Introduction
Lung cancer presents with the characteristics of
frequent aberrance in driver genes, particularly the epidermal
growth factor receptor (EGFR) gene (1). Non-small cell lung cancer (NSCLC) is
responsible for ≤80% of all cases of lung cancer, and advanced
disease is commonly found at the time of diagnosis. Gefitinib and
erlotinib are EGFR tyrosine kinase inhibitors (EGFR-TKI) that have
exhibited marked therapeutic effects against NSCLC with activating
mutations in EGFR, such as exon 19 deletions and L858R point
mutations (2). However, resistance
will eventually develop in all patients after varying periods of
time. Acquired resistance to gefitinib is most commonly conferred
upon a patient by the EGFR T790M mutation, which has been
detected in 50% of NSCLC cases with acquired resistance and in cell
line models that have been selected for gefitinib resistance
(3). Due to the limited treatment
options available for individuals with advanced lung cancer, a
requirement exists for the identification of novel therapeutic
strategies.
Traditional Chinese medicine is used as a component
of complementary and alternative medicine in the treatment of a
number of diseases (4). Garcinia
hanburyi, a plant belonging to the Guttiferae family, is a
small tree that is found distributed throughout India, Cambodia and
the southern regions of China (5).
Garcinia hanburyi exudes gamboge resin, which contains
gambogic acid (GA) as its main active ingredient; GA has been
introduced as an effective anticancer drug (6,7). The
potent anticancer activity of GA is mainly dependent on the
resulting activation of the impaired apoptosis pathways via
downregulation of telomerase in cancer cells (8). Furthermore, GA strongly inhibits
angiogenesis via vascular endothelial growth factor suppression
(9). The aim of the present study was
to investigate whether a combination of gefitinib and GA
administration can overcome EGFR T790M-mediated resistance
in patients with NSCLC.
Materials and methods
Ethics statement
All experiments were approved by the Animal User and
Ethical Committees at Shandong University (Jinan, Shandong,
China).
Compound
Gefitinib was obtained from Sellech Chemicals
(Houston, TX, USA). Gambogic acid was purchased from Santa Cruz
Biotechnology Inc. (Dallas, TX, USA).
Cell line
The NCI-H1975 EGFR T790M mutation human NSCLC cell
line was obtained from the American Type Culture Collection
(Manassas, VA, USA). The cells were cultured in RPMI 1640 medium
supplemented with 10% fetal bovine serum, 100 U/ml penicillin, 100
mg/ml streptomycin and 2 mmol/l L-glutamine (Invitrogen Life
Technologies, Carlsbad, CA, USA), and maintained at 37°C in a
humidified atmosphere with 5% CO2.
Efficacy study in vivo
Female, 7–8-week-old, BALB/C nude mice were
purchased from Vital River Laboratories (Beijing, China). The mice
were maintained in super pathogen-free conditions and housed in
barrier facilities using a 12-h light/dark cycle, with food and
water ad libitum. The mice were subcutaneously injected with
1×107 NCI-H1975 cells suspended in 100 µl of Matrigel (BD
Biosciences, Milan, Italy). The tumor volume (TV) was measured and
recorded during the treatment period using the following formula:
TV = length × width2 / 2. When the tumor volume reached ~150 mm3,
the mice were randomly divided into four groups (n=10 for each
group) and received normal saline (vehicle group, daily intravenous
injection), gefitinib (100 mg/kg, daily oral administration), GA
solution (8 mg/kg, daily intravenous injection) or a combination
treatment of gefitinib and GA for 28 days. Gefitinib was dissolved
in 0.1% Tween 80 prior to use.
Western blot analysis
The tumors in each group were collected 4 h after
the last treatment with gefitinib, GA or the combination treatment
on day 28 of the efficacy study. Western blotting was performed as
described previously (10). Proteins
(50 µg) were separated by 12% SDS-PAGE and then transferred onto
0.45-µm polyvinylidine fluoride membranes (Bio-Rad Laboratories,
Inc., Hercules, CA, USA). The membranes were blocked in
phosphate-buffered saline containing 5% non-fat dry milk for 1 h,
then incubated at 4°C overnight with the following rabbit
anti-human monoclonal primary antibodies: AKT (dilution, 1:1,000;
catalog no. 4685), phosphorylated (p)-AKT (Ser473; dilution,
1:1,000; catalog no. 4058), ERK1/2 (dilution, 1:1,000; catalog no.
4695), p-ERK1/2 (Thr202/Tyr204; dilution, 1:1,000; catalog no.
14474), MEK1/2 (dilution, 1:1,000; catalog no. 13033), p-MEK1/2
(dilution, 1:1,000; catalog no. 2338), Bax (dilution, 1:1,000;
catalog no. 5023) and Bcl-2 (dilution, 1:1,000; catalog no. 2870)
(Cell Signaling Technology, Inc., Danvers, MA, USA). This was
followed by incubation with horseradish peroxidase-conjugated
anti-rabbit secondary antibody (EMD Millipore, Billerica, MA,
USA).
Caspase activity assay
Caspase-3, 8 and 9 activity was evaluated by
fluorometric detection of apoptosis with caspase-3 (cat. no.
KA0740),-8 (cat. no. KA0756) and -9 (cat. no. KA0761) colorimetric
protease kits (Abnova, Walnut, CA, USA) according to the
manufacturer's instructions. Briefly, the tumor tissues were
obtained and lysed in 100µl cell lysis buffer (Cell Signaling
Technology, Inc.), and 200 µg protein was incubated with 5 µl 4 mM
pNA-conjugated substrate (DEVD-pNA for caspase-3,
LETD-pNA for caspase-8 and LEHD-pNA for caspase-9;
Abnova) at 37°C for 2 h. The amount of pNA released was measured at
405 nm using a UV-Vis Flour spectrophotometer (CRAIC Technologies,
Inc., San Dimas, CA, USA). Next, tissue lysates were centrifuged at
3,000 × g, resuspended in 100 µl apopain lysis buffer and vortexed
gently. The suspensions were frozen and thawed 4–5 times using
liquid nitrogen and a 37°C water bath, respectively.
Statistical analysis
Data are expressed as the mean ± standard deviation.
InStat, version 1.14 (GraphPad Software, Inc., San Diego, CA, USA)
was used for statistical analysis, and a two-way unweighted mean
analysis of variance test was used to determine the statistical
significance. P<0.05 was considered to indicate a statistically
significant difference.
Results
Gefitinib in combination with GA
treatment has a synergistic effect on gefitinib-resistant NCI-H1975
tumor growth in vivo
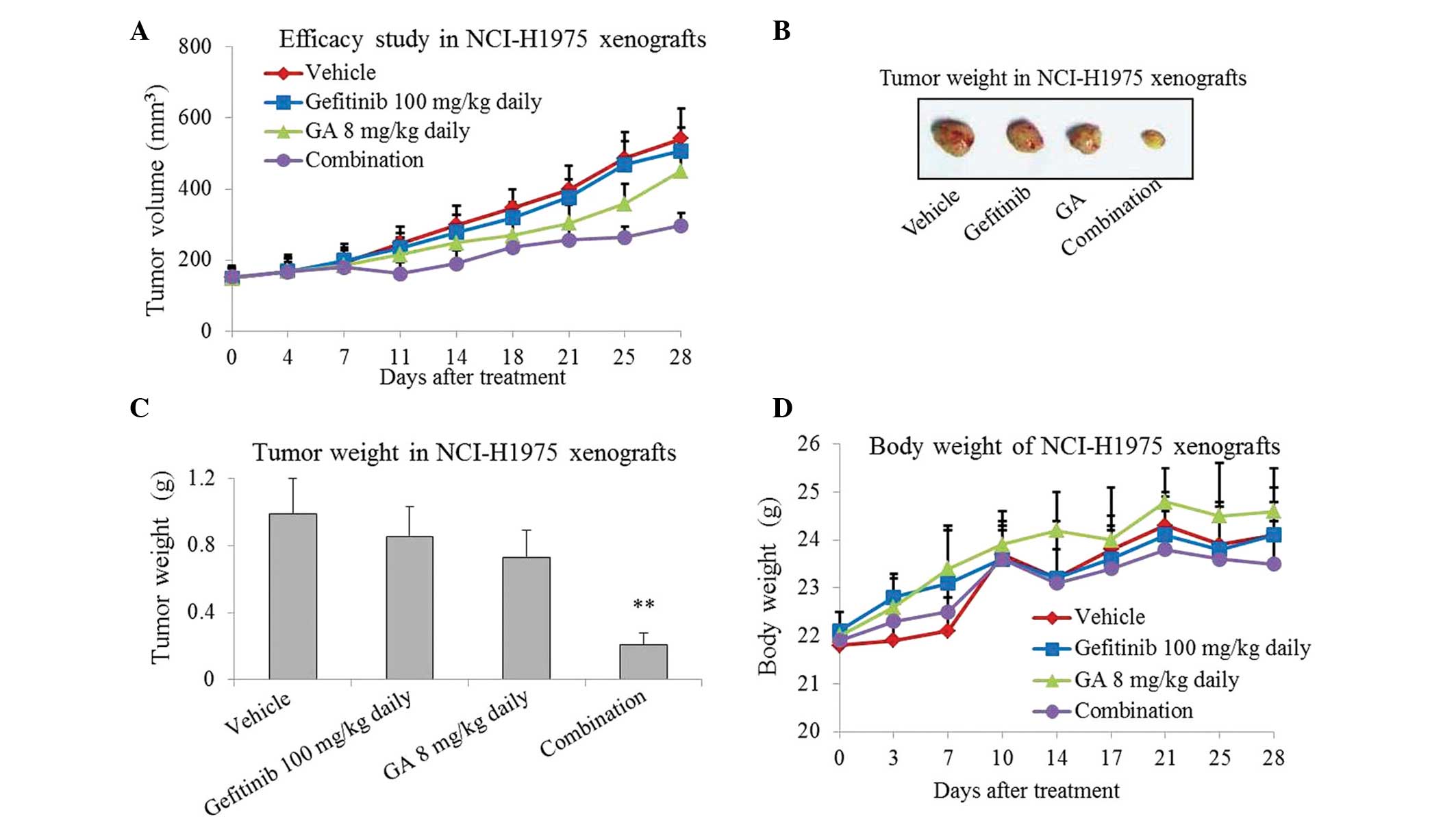
In order to investigate the inhibitory effect of
gefitinib or/and GA on tumor growth in vivo, gefitinib, GA and
gefitinib plus GA were used to treat NCI-H1975 xenografts for 4
weeks. As presented in Fig. 1A–C,
treatment with gefitinib or GA for 4 weeks slightly inhibited tumor
growth in the NCI-H1975 xenografts. However, more marked inhibition
was caused by the combined treatment, which resulted in a ~70%
reduction in tumor growth (P=0.008; Fig.
1C). The mice tolerate the single-agent and combination
treatments well, with no weight loss or other signs of acute or
delayed toxicity (Fig. 1D).
Effect of the combined treatment on
PI3K and ERK signaling pathways in gefitinib-resistant NCI-H1975
xenografts
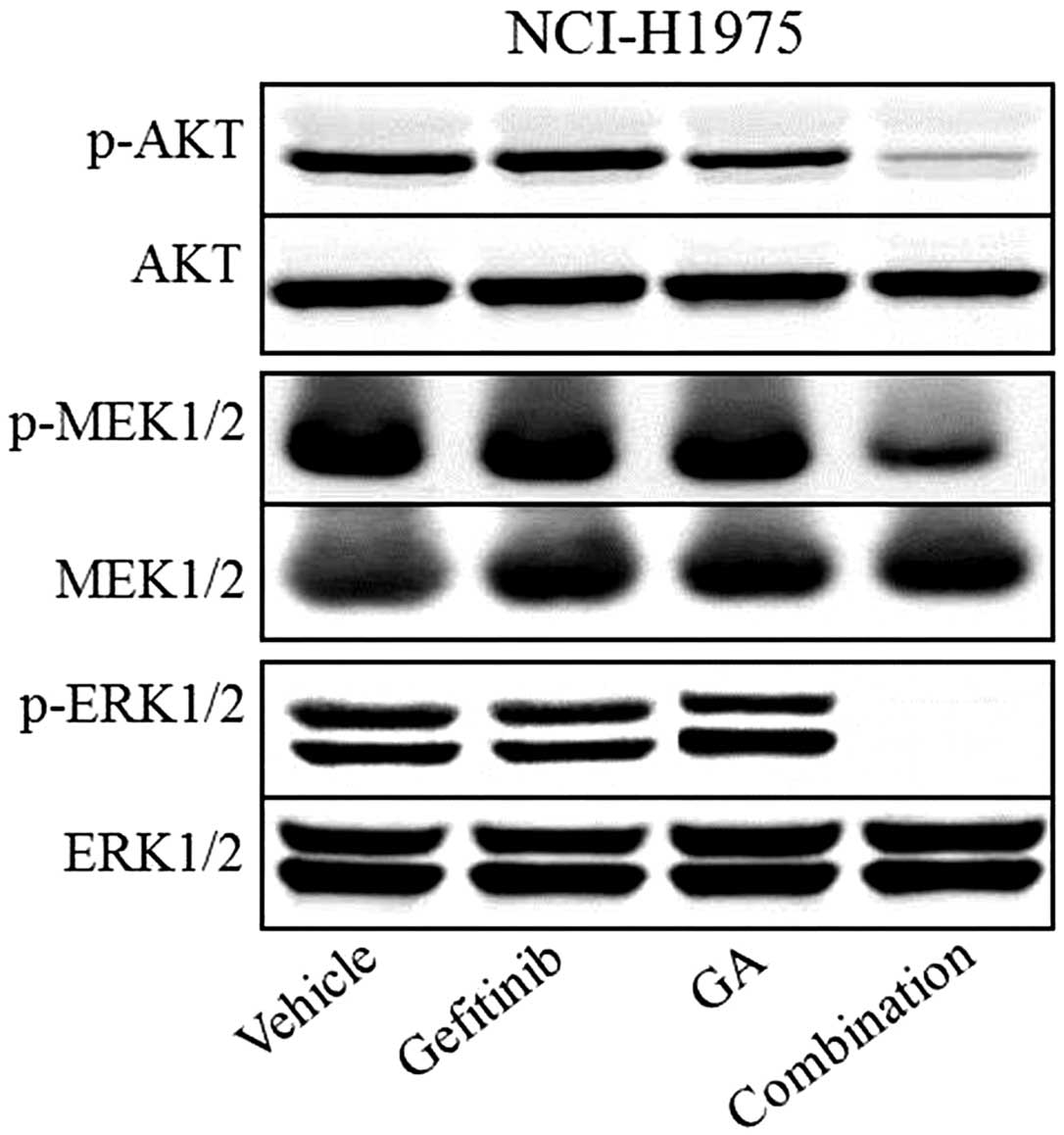
Western blot analysis was used to assess the effect
of the two compounds on downstream molecules of the PI3K and ERK
pathways. The results showed that p-AKT, p-MEK1/2 and p-ERK1/2
appeared to be inhibited by gefitinib and GA combination treatment,
whereas the total protein levels of AKT, MEK1/2 and ERK1/2 remained
unchanged in each of the groups (Fig.
2). Western blot analysis also showed that single-agent
treatment with gefitinib or GA only exhibited a slightly inhibitory
effect on the phosphorylation of AKT, MEK1/2 and ERK1/2 in the
NCI-H1975 xenografts.
Effect of combined treatment-induced
apoptosis in gefitinib-resistant NCI-H1975 xenografts
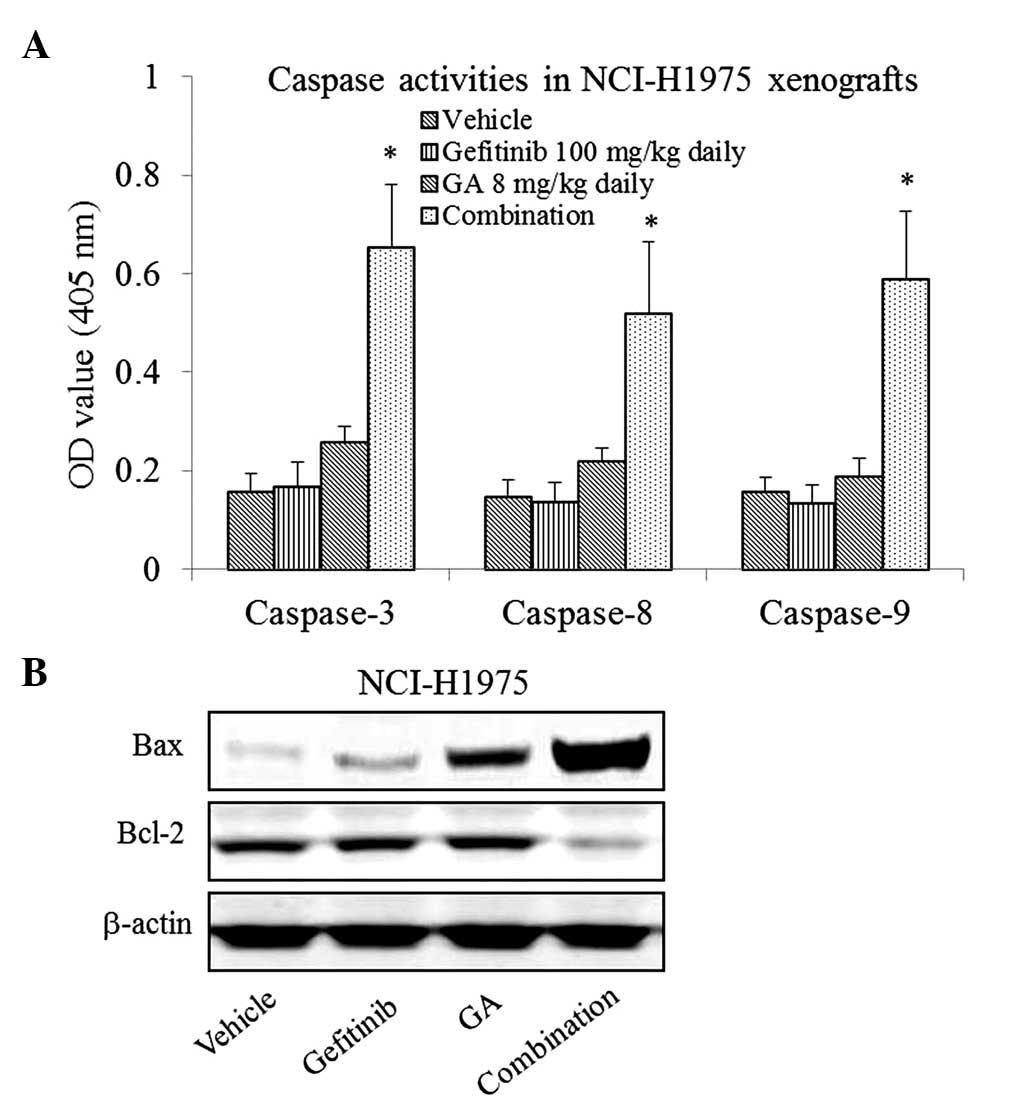
To study whether gefitinib in combination with GA
would induce apoptosis in the gefitinib-resistant NCI-H1975
xenografts, the activity of caspase-3, 8 and 9 were measured by
colorimetric assay. As presented in Fig.
3A, the results indicated that the gefitinib and GA
single-agent treatments exhibited no significant effect on
caspase-3, 8 and 9 activity in the NCI-H1975 xenografts (P>0.05
compared with the vehicle), whereas the combined treatment led to a
significant increase (P<0.05 compared with the vehicle).
The results of western blotting also showed that the
expression of Bax was upregulated, whereas the expression of Bcl-2
was downregulated by the combination treatment (Fig. 3B).
Discussion
The resistance to the EGFR-TKIs gefitinib and
erlotinib ultimately develops in all patients with metastatic EGFR
mutant lung cancer. The most commonly observed mechanism for this
involves the acquisition of cells harboring a second-site mutation,
T790M (11). Irreversible EGFR-TKIs,
such as BIBW2992, that can overcome the T790M-mediated resistance
to gefitinib have been developed (12), however, clinical trials have failed to
show that monotherapy with such irreversible EGFR-TKIs leads to any
benefit in those patients with NSCLC refractory to gefitinib
(13). Thus, effective therapies for
these patients are urgently required. Certain studies have
demonstrated the significant anti-proliferative and pro-apoptotic
effects of GA on a range of human cancer cell types in vitro
and in vivo (14,15). In the current study, gefitinib in
combination with GA was found to have a synergistic inhibitory
effect on gefitinib-resistant NCI-H1975 tumor growth, whereas
single-agent treatment with gefitinib or GA only exhibited a slight
inhibitory effect on tumor growth.
It is established that apoptosis caused by
mitochondria is involved in the activation of caspases and
Fas-associated death domain protein activation. In the former case,
caspase-9 is activated by mitochondrial permeability transitions
(ψm), which are mediated by cytochrome c release and a
reduction in the Bcl-2/Bax ratio (16). In the latter case, Fas-associated
death domain protein activates caspase-8, which in turn activates
downstream executioners caspase-3 or -7. Xu et al (17) reported that GA causes the induction of
mitochondria-dependent apoptosis via Bcl-2 and Bax modulation in
mantle cell lymphoma JeKo-1 cells. However, in the current study,
single-agent treatment with GA could not induce apoptosis in the
NCI-H1975 xenografts. It was notable that the combined treatment
caused significantly increased levels of caspase 3, 8 and 9
activity, and that an increased expression ratio of Bax/Bcl-2 was
also observed in the tumor tissues. The detailed mechanisms behind
this require further investigation.
In conclusion, the enhancement of apoptosis caused
by treatment with the combination of gefitinib and GA was effective
in the suppression of gefitinib-resistant tumor growth caused by a
EGFR T790M secondary mutation. These findings provide a
promising future strategy for the treatment of gefitinib-resistant
NSCLC.
References
|
1
|
She J, Yang P, Hong Q and Bai C: Lung
cancer in China: Challenges and interventions. Chest.
143:1117–1126. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Weir HK, Thun MJ, Hankey BF, Ries LA, Howe
HL, Wingo PA, Jemal A, Ward E, Anderson RN and Edwards BK: Annual
report to the nation on the status of cancer, 1975–2000, featuring
the uses of surveillance data for cancer prevention and control. J
Natl Cancer Inst. 95:1276–1299. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shimamura T, Li D, Ji H, Haringsma HJ,
Liniker E, Borgman CL, Lowell AM, Minami Y, McNamara K, Perera SA,
et al: Hsp90 inhibition suppresses mutant EGFR-T790M signaling and
overcomes kinase inhibitor resistance. Cancer Res. 68:5827–5838.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yang H, Chen D, Cui QC, Yuan X and Dou QP:
Celastrol, a triterpene extracted from the Chinese ‘Thunder of God
Vine’ is a potent proteasome inhibitor and suppresses human
prostate cancer growth in nude mice. Cancer Res. 66:4758–4765.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sukpondma Y, Rukachaisirikul V and
Phongpaichit S: Antibacterial caged-tetraprenylated xanthones from
the fruits of Garcinia hanburyi. Chem Pharm Bull (Tokyo).
53:850–852. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wu ZQ, Guo QL, You QD, Zhao L and Gu HY:
Gambogic acid inhibits proliferation of human lung carcinoma SPC-A1
cells in vivo and in vitro and represses telomerase activity
and telomerase reverse transcriptase mRNA expression in the cells.
Biol Pharm Bull. 27:1769–1774. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Guo QL, Lin SS, You QD, Gu HY, Yu J, Zhao
L, Qi Q, Liang F, Tan Z and Wang X: Inhibition of human telomerase
reverse transcriptase gene expression by gambogic acid in human
hepatoma SMMC-7721 cells. Life Sci. 78:1238–1245. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhao L, Guo QL, You QD, Wu ZQ and Gu HY:
Gambogic acid induces apoptosis and regulates expressions of Bax
and Bcl-2 protein in human gastric carcinoma MGC-803 cells. Bio
Pharm Bull. 27:998–1003. 2004. View Article : Google Scholar
|
|
9
|
Yi T, Yi Z, Cho SG, Luo J, Pandey MK,
Aggarwal BB and Liu M: Gambogic acid inhibits angiogenesis and
prostate tumor growth by suppressing vascular endothelial growth
factor receptor 2 signaling. Cancer Res. 68:1843–1850. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sano T, Takeuchi S, Nakagawa T, Ishikawa
D, Nanjo S, Yamada T, Nakamura T, Matsumoto K and Yano S: The novel
phosphoinositide 3-kinase-mammalian target of rapamycin inhibitor,
BEZ235, circumvents erlotinib resistance of epidermal growth factor
receptor mutant lung cancer cells triggered by hepatocyte growth
factor. Int J Cancer. 133:505–513. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pao W, Miller VA, Politi KA, Riely GJ,
Somwar R, Zakowski MF, Kris MG and Varmus H: Acquired resistance of
lung adenocarcinomas to gefitinib or erlotinib is associated with a
second mutation in the EGFR kinase domain. PLoS Med. 2:e732005.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li D, Ambrogio L, Shimamura T, Kubo S,
Takahashi M, Chirieac LR, Padera RF, Shapiro GI, Baum A,
Himmelsbach F, et al: BIBW2992, an irreversible EGFR/HER2 inhibitor
highly effective in preclinical lung cancer models. Oncogene.
27:4702–4711. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wong KK, Fracasso PM, Bukowski RM, Lynch
TJ, Munster PN, Shapiro GI, Jänne PA, Eder JP, Naughton MJ, Ellis
MJ, et al: A phase I study with neratinib (HKI-272), an
irreversible pan ErbB receptor tyrosine kinase inhibitor, in
patients with solid tumors. Clin Cancer Res. 15:2552–2558. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhao Q, Yang Y, Yu J, You QD, Zeng S, Gu
HY, Lu N, Qi Q, Liu W, Wang XT, et al: Posttranscriptional
regulation of the telomerase hTERT by gambogic acid in human
gastric carcinoma 823 cells. Cancer Lett. 262:223–231. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pandey MK, Sung B, Ahn KS, Kunnumakkara
AB, Chaturvedi MM and Aggarwal BB: Gambogic acid, a novel ligand
for transferrin receptor, potentiates TNF-induced apoptosis through
modulation of the nuclear factor-kappaB signaling pathway. Blood.
110:3517–3525. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Adrain C, Creagh EM and Martin SJ: Defying
death: Showing Bcl-2 the way home. Nat Cell Biol. 5:9–11. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xu J, Zhou M, Ouyang J, Wang J, Zhang Q,
Xu Y, Xu Y, Zhang Q, Xu X and Zeng H: Gambogic acid induces
mitochondria-dependent apoptosis by modulation of Bcl-2 and Bax in
mantle cell lymphoma JeKo-1 cells. Chin J Cancer Res. 25:183–191.
2013.PubMed/NCBI
|