzzy
and the soft tissue around the mass did not present abnormal signal
intensity. A number of of small dots distributed around the left
humeral neck exhibited long signal intensity on T1- and T2-weighted
sequences, and high signals in the fat suppression sequences.
Furthermore, a small amount of effusion was observed in the left
shoulder joint capsule (Fig. 1C). An
MRI scan demonstrated no enlarged lymph nodes or distant
metastases.
Notably, a mass along the inner surface of the left
shoulder scapula with polycystic expansion and bone destruction was
identified, which was diagnosed as osteofibrous dysplasia of the
scapula. Subsequently, tumorectomy was performed by bone tumor
specialists, since the patient did not present any surgery
contraindications. Sterile drapes were disinfected and paved
routinely in the right lateral position to expose the operative
field following the administration of anesthesia. The left acromion
was selected as the center transverse incision, followed by skin,
subcutaneous, superficial fascia and scapular muscle incisions. The
fascia and soft tissue were stripped to expose the acromion, which
exhibited expansive bone destruction within a polycystic intratumor
with an uneven surface and rich blood supply. The tumor was excised
completely and tissue lesions were removed for pathological
examination. A wound drainage tube was inserted and each layer of
tissue was sutured to allow complete hemostasis. The estimated
blood loss was 200 ml and no blood transfusions were required
during surgery.
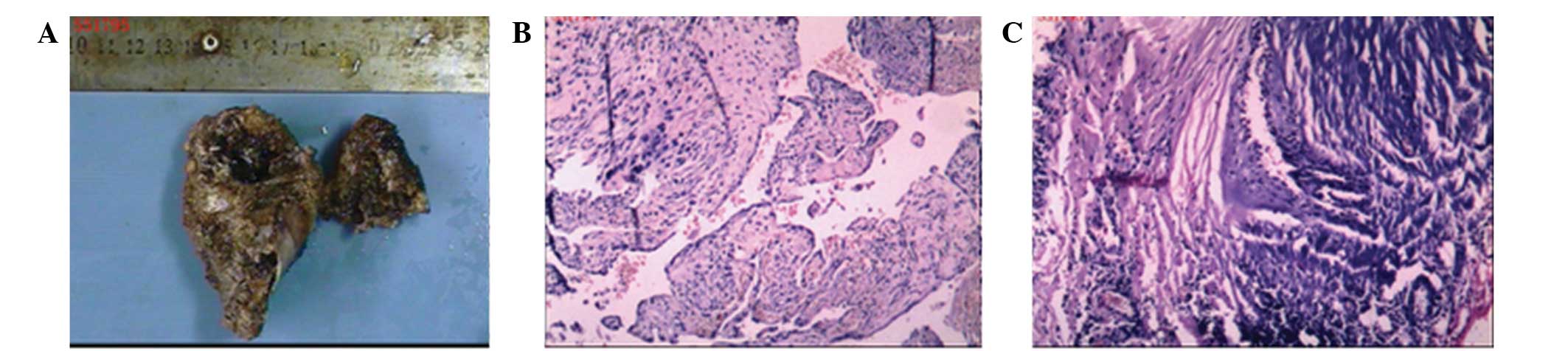
Pathological examination revealed two taupe bones
with an irregular shape, measuring between 3.5×3.5×1.5
cm3 and 7×5.0×3.2 cm3, and numerous gray-red
broken floccules enclosing the medullary cavity. (Fig. 2A) Light microscopy revealed
hyperplasia of the blood vessels between the bone and soft tissue
(Fig. 2C), with pipe cavities of
different sizes and uneven tube walls, exhibiting hemorrhage,
thrombosis and recanalization (Fig.
2B). Based on these findings, the tumor was finally diagnosed
as a benign left acromion hemangioma originating from the scapula.
The patient underwent follow-up X-ray examination 1 year after
surgery, which revealed that symptoms had improved and no evidence
of recurrence was identified (Fig.
1D).
Discussion
Hemangioma of the scapula is an uncommon benign
vascular tumor, and to the best of our knowledge, no cases have
been reported in the literature to date. Thus, the etiology remains
unclear. Furthermore, it remains unclear whether all lesions are
true neoplasms or should be regarded as hamartomas (2,21).
Histologically, four types of hemangioma exist: Capillary,
cavernous, venous and mixed. Capillary hemangiomas consist of
numerous tortuous small vascular channels lined with epithelium,
while the more common cavernous hemangiomas present large dilated
vessels lined with a single layer of endothelial cells surrounded
by a fibrous stromal layer (22). In
general, hemangiomas have a good prognosis with a low recurrence
rate (23,24). Although capillary hemangioma of the
scapula is extremely rare, it should be considered in the
differential diagnosis of scapula tumors. The differential
diagnosis may include malignant scapula tumors, such as
chondrosarcoma, Ewing's sarcoma, and myeloma or benign scapula
tumors, including fibrous dysplasia, osteochondroma, eosinophilic
granuloma and aneurysmal bone cysts (22).
Generally, as degradation may occur during the
growth of bone hemangioma, the vascular tissue may be replaced by
fibrous tissue and achieve self-healing (1). Therefore, asymptomatic patients must be
followed-up, without receiving any therapy temporarily. In
addition, patients with symptoms or pathological lesions should be
followed up as they have a high risk of fracture (25). However, symptomatic patients should
receive surgical resection with intralesional ethanol injection,
selective arterial embolization, percutaneous vertebroplasty and
radiotherapy treatment. It is important to avoid impairing the
tumor during surgery as this may lead to severe hemorrhage
(26). Thus, preoperative
embolization may be beneficial to avoid intraoperative bleeding
(27).
With the exception of hemorrhage, the adverse
effects of surgery include incomplete removal and long recovery
periods (28). The prognosis depends
on tumor location and tumor size. Furthermore, it has been reported
that the coracoclavicular ligament is a critical component of the
acromioclavicular joint, although each component of the joint
provides acromioclavicular joint stability in different directions
(29). Partial resection, which leads
to the destruction of the acromioclavicular joint, does not affect
the function of shoulder joint as long as the coracoclavicular
ligament is preserved. Surgical excision is the treatment of choice
for patients with hemangiomas that have infiltrated the bone and
soft tissue, while histological examination confirms diagnosis. In
addition, needle biopsy may indicate a definite diagnosis. However,
this procedure may be difficult in certain cases, and should be
avoided due to the risk of hemorrhage or seeding of the needle
tract, unless multiple myeloma or metastatic disease is highly
suspected (30,31).
Hemangioma patients are usually asymptomatic and the
tumor is often identified incidentally on routine chest
roentgenograms. Subsequent CT and MRI scans may reveal the extent
of cortical destruction more clearly. The presence of fat density
on MRI examinations, well-defined rather than infiltrative borders
and sclerotic margins, and a honeycomb appearance are highly
suggestive of bone hemangiomas (32–34). Ching
et al reported that bone hemangiomas commonly exhibit
hypointensity on T1-weighted images and hyper intensity on
T2-weighted images depending on the quantity of vascularity and
adipose tissue (16). Therefore, it
is crucial to evaluate every radiological abnormality carefully and
possible accompanying disorders should not be ignored when the
diagnosis of the disease is confirmed.
In conclusion, accurate diagnosis is essential for
the surgical management of hemangioma. Notably, the majority of
bone hemangioma cases are accompanied by slight pain; however, the
symptoms of the present case included local tenderness, an osseous
lump and limited shoulder movement. As it is difficult to identify
such lesions on CT and MRI scans, the present case was
misdiagnosed, as rare conditions were not considered. Histological
observations may help establish a definitive diagnosis; however,
the preoperative clinical manifestations and details of the
differential diagnosis were overlooked in the present case. Patient
age, tumor location, tumor size, possible pathological type and the
other aspects must be considered during preoperative assessment due
to the high risk of hemorrhage that is associated with bone
hemangioma surgery. Thus, intraoperative precautions must be
performed to prevent hemorrhagic shock and other adverse
consequences during surgery. In the current study, the patient was
misdiagnosed with osteofibrous dysplasia based on the clinical
manifestations. The results of X-ray examination indicated a
diagnosis of left acromion osteofibrous dysplasia; however,
three-dimensional computed tomography (CT) and magnetic resonance
imaging (MRI) examinations revealed a mass along the inner surface
of the left shoulder scapula, with polycystic expansion and bone
destruction. Therefore, clinical examination and careful
consideration of patient history are key factors for the
identification of diseases, particularly tumors. Further diagnostic
pathological examination may clarify the diagnosis (35). However, definitive preoperative
diagnosis of hemangioma is of great significance to guide
treatment. In the present case, the patient was followed-up for 1
year and has achieved full recovery. The current report analyzed
the clinical and imaging features of the scapula hemangioma to
highlight possible diagnostic and management methods. The results
indicated that careful evaluation of all radiological abnormalities
is crucial, to avoid potential diseases being overlooked.
Furthermore, this study may increase clinical awareness with regard
to hemangioma.
References
|
1
|
Syrimpeis V, Vitsas V and Korovessis P:
Lumbar vertebral hemangioma mimicking lateral spinal canal
stenosis: Case report and review of literature. J Spinal Cord Med.
37:237–242. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hart JL, Edgar MA and Gardner JM: Vascular
tumors of bone. Semin Diagn Pathol. 31:30–38. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shimizu K, Yamashita Y, Hihara J, et al:
Cavernous hemangioma of the rib. Ann Thorac Surg. 74:932–934. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Okumura T, Asamura H, Kondo H, Matsuno Y
and Tsuchiya R: Hemangioma of the rib: a case report. Jpn J Clin
Oncol. 30:354–357. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gologorsky Y, Shrivastava RK, Panov F,
Mascitelli J, Signore AD, Govindaraj S, Smethurst M and Bronster
DJ: Primary intraosseous cavernous hemangioma of the clivus: case
report and review of the literature. J Neurol Surg Rep. 74:17–22.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Politi M, Romeike BF, Papanagiotou P, et
al: Intraosseous hemangioma of the skull with dural tail sign:
radiologic features with pathologic correlation. AJNR Am J
Neuroradiol. 26:2049–2052. 2005.PubMed/NCBI
|
|
7
|
Schrock WB, Wetzel RJ, Tanner SC and Khan
MA: Aggressive hemangioma of the thoracic spine. J Radiol Case Rep.
5:7–13. 2011.PubMed/NCBI
|
|
8
|
Tucer B, Ekici MA, Menku A, et al:
Surgical management of symptomatic T8 vertebral hemangioma: case
report and review of the literature. Turk Neurosurg. 23:680–684.
2013.PubMed/NCBI
|
|
9
|
Oruc M, Atay AE, Karabulut P, et al: An
unusual case of cavernous hemangioma of the rib in a young man with
lung tuberculosis: a brief review and case report. Intern Med.
52:1263–1265. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Agarwal V, Sreedher G, Weiss KR and Hughes
MA: Sacroplasty for symptomatic sacral hemangioma: a novel
treatment approach. A case report. Interv Neuroradiol. 19:245–249.
2013.PubMed/NCBI
|
|
11
|
Mridha AR, Kinra P, Sable M, et al:
Epithelioid hemangioma of distal femoral epiphysis in a patient
with congenital talipes equinovarus. Malays J Pathol. 36:63–66.
2014.PubMed/NCBI
|
|
12
|
Park BH, Hwang E and Kim CH: Primary
intraosseous hemangioma in the frontal bone. Arch Plast Surg.
40:283–285. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Atcı IB, Albayrak S, Yılmaz N, et al:
Cavernous hemangioma of the parietal bone. Am J Case Rep.
14:401–404. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Aneja A, Bhattacharyya S, Mydlo J and
Inniss S: Testicular seminomatous mixed germ cell tumor with
choriocarcinoma and teratoma with secondary somatic malignancy: a
case report. J Med Case Rep. 8:12014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gologorsky Y, Shrivastava RK, Panov F,
Mascitelli J, Signore AD, Govindaraj S, Smethurst M and Bronster
DJ: Primary intraosseous cavernous hemangioma of the clivus: case
report and review of the literature. J Neurol Surg Rep. 74:17–22.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ching BC, Wong JS, Tan MH and Jara-Lazaro
AR: The many faces of intraosseous haemangioma: a diagnostic
headache. Singapore Med J. 50:e195–e198. 2009.PubMed/NCBI
|
|
17
|
Nakamura H, Kawasaki N, Taguchi M and
Kitamura H: Cavernous hemangioma of the rib diagnosed
preoperatively by percutaneous needle biopsy. Gen Thorac Cardiovasc
Surg. 55:134–137. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen KC, Wu CT, Pan CT and Lee YC:
Metachronous multiple chest wall osseous hemangiomas. J Thorac
Cardiovasc Surg. 133:838–839. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tew K, Constantine S and Lew WY:
Intraosseous hemangioma of the rib mimicking an aggressive chest
wall tumor. Diagn Interv Radiol. 17:118–121. 2011.PubMed/NCBI
|
|
20
|
Jung JY, Jee WH, Hong SH, et al: MR
findings of the osteofibrous dysplasia. Korean J Radiol.
15:114–122. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kamala KA, Ashok L and Sujatha GP:
Cavernous hemangioma of the tongue: A rare case report. Contemp
Clin Dent. 5:95–98. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gourgiotis S, Piyis A and Panagiotopoulos
N: Cavernous hemangioma of the rib: A rare diagnosis. Case Rep Med.
2010:2540982010.PubMed/NCBI
|
|
23
|
Verbeke SL and Bovée JV: Primary vascular
tumors of bone: a spectrum of entities? Int J Clin Exp Pathol.
4:541–551. 2011.PubMed/NCBI
|
|
24
|
Mallınson P, Coupal T, Hayes M, et al:
Osteosarcoma arising from a haemangioma: case report and review of
the literature. Turk Patoloji Derg. 30:137–141. 2014.PubMed/NCBI
|
|
25
|
Abrão FC, Tamagno M, Canzian M, Fernandez
Â, Bibas J, Fernandes PM and Jatene FB: Hemangioma of the rib. Ann
Thorac Surg. 91:595–596. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hao YJ, Yu L, Zhang Y, Wang LM and Li JZ:
Surgical treatment of cervical vertebral hemangioma associated with
adjacent cervical spondylotic myelopathy. Spine J. 13:1774–1779.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Naama O, Gazzaz M, Akhaddar A, et al:
Cavernous hemangioma of the skull: 3 case reports. Surg Neurol.
70:654–659. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Heiss JD, Doppman JL and Oldfield EH:
Brief report: relief of spinal cord compression from vertebral
hemangioma by intralesional injection of absolute ethanol. N Engl J
Med. 331:508–511. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yoo YS, Seo YJ, Noh KC, Patro BP and Kim
DY: Arthroscopically assisted anatomical coracoclavicular ligament
reconstruction using tendon graft. Int Orthop. 35:1025–1030. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Clements RH, Turnage RB and Tyndal EC:
Hemangioma of the rib: a rare diagnosis. Am Surg. 64:1027–1029.
1998.PubMed/NCBI
|
|
31
|
Okumura T, Asamura H, Kondo H, et al:
Hemangioma of the rib: a case report. Jpn J Clin Oncol. 30:354–357.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ulku R, Onat S, Avci A and Ozmen CA:
Resection of intercostal hemangioma with involved chest wall and
ribs: in an 11-year old girl. Tex Heart Inst J. 37:486–489.
2010.PubMed/NCBI
|
|
33
|
Ly JQ and Sanders TG: Case 65: hemangioma
of the chest wall. Radiology. 229:726–729. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chawla A, Singrakhia M, Maheshwari M, Modi
N and Parmar H: Intraosseous hemangioma of the proximal femur:
imaging findings. Br J Radiol. 79:e64–e66. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zou F, Dai M, Zhang B and Nie T:
Misdiagnosis of a giant intrapelvic schwannoma: A case report.
Oncol Lett. 6:1646–1648. 2013.PubMed/NCBI
|