Introduction
Breast cancer in males is extremely rare and
accounts for ~1% of all malignant breast neoplasm cases (1,2). Accessory
breasts are observed in 2–6% of the general population (3), and these tissues may present in various
positions along the milk line, but most frequently in the axillary
region (4). Accessory breast
carcinoma is a rare form of breast cancer usually occurring in the
axilla or inguinal region, where there are abundant lymph nodes and
capillaries, and the incidence rate is 0.2–0.6% (5). There are few cases of accessory breast
carcinoma in males reported in the literature (6–8). The
principal malignancy identified in accessory breast tissue, as with
normal breasts, is invasive ductal carcinoma (79%). In the majority
of previously documented cases, treatment regimens for accessory
breast carcinoma follow the guidelines for breast cancer (9–11). Early
diagnosis of this carcinoma is difficult due to its rarity and a
general lack of awareness among physicians and patients (9,10,12). Thus, metastasis occurs at an early
stage and the prognosis of patients is often poor, however, due to
limited follow-up data and small sample sizes of previous studies,
an accurate prognosis for accessory breast carcinoma is difficult
to estimate (12).
The present study reported the case of a 56-year-old
male accessory breast cancer patient who underwent a series of four
surgical excisions of a primary ectopic breast carcinoma and
developed local lymph node and opposite supraclavicular lymph node
metastasis. Subsequently, the patient developed pulmonary and bone
metastasis. The patient was successfully treated with an endocrine
therapy regimen (anastrozole and goserelin). Furthermore, the
present study evaluated the best approach for the treatment of
accessory breast neoplasm in male patients.
Case report
A 56-year-old Chinese male was referred to a local
hospital in July 2005, complaining of a mung bean-sized mass under
the right axilla. The patient underwent mass resection; however,
the resected tissue was not pathologically examined as it was
considered to be lipoma by the doctor, and thus no official
diagnosis was recorded. On April 23rd, 2006, a second mung
bean-sized mass was detected at the same site, which was again
excised with no pathological examination. In October 2007, the
patient presented at the China-Japan Union Hospital Affiliated to
Jilin University (Changchun, China) with a further, yolk-sized
painful mass at the same site. Tumor resection was performed, and
the macroscopic appearance of the lesion indicated a
poorly-differentiated adenocarcinoma in dermis and subcutaneous
tissues. Immunohistochemical analysis [with (-), negative; (+),
positive; and (+++), strongly positive] demonstrated that the mass
was cytokeratin (CK)20 (-), estrogen receptor (ER) (+++), C-erbB-2
(-), thyroid transcription factor 1 (TTF-1) (-) and gross cystic
disease fluid protein 15 (GCDFP-15) (focal+). According to the
clinical performance and pathological findings, the mass was
considered to be an adenocarcinoma from the accessory breast
tissue. Next, the patient received right breast modified radical
mastectomy and left breast simple excision. Postoperative pathology
results indicated reactive hyperplasia of giant cell and fibrillar
connective tissue, observed in the right breast. A total of 3 out
of the 17 dissected axillary lymph nodes were positive for
metastatic carcinoma. There was no cancer in the left breast
tissue. The patient was diagnosed with right accessory breast
carcinoma (PT1N1M0) (13), right
axilla metastatic carcinoma and hypertrophy of the left breast.
Subsequently, the patient received six cycles of adjuvant
chemotherapy [docetaxel, 140 mg intravenously (i.v.) and
cyclophosphamide (CTX), 1 g i.v.; on day 1 then once every 3 weeks]
along with radiation therapy.
On April 20th, 2011, the patient incidentally
detected a further mung bean-sized mass adjacent to the his left
subclavian and, at the same time, experienced lower-limb bone pain.
This mass was then resected and the pathological results indicated
a poorly-differentiated metastatic carcinoma. Immunohistochemical
analysis revealed that the mass was CK (focal+), vimentin (+), CK7
(+), CD117 (+), E-calpain (+), ER (3+), progesterone receptor (PR)
(+++) and CD56 (+); however, the tissue was negative for TTF-1,
Syn, HMB45, chromogranin A and human epidermal growth factor
receptor-2. The patient was administered two adjuvant chemotherapy
(AC) cycles (AC regimen: Pirarubicin, 40 mg, day 1 and 2; and CTX,
1 g i.v., on day 1, then once every 3 weeks). However, following
chemotherapy, the bone pain was not relieved. On May 24th, 2011, a
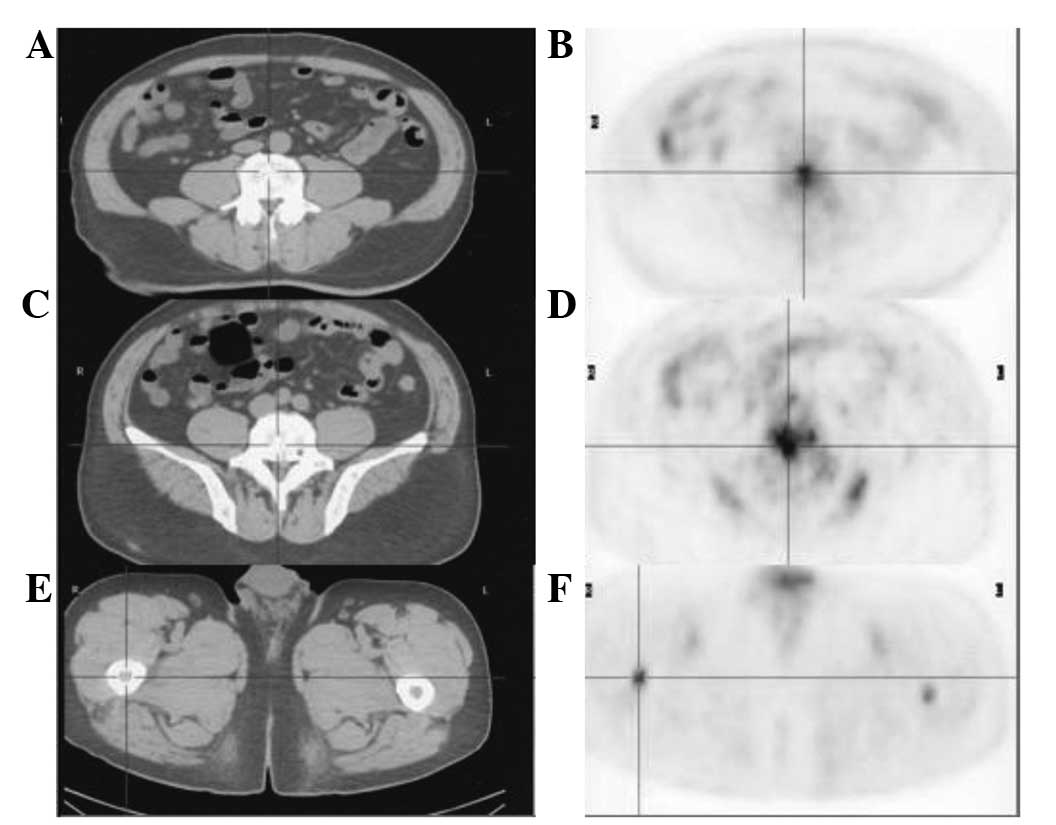
positron emission tomography-computed tomography (PET-CT)
examination revealed multiple metabolic enlarged lymph nodes with
high fluorodeoxyglucose uptake under the left collarbone, in the
mediastinum and in the two hilus pulmonis, as well as multiple
nodules in the two lungs and pleuras at both sides, a subcutaneous
nodule on the right back and multiple high metastatic lesions of
the bone (Figs. 1 and 2). Subsequently, the treatment was changed
from chemotherapy to endocrine therapy (anastrozole and goserelin
regimen: Anastrozole, 1 mg orally, daily; goserelin, 3.6 mg
subcutaneously, once every 28 days). Endocrine therapy treatment is
on-going since June 29th, 2011, and zoledronic acid (4 mg i.v.,
once every 28 days) is also administered every 28 days. The
lower-limb bone pain was relieved following hormone therapy. On May
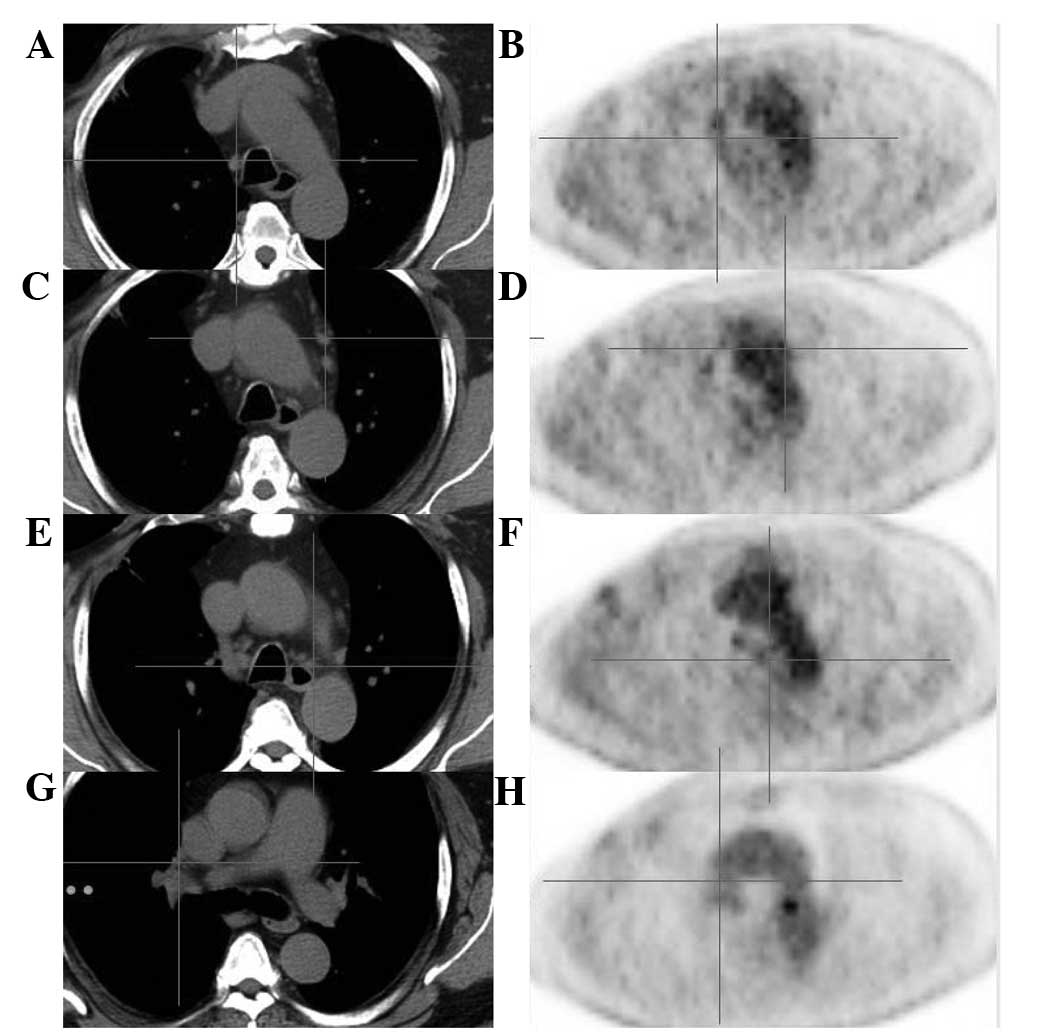
24th 2012, a PET-CT scan revealed that the disease had improved
significantly. Fig. 3 demonstrates
that the enlarged mediastinal lymph nodes were reduced in size, and
the metabolic level was reduced. Fig.
4 indicates that the metabolic levels of the osteoblastic
metastases were reduced also. The enlarged lymph nodes were much
smaller, and the metabolic levels of the lymph nodes and
osteoblastic metastases were reduced compared with previous
examinations. The most commonly used markers for breast cancer are
cancer antigen 153 and carcinoembryonic antigen, thus, levels of
these antigens are presented in Table
I. The increased levels of serum alkaline phosphatase are
associated with bone metastasis. At the latest follow-up in 2014,
the patient remained stable with no evidence of progression
(Figs. 5 and 6).
 | Table I.Laboratory test results. |
Table I.
Laboratory test results.
|
|
| Current case |
|---|
|
|
|
|
|---|
| Markers | Normal range | Prior to therapy | 1 year after
therapy |
|---|
| Carcinoembryonic
antigen, ng/ml | 0.50–9.60 |
28.18 | 11 |
| Cancer antigen 15-3,
U/ml |
0.10–31.30 |
31.12 | 16 |
| Serum alkaline
phosphatase, IU/l |
30.0–120.0 | 188.00 | 145 |
The current study was approved by the ethics
committee of China-Japan Union Hospital Affiliated to Jilin
University, and written informed consent was obtained from the
patient prior to the study.
Discussion
The presence of ectopic breast tissue is reported in
2–6% of the general population with the majority of cases being
located in the axillary region (3).
Accessory breast carcinoma in males is extremely rare, with the
most common clinical manifestation being accessory breast carcinoma
of the axilla (12,15). The most frequent histological type of
this lesion is invasive ductal carcinoma (worldwide incidence, 72%)
(16,17).
In the majority of studies, treatment regimens for
accessory breast carcinoma follow the guidelines for breast cancer
treatment (9,10,11).
Similar to breast cancer, accessory breast cancer is also
surgically treated and supplemented with preoperative or
postoperative chemotherapy (18).
However, external radiotherapy should be considered in order to
increase local control. Hormonal therapy is offered depending on
the tumor and patients characteristics, following the same
guidelines with anatomic breast carcinoma (19). Yamamura et al (8) have reported a case of male breast cancer
originating in an accessory mammary gland in the axilla, which was
successfully treated with hormone therapy (tamoxifen at 20 mg/day)
(8). In the case reported in the
present study, the disease was not relieved following two cycles of
adjuvant chemotherapy with the AC regimen. Based on the results of
immunohistochemical analysis, which revealed that the tumor was ER
(+++) and PR (+++), the patient was treated with endocrine therapy
(anastrozole and goserelin), which was an effective and tolerated
therapy for the current patient.
Prognosis of accessory breast carcinoma is difficult
to establish, primarily due to absent or limited follow-up data,
and small sample sizes of previous studies (12). The disease follows similar prognostic
indices as those of anatomic breast carcinoma (20). Certain authors have reported that
carcinoma of accessory breast tissue may metastasize to the lymph
nodes earlier and more frequently compared with anatomic breast
carcinoma, since it usually occurs under the axilla or in the
inguinal region, where there is an abundance of lymph nodes and
capillaries (19,21).
In conclusion, the present study reported a case of
male accessory breast cancer in a patient with delayed diagnosis at
a locally advanced stage; however, the patient was successfully
treated with endocrine therapy. Currently, the patient is under
follow-up observation, without any progression of the accessory
breast cancer. The current case demonstrates that, although
accessory breast cancer in males is extremely rare, the possibility
of this disease should be considered when establishing a diagnosis.
Investigation of further cases of accessory breast cancer in male
patients will provide an improved understanding of the underlying
mechanism, etiology, treatment and prognosis of this disease.
References
|
1
|
Fentiman IS, Fourquet A and Hortobagyi GN:
Male breast cancer. Lancet. 367:595–604. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gennari R, Curigliano G, JereczekFossa BA,
Zurrida S, Renne G, Intra M, Galimberti V, Luini A, Orecchia R,
Viale G, et al: Male breast cancer: A special therapeutic problem.
Anything new? (Review). Int J Oncol. 24:663–670. 2004.PubMed/NCBI
|
|
3
|
Gutermuth J, Audring H, Voit C and Haas N:
Primary carcinoma of ectopic axillary breast tissue. J Eur Acad
Dermatol Venereol. 20:217–221. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Grossl NA: Supernumerary breast tissue:
Historical perspectives and clinical features. South Med J.
93:29–32. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Matsuoka H, Ueo H, Kuwano H, Sugimachi K
and Inokuchi K: A case of carcinoma originating from accessory
breast tissue of the axilla. Gan No Rinsho. 30:387–391.
1984.PubMed/NCBI
|
|
6
|
Takeyama H, Takahashi H, Tabei I, Fukuchi
O, Nogi H, Kinoshita S, Uchida K and Morikawa T: Malignant neoplasm
in the axilla of a male: Suspected primary carcinoma of an
accessory mammary gland. Breast Cancer. 17:151–154. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gao YG, Zhang SH and Wang Y: A case of
accessory mammary cancer in a male patient and a literature review.
Eur J Gynaecol Oncol. 35:452–455. 2014.PubMed/NCBI
|
|
8
|
Yamamura J, Masuda N, Kodama Y, Yasojima
H, Mizutani M, Kuriyama K, Mano M, Nakamori S and Sekimoto M: Male
breast cancer originating in an accessory mammary gland in the
axilla: A case report. Case Rep Med. 2012:2862102012.PubMed/NCBI
|
|
9
|
Madej B, Balak B, Winkler I and Burdan F:
Cancer of the accessory breast - a case report. Adv Med Sci.
54:308–310. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
KahramanCetintas S, TuranOzdemir S, Topal
U, Kurt M, Gokgoz S, Saraydaroglu O and Ozkan L: Carcinoma
originating from aberrant breast tissue. A case report and review
of the literature. Tumori. 94:440–443. 2008.PubMed/NCBI
|
|
11
|
Markopoulos C, Kouskos E, Kontzoglou K,
Gogas G, Kyriakou V and Gogas J: Breast cancer in ectopic breast
tissue. Eur J Gynaecol Oncol. 22:157–159. 2001.PubMed/NCBI
|
|
12
|
Evans DM and Guyton DP: Carcinoma of the
axillary breast. J Surg Oncol. 59:190–195. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Singletary SE and Connolly JL: Breast
cancer staging: Working with the sixth edition of the AJCC Cancer
Staging Manual. CA Cancer J Clin. 56:37–47; quiz 50-31. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rusch VW, Asamura H, Watanabe H, et al:
Members of IASLC Staging Committee: The IASLC lung cancer staging
project: A proposal for a new international lymph node map in the
forthcoming seventh edition of the TNM classification for lung
cancer. J Thorac Oncol. 4:568–577. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Amsler E, SigalZafrani B, Marinho E and
Aractingi S: Ectopic breast cancer of the axilla. Ann Dermatol
Venereol. 129:1389–1391. 2002.(In French). PubMed/NCBI
|
|
16
|
Visconti G, Eltahir Y, Van Ginkel RJ, Bart
J and Werker PM: Approach and management of primary ectopic breast
carcinoma in the axilla: Where are we? A comprehensive historical
literature review. J Plast Reconstr Aesthet Surg. 64:e1–e11. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yerra L, Karnad AB and Votaw ML: Primary
breast cancer in aberrant breast tissue in the axilla. South Med J.
90:661–662. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hao JY, Yang CC, Liu FF, Yang YL, Li S, Li
WD, Li YQ, Lang RG, Fan Y, Paulos E, et al: Accessory breast cancer
occurring concurrently with bilateral primary invasive breast
carcinomas: A report of two cases and literature review. Cancer
Biol Med. 9:197–201. 2012.PubMed/NCBI
|
|
19
|
Routiot T, Marchal C, Verhaeghe JL,
Depardieu C, Netter E, Weber B and Carolus JM: Breast carcinoma
located in ectopic breast tissue: A case report and review of the
literature. Oncol Rep. 5:413–417. 1998.PubMed/NCBI
|
|
20
|
Marshall MB, Moynihan JJ, Frost A and
Evans SR: Ectopic breast cancer: Case report and literature review.
Surg Oncol. 3:295–304. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
NihonYanagi Y, Ueda T, Kameda N and
Okazumi S: A case of ectopic breast cancer with a literature
review. Surg Oncol. 20:35–42. 2011. View Article : Google Scholar : PubMed/NCBI
|