Introduction
Renal cell carcinoma is the most common primary
malignant tumor of the kidney, accounting for 85–90% of malignant
kidney tumors (1) and 3% of all
malignant tumors, with an incidence that is increasing yearly
(2). Angiomyolipoma is the most
common benign tumor of the kidney, comprising 7–9% of kidney
tumors. The accurate evaluation of tumor behavior is critical in
selecting an appropriate therapy and determining the prognosis. At
present, the most commonly employed imaging modalities for renal
tumors include computed tomography (CT), magnetic resonance imaging
(MRI) and contrast-enhanced imaging. To differentiate between
benign and malignant tumors or tumor stages, tissue density is
measured on CT, the tissue signal intensity if measured on plain
MRI and the pattern of contrast enhancement is examined on enhanced
images. Renal angiomyolipoma usually contains significant fat and
therefore, shows typical characteristics of fat on CT or MRI. The
differential diagnosis of renal carcinoma is usually not difficult,
but lesions with little or no fat and small tumors are difficult to
discern on conventional CT, plain MRI and contrast-enhanced imaging
(3–5).
In addition, certain patients are allergic to the contrast material
used during enhanced CT (6,7). Contrast-enhanced MRI may produce fewer
allergic reactions, but it can also accelerate the progression of
nephrogenic systemic fibrosis in patients with renal insufficiency
(8–10).
With recent developments in imaging technology and
the increased use of software for high-field MRI (1.5T and 3.0T),
diffusion-weighted MRI (DWI) has gained wide use clinically. This
diagnostic technique offers the advantage of good safety and does
not require a contrast agent. DWI is the only non-invasive
functional imaging able to assess the water diffusion status in
vivo, and as a result, it is widely used in the examination of
the central nervous system, particularly for the early diagnosis of
cerebral infarction (11) and the
staging of astrocytoma. The apparent diffusion coefficient (ADC) is
an important index that is measured during DWI. In recent years,
follow-up DWI has been increasingly employed in imaging the
abdominal organs, breast, pancreas, liver and kidney (12–16). DWI
provides functional information through three parameters: The
dispersion diagram, the ADC diagram and the ADC value, which has
gained significant attention due to its role in tumor diagnosis
(17).
In the present study, a retrospective analysis was
performed on 54 cases of renal carcinoma and 31 cases of
angiomyolipoma assessed by DWI in order to investigate the utility
of DWI in the differential diagnosis of common benign and malignant
tumors in the kidney.
Materials and methods
General data
The complete medical records of 85 renal tumor cases
presenting to the Linyi People's Hospital (Linyi, Shangdong, China)
between March 2006 and December 2010 were collected. All patients
underwent plain MRI and DWI pre-operatively, and the diagnosis was
confirmed surgically by the Department of Urology. Among the 85
patients, 59 were male and 26 were female; the mean patient age was
53.8 years (range, 23–81 years). All 85 cases presented with
unilateral renal tumors, 48 on the right and 37 on the left. The
upper pole of the kidney was affected in 30 cases, the lower pole
in 37 cases and the renal hilus in 18 cases. Clinical signs were
absent in 38 cases, and in the remainder, the following physical
findings were observed: Gross hematuria (n=14); lower back pain
(n=13); lower back pain with hematuria (n=15); and a waist mass
(n=5). Of the 54 cases of renal cell carcinoma, 8 patients
underwent tumor enucleation, 37 patients underwent a nephrectomy
and 9 patients underwent a radical nephrectomy. Of the 31 cases of
renal angiomyolipoma, 4 patients underwent a nephrectomy and 27
patients underwent tumor enucleation. Tumors were diagnosed on
post-operative histopathological staging as follows: Clear cell
renal cell carcinoma (n=46); papillary carcinoma (n=7); chromophobe
cell carcinoma (n=1); and renal angiomyolipoma (n=31). This study
was conducted in accordance with the declaration of Helsinki and
with approval from the Ethics Committee of Linyi People's Hospital.
Written informed consent was obtained from all participants.
MRI examination
All 85 patients underwent plain MRI and DWI (1.5T;
Twin Speed Infinity with Excite II; GE Healthcare Life Sciences,
Pittsburgh, PA, USA). The patient was placed in the supine position
and the phased array surface coil was positioned at the abdomen,
with the Torso PA coils at the abdominal wall and side. The
abdominal wall was secured in front of the coil using a bandage,
and patients were trained to breathe deeply prior to holding their
breath in order to reduce motion artifacts during scanning. Axial
MRI was performed in a fast recovery fast spin echo (SE)
accelerated sequence [axial T1-weighted imaging (WI), T2WI and fat
suppression] and a coronal fast imaging employing steady state
acquisition sequence. A single-shot SE echo planar imaging was
obtained during axial DWI scanning. The two scans were performed
with the same body position, and layer thickness on axial T2WI, and
imaging parameters were as follows: b=0 or 800 sec/mm2;
stimulated repetition time/echo time of 4,000/56 msec one time;
received bandwidth of 125 kHz; thickness/distance of 5/0 mm; field
of view of 36×34 cm; and a matrix of 128×128 cm. Sensitive gradient
pulses were applied in the X-, Y- and Z-axes. Patients were
required to breath-hold during scanning.
Image transmission
The DWI images were transferred to an AW4.0
workstation and processed using FuncTool 2.0 software (both GE
Healthcare Life Sciences).
Image processing
During processing, the threshold was defined by
removing fat, bone and gas regions from the image, and the b-value
was entered, resulting in the DWI and ADC maps.
The region of interest (ROI) measured 60–100
mm2. Using the conventional axial T2WI and DWI images as
a reference, the ROI was set at the same size in three successive
levels of the ADC diagram, and the ADC was measured. The mean value
was designated as the final value. In lesions with a homogeneous
signal, the ROI avoided the margins as much as possible in order to
reduce the partial volume effect. In lesions with an inhomogeneous
signal, the ROI was positioned in the solid region of the lesion,
and cystic change, necrosis, hemorrhage and calcification areas
were avoided when possible.
The ADC values were measured and recorded, and the
ADC diagram was stored. The ADC was calculated according to the
following formula: ADC = (S0 / S1) / (b1 − b0), where b0 was 0
sec/mm2, b1 was 800 sec/mm2, S0 was the DWI
signal intensity at a b-value of 0 sec/mm2 and S1 was
the DWI signal strength with a b-value of 800
sec/mm2.
Statistical analysis
The normal distribution of ADC values was evaluated
in the renal cell carcinoma group and the renal angiomyolipoma
group using SPSS14.0 statistical analysis software (SPSS, Inc.,
Chicago, IL, USA). Subsequent to confirming that the data were
normally distributed, the mean ADC values were compared between the
two groups using an analysis of variance. P<0.05 was considered
to indicate a statistically significant difference.
Results
MRI, DWI, and ADC in the renal
angiomyolipoma group
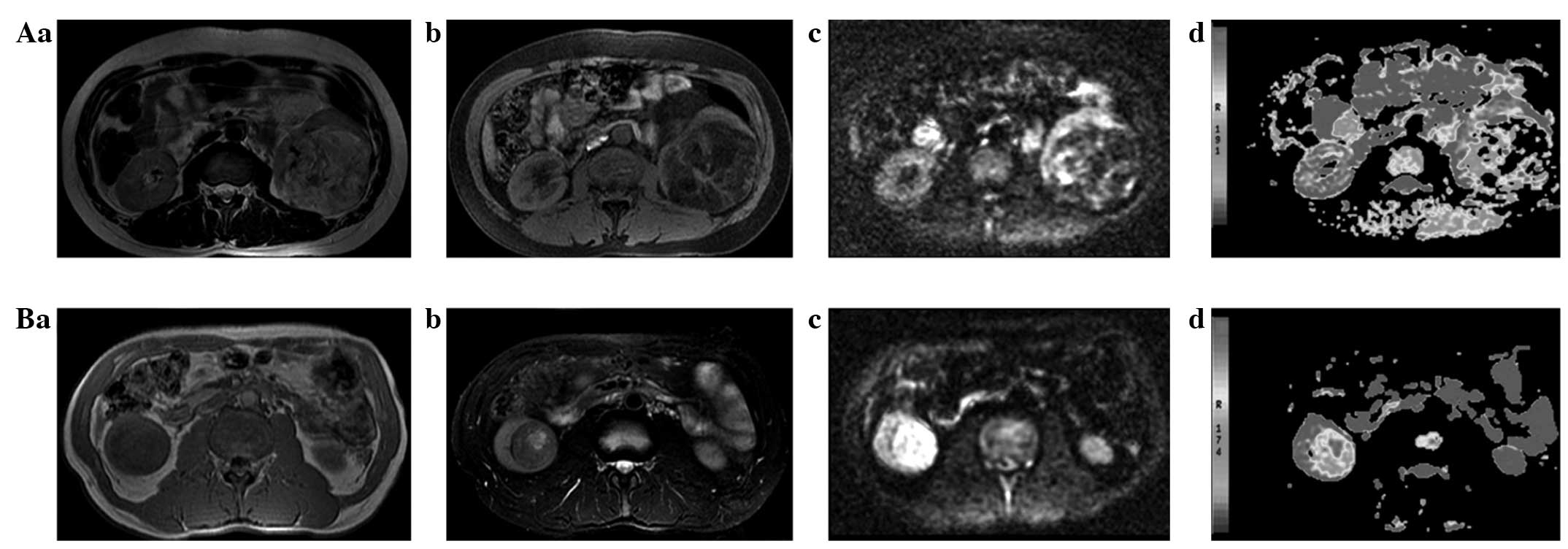
On T1WI, the 31 angiomyolipoma cases showed a high
partial fat signal intensity and a low signal intensity in the soft
tissue. When the fat signal was suppressed, it showed a low signal
intensity on T2 images, while the soft tissues showed a slightly
high signal intensity. The corresponding DWI image showed a lower
fat signal intensity and slightly higher signal intensity for soft
tissue; the fat signal intensity on the pseudo-color ADC map was
high, the soft tissue was slightly low and the signal intensity was
inhomogeneous (Fig. 1A).
MRI, DWI and ADC in the renal cell
carcinoma group
Among the 54 renal cell carcinoma cases, 18 cases
showed a homogeneous low signal intensity on T1WI images, a
homogeneous high signal intensity on T2WI and a homogeneous high
signal intensity on DWI. The corresponding pseudo-color ADC map
showed a slightly low signal intensity and a uniform signal
strength. In 36 cases containing necrosis, cystic change or
hemorrhage, the T1WI showed an inhomogeneous low signal intensity,
and the T2WI and DWI showed an inhomogeneous high signal intensity.
The corresponding pseudo-color ADC map showed a heterogeneous
slightly low signal intensity (Fig.
1B).
ADC comparison
As detailed in Table
I, the ADC for the renal cell carcinoma group was significantly
lower than that in the renal angiomyolipoma group (P<0.05).
 | Table I.Comparison of the ADC in the renal
cell carcinoma and renal angiomyolipoma groups when the b-value was
set at 800 sec/mm2. |
Table I.
Comparison of the ADC in the renal
cell carcinoma and renal angiomyolipoma groups when the b-value was
set at 800 sec/mm2.
| Group | n | ADC, x10−3
mm2/sec | F | P-value |
|---|
| Renal
angiomyolipoma | 31 | 1.8271±0.3486 | 25.718 | 0.000 |
| Renal carcinoma | 54 | 1.4181±0.1643 |
|
|
Discussion
The present study demonstrated that the ADC in renal
cell carcinoma is lower than that in renal angiomyolipoma, which is
consistent with the results of studies by Doğanay et al
(18), Mytsyk et al (19) and Zhang et al (20), which found that the ADC was lower in
malignant kidney tumors than in benign tumors. Doğanay et al
reported a mean ADC of 2.21±0.63×10−3 mm2/sec
for renal cell carcinoma and 2.55±0.49×10−3
mm2/sec for benign tumors. Mytsyk et al reported
a similar trend of 2.11±0.25×10−3 mm2/sec in
renal cell carcinoma and 2.36±0.32×10−3
mm2/sec in angiomyolipoma. Zhang et al reported a
mean ADC of 1.264±0.271×10−3 mm2/sec for
renal cell carcinoma and 1.717±0.431×10−3
mm2/sec for angiomyolipoma. The present analysis showed
a mean ADC of 1.4181±0.1643×10−3 mm2/sec for
renal cell carcinoma and 1.8271±0.3486×10−3
mm2/sec for renal angiomyolipoma.
The diagnosis of tumors on DWI is based on
organizational, structural, cell density and karyoplasmic ratio
changes within lesions, as well as changes to the large molecular
distribution in the intra- and extracellular spaces; these
variations alter the Brownian motion of water molecules, generating
an abnormality in the DWI signal (21). The cell concentration increases in
renal cell carcinoma, which decreases the extracellular clearance
and slows the extracellular water molecular motion (22). In addition, the cell nuclei are
relatively larger and the cytoplasm lessened, which further reduces
water molecular motion within cells. These mechanisms explain the
lower ADC value in renal cell carcinoma compared with normal
tissues. The ADC is an index that measures the magnitude of random
molecular motion within a given volume and is calculated as
follows: ADC = ln(low S / high S) / (high b − low b), where low S
and high S represent the signal intensity measured by DWI
corresponding to a low b and high b, respectively, and b is a
diffusion gradient factor. By calculating the ADC at each voxel and
arranging the results in a grayscale image, an ADC diagram can be
generated, providing a map of the ADCs of the entire lesion. In the
ADC map, high signal areas represent high dispersion; the ADC value
is high, while the signal on the corresponding DWI map is low. By
contrast, a low signal zone on the ADC map represents a low
dispersion region, indicating a low ADC value and a high signal on
the corresponding DWI map. In addition, the ADC map eliminates the
effect of T2 transmission and does not depend on the strength of
the magnetic field and gradient; thus, the ADC value reflects the
magnitude of molecular water movement in tissues, and can be
measured and compared graphically (23).
The present study has two notable limitations.
Firstly, the majority of the renal angiomyolipomas contained
significant amounts of fat, and only a few lesions contained little
or no fat. Furthermore, other common benign renal tumors, such as
renal oncocytoma, were not included in this small study.
This study indicates that DWI may be valuable in the
differential diagnosis of benign and malignant renal tumors in the
kidney; it can differentiate between benign and malignant tumors,
and could prove particularly useful for small renal vascular
leiomyoma, tumors with little to no fat and renal cell carcinoma.
DWI should be considered an important supplementary tool in the
diagnosis and differentiation of renal tumors.
References
|
1
|
Lam JS, Shvarts O and Pantuck AJ: Changing
concepts in the surgical management of renal cell carcinoma. Eur
Urol. 45:692–705. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kirkali Z and Öber C: Clinical aspects of
renal cell carcinoma. EAU Update Series. 1:189–196. 2003.
View Article : Google Scholar
|
|
3
|
Fujii Y, Komai Y, Saito K, Iimura Y,
Yonese J, Kawakami S, Ishikawa Y, Kumagai J, Kihara K and Fukui I:
Incidence of benign pathologic lesions at partial nephrectomy for
presumed RCC renal masses: Japanese dual-center experience with 176
consecutive patients. Urology. 72:598–602. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jeon HG, Lee SR, Kim KH, Oh YT, Cho NH,
Rha KH, Yang SC and Han WK: Benign lesions after partial
nephrectomy for presumed renal cell carcinoma in masses 4 cm or
less: Prevalence and predictors in Korean patients. Urology.
76:574–579. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Xiong YH, Zhang ZL, Li YH, Liu ZW, Hou GL,
Liu Q, Yun JP, Zhang XQ and Zhou FJ: Benign pathological findings
in 303 Chinese patients undergoing surgery for presumed localized
renal cell carcinoma. Int J Urol. 17:517–521. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Loh S, Bagheri S, Katzberg RW, Fung MA and
Li CS: Delayed adverse reaction to contrast-enhanced CT: A
prospective single-center study comparison to control group without
enhancement. Radiology. 255:764–771. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Katzberg RW and Newhouse JH: Intravenous
contrast medium-induced nephrotoxicity: Is the medical risk really
as great as we have come to believe? Radiology. 256:21–28. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Marckmann P, Skov L, Rossen K, Dupont A,
Damholt MB, Heaf JG and Thomsen HS: Nephrogenic systemic fibrosis:
Suspected causative role of gadodiamide used for contrast-enhanced
magnetic resonance imaging. J Am Soc Nephrol. 17:2359–2362. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Deo A, Fogel M and Cowper SE: Nephrogenic
systemic fibrosis: A population study examining the relationship of
disease development to gadolinium exposure. Clin J Am Soc Nephrol.
2:264–267. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
AbuAlfa AK: Nephrogenic systemic fibrosis
and gadolinium-based contrast agents. Adv Chronic Kidney Dis.
18:188–198. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Artz NS, Sadowski EA, Wentland AL, Grist
TM, Seo S, Djamali A and Fain SB: Arterial spin labeling MRI for
assessment of perfusion in native and transplanted kidneys. Magn
Reson Imaging. 29:74–82. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Irie H, Kamochi N, Nojiri J, Egashira Y,
Sasaguri K and Kudo S: High b-value diffusionweighted MRI in
differentiation between benign and malignant polypoid gallbladder
lesions. Acta Radiol. 15:236–240. 2011. View Article : Google Scholar
|
|
13
|
Chandarana H, Lee VS, Hecht E, Taouli B
and Sigmund EE: Comparison of biexponential and monoexponential
model of diffusion weighted imaging in evaluation of renal lesions:
Preliminary experience. Invest Radiol. 46:285–291. 2011.PubMed/NCBI
|
|
14
|
Rheinheimer S, Stieltjes B, Schneider F,
Simon D, Pahernik S, Kauczor HU and Hallscheidt P: Investigation of
renal lesions by diffusion-weighted magnetic resonance imaging
applying intravoxel incoherent motion-derived parameters-initial
experience. Eur J Radiol. 81:e310–e316. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sandrasegaran K, Sundaram CP, Ramaswamy R,
Akisik FM, Rydberg MP, Lin C and Aisen AM: Usefulness of
diffusion-weighted imaging in the evaluation of renal masses. AJR
Am J Roentgenol. 194:438–445. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Baliyan V, Das CJ, Sharma S and Gupta AK:
Diffusion-weighted imaging in urinary tract lesions. Clin Radiol.
69:773–782. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen S, Ikawa F, Kurisu K, Arita K, Takaba
J and Kanou Y: Quantitative MR evaluation of intracranial
epidermoid tumors by fast fluid-attenuated inversion recovery
imaging and echo-plannar diffusion-weighted imaging. AJNR Am J
Neuroradiol. 22:1089–1096. 2001.PubMed/NCBI
|
|
18
|
Doğanay S, Kocakoç E, Ciçekçi M, Ağlamiş
S, Akpolat N and Orhan I: Ability and utility of diffusion-weighted
MRI with different b values in the evaluation of benign and
malignant renal lesions. Clin Radiol. 66:420–425. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mytsyk Y, Borys Y, Komnatska I, Dutka I
and Shatynska-Mytsyk I: Value of the diffusion-weighted MRI in the
differential diagnostics of malignant and benign kidney
neoplasms-our clinical experience. Pol J Radiol. 79:290–295. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang YL, Yu BL, Ren J, Qu K, Wang K,
Qiang YQ, Li CX and Sun XW: EADC values in diagnosis of renal
lesions by 3.0 T diffusion-weighted magnetic resonance imaging:
Compared with the ADC values. Appl Magn Reson. 44:349–363. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zimmerman RD: Is there a role for
diffusion-weighted imaging in patients with brain tumors or is the
‘bloom off the rose’? AJNR Am J Neuroradiol. 22:1013–1014.
2001.PubMed/NCBI
|
|
22
|
Wang J, Takashima S, Takayama F, Kawakami
S, Saito A, Matsushita T, Momose M and Ishiyama T: Head and neck
lesions: Characterization with diffusion-weighted echo-planar MR
imaging. Radiology. 220:621–630. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Huisman TA: Diffusion-weighted imaging:
Basic concepts and applicationin cerebral stroke and head trauma.
Eur Radiol. 13:2283–2297. 2003. View Article : Google Scholar : PubMed/NCBI
|