Introduction
Gastric cancer (GC) is the fourth most common type
of cancer and the second leading cause of cancer-associated
mortality worldwide (1). Metastasis
remains the major cause of mortality in patients with GC, despite
advancements in the understanding and treatment of cancer (2,3).
Therefore, exploring the molecular mechanisms underlying GC
metastasis may provide novel insights into tumor treatment and
predicting patient prognosis. Chemokine (C-X-C motif) ligand 1
(CXCL1), also termed GRO-1 oncogene, is a member of the G
protein-coupled receptor family that binds specifically to CXC
chemokine receptor 2 (CXCR2) (4).
CXCL1 is overexpressed in melanoma tumors and is involved in
malignant transformation (5). In
addition, CXCL1 activates nuclear factor (NF)-κB signaling through
epidermal growth factor receptor-transactivated Akt and contributes
to CXCR2-mediated ovarian cancer progression (6). CXCL1 was also found to contribute to
prostate tumor cell migration and invasion through the activation
of NF-κB (7). In GC, overexpression
of CXCL1 and its receptor CXCR2 were found to be associated with
tumor metastasis and a poor prognosis (8). However, the role of CXCL1 as a
prognostic biomarker and the mechanism in tumor metastasis remain
unknown. Epithelial mesenchymal transition (EMT) is a process that
results in epithelial cells losing intercellular adhesion and
obtaining myofibroblastic features and is critical for tumor
invasion and metastasis (9). Snail is
a zinc-finger transcription factor that may promote EMT, and
overexpression of Snail is associated with lymph node metastasis
and a poor prognosis in patients with GC (10,11). In
GC, NF-κB activation by tumor necrosis factor-Z, cyclooxygenase-2
and Rho guanosine diphosphate-dissociation inhibitor 2 increase
Snail expression, and Snail downregulates E-cadherin and promotes
EMT (12–14).
In the present study, the prognosis value of the
expression of CXCL1 and Snail was investigated and the correlation
between CXCL1 and Snail expression was evaluated. The current study
aimed to identify a prognostic biomarker in GC and suggest a
possible mechanism for the effect of CXCL1 in tumor metastasis.
Materials and methods
Patients and samples
In total, 127 patients with GC that underwent
radical resection at the Department of Gastrointestinopancreatic
Surgery of the First Affiliated Hospital of Sun Yat-Sen University
(Guangzhou, Guangdong, China) between 2005 and 2007 were enrolled
in the present study. The staging of the resected specimens was
performed using the tumor-node-metastasis (TNM) classification of
malignant tumors established by the International Union Against
Cancer (15). No patients received
adjuvant therapy, such as radiotherapy, chemoradiotherapy or
chemotherapy, or other medical interventions. The present study was
approved by the Research Ethics Committee of Sun Yat-Sen
University. Prior to enrollment in the present study, written
informed consent was obtained from all patients.
Immunohistochemistry
Tissue specimens were fixed in 10% neutral buffered
formaldehyde and were embedded in paraffin. All samples were
histologically reviewed by hematoxylin and eosin staining. Tissue
sections were cut into 4-µm thick slices and slides were coated
with 3-aminopropyltriethoxysilane (Guangzhou Xiuwei Commerce Co.,
Ltd., Guangzhou, China). Mouse anti-human CXCL1 monoclonal antibody
(dilution, 1:50; catalog no., MAB275; R&D Systems, Inc.,
Minneapolis, MN, USA) and rabbit anti-human Snail monoclonal
antibody (dilution, 1:50; catalog no., ab180714; Abcam, Cambridge,
UK) were used. The slides containing paraffin-embedded tissue
sections were baked at 65°C for 2 h, deparaffinized in xylene (3
times for 10 min each) and then rehydrated in ethanol (100, 95 and
75% graded series). Subsequently, the sections were autoclaved at
121°C for 8 min in citrate buffer (10 mmol/l sodium citrate; pH
6.0) for antigen retrieval. Endogenous peroxidase activity was
blocked by incubating the slides in 0.3% H2O2
solution. The sections were blocked with normal goat serum to block
non-specific binding and then incubated with the aforementioned
rabbit anti-Snail and mouse anti-CXCL1 antibodies overnight at 4°C.
Subsequently, secondary antibody (goat anti-rabbit/mouse antibody;
1:100 dilution; cat no. CW2069A; CWBio, Beijing, China) was
incubated with the tissue sections for 20 min. Finally, the slides
were counterstained with hematoxylin (Loogene Biotechnology Co.,
Ltd., Beijing, China), dehydrated with ethanol, permeabilized with
dimethylbenzene and mounted for assessment.
Evaluation of immunohistochemical
variables
Two independent observers, who were blinded to the
clinical outcomes of the cases, performed the immunohistochemical
analysis (16). Subsequent to
counting 1,000 tumor cells (high-power field, x400), the percentage
of cancer cells with Snail-positive nuclei was recorded. Nuclear
expression of Snail was graded by classifying the extent of
positive nuclear staining as ≤10, 11–25, 26–50, 51–75 and >75%.
The H-scoring system was used for semi-quantitative analysis of
CXCL1 expression in the GC tissue specimens (17).
Statistical analysis
Actuarial OS rates were calculated using the
Kaplan-Meier method and differences between the survival curves
were analyzed using the log-rank test. The survival time was
recorded in days and was defined as the time between the surgical
procedure and mortality or the last review. Univariate and
multivariate analyses based on the Cox proportional hazards
regression model were used to determine the association between the
survival time and multiple clinicopathological variables. In order
to compare the individual variables, the χ2 test was
performed. Spearman's rank correlation coefficient and Fisher's
exact test were used to explore the association between CXCL1 and
Snail expression. P<0.05 was considered to indicate a
statistically significant difference. All analyses were performed
using SPSS 19.0 software (IBM, Armonk, NY, USA).
Results
Patient characteristics
The clinicopathological characteristics of all
patients are summarized in Table I.
The patients consisted of 92 males and 35 females. The median age
was 57 (26–87) years old. The mean follow-up time of the patients
was 42.3±2.6 months.
 | Table I.Clinicopathological characteristics of
127 patients with gastric cancer. |
Table I.
Clinicopathological characteristics of
127 patients with gastric cancer.
| Characteristic | Total, n (%) |
|---|
| Gender |
|
| Male | 92
(72.4) |
|
Female | 35
(27.6) |
| Age |
|
| <60
years | 77 (60.6) |
| ≥60
years | 50 (39.4) |
| Location |
|
| Upper
third | 42
(33.1) |
| Middle
third | 28
(22.0) |
| Lower
third | 46
(36.2) |
|
Whole | 11 (8.7) |
| Tumor size (maximal
diameter) |
|
| <5
cm | 64
(50.4) |
| ≥5
cm | 63
(49.6) |
| pT |
|
| T1-2 | 41
(32.3) |
| T3–4 | 86
(67.7) |
| pN |
|
| No | 29
(22.8) |
| Yes | 98
(77.2) |
| TNM |
|
| I/II | 39
(30.7) |
|
III/IV | 88
(69.3) |
| Distant
metastasis |
|
| No | 100 (78.7) |
| Yes | 27
(21.3) |
| Liver metastasis |
|
| No | 123 (96.9) |
| Yes | 4
(3.1) |
| Peritoneal
seeding |
|
| No | 109 (85.8) |
| Yes | 18
(14.2) |
| Histological
type |
|
|
Well-differentiated | 4
(3.1) |
|
Moderately-differentiated | 37
(29.1) |
|
Poorly-differentiated | 86
(67.8) |
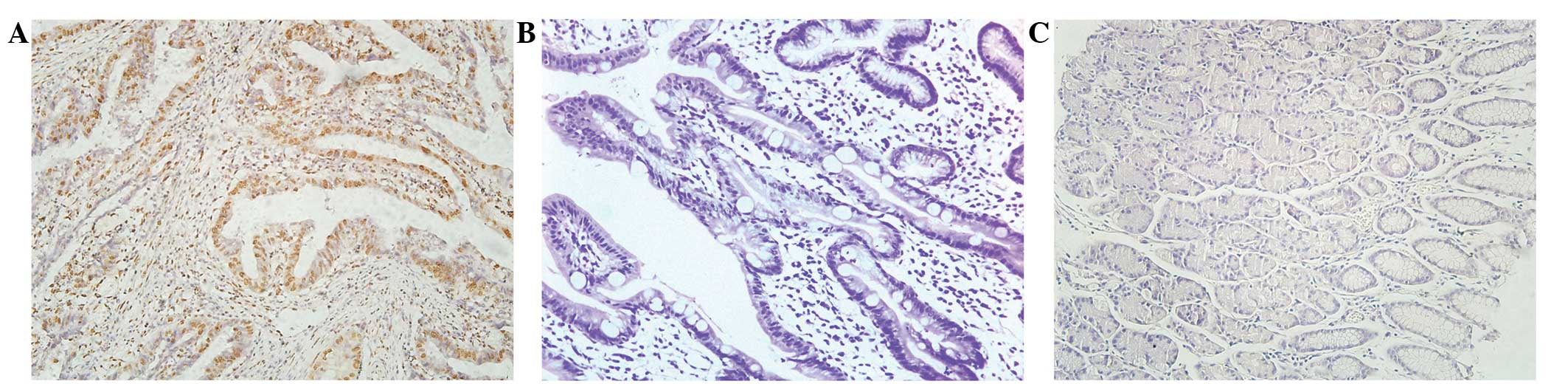
Expression of CXCL1 and Snail in GC
tissues
The expression levels of CXCL1 and Snail in tumor
and para-carcinoma tissues obtained from patients with GC were
examined by immunohistochemical analysis. Figs. 1 and 2
revealed the expression of CXCL1 and Snail in the para-carcinoma
tissue of patients with GC. Fig. 1B and
E exhibits the staining of CXCL1 and Snail in the tumor tissue
of patients with GC. Fig. 1C and F is
representative of strong staining for CXCL1 and Snail in the tumor
tissue of patients with GC. The dark brown immunostaining was
frequently observed in tumor cells, whereas extremely few
incidences of brown staining appeared in the para-carcinoma tissue
of patients with GC. A receiver operating characteristic curve was
used to determine the cut-off value for CXCL1 and Snail-positive
cases. The cut-off value for tissue specimens with CXCL1 scores is
>97.2%, thus, scores of ≤97.2% indicated no expression; this
resulted in 62.2% patients being defined as expressing CXCL1, while
the remaining patients (37.8%) had scores that resulted in the
tissue being classified as not expressing CXCL1. Positive nuclear
staining for Snail at levels of ≤75% (55.9%) and >75% (44.1%)
was defined as demonstrating the absence and presence of Snail
expression, respectively.
CXCL1 and Snail expression was
associated with overall survival in GC
Cox regression and Kaplan-Meier analysis were
performed to evaluate the prognostic effects of low or high
expression of CXCL1 and Snail in GC tissue specimens. Univariate
analysis revealed that neither patient age nor patient gender
demonstrated any prognostic significance for the OS rate of
patients with GC. However, the tumor size (P<0.001), presence of
lymph node metastasis (P<0.001), TNM staging (P<0.001), depth
of tumor infiltration (P<0.001), presence of distant metastasis
(P<0.001) and development of peritoneal dissemination (P=0.001)
were significantly associated with the OS rate (Table II). The overexpression of CXCL1
(P<0.001) and Snail (P<0.001) were significantly correlated
with the OS rate (Table II; Fig. 2). Multivariate analysis revealed that
high expression of CXCL1 (P=0.011; Table III) and Snail (P=0.013; Table III) were independent prognostic
factors for worse OS rates. The patients demonstrating increased
expression of CXCL1 and Snail were revealed by univariate and
multivariate analyses to possess worse prognoses compared with the
other groups (P=0.001 and P=0.005, respectively; Tables II and III; Fig.
3).
 | Table II.Univariate analysis of factors
associated with the overall survival rate. |
Table II.
Univariate analysis of factors
associated with the overall survival rate.
|
| Overall
survival |
|
|---|
|
|
|
|
|---|
| Variables | HR | 95% CI | P-value |
|---|
| Age |
|
|
|
| <60
years vs. ≥60 years | 0.885 | 0.554–1.414 | 0.610 |
| Gender |
|
|
|
| Male
vs. female | 1.120 | 0.663–1.893 | 0.671 |
| Tumor size |
|
|
|
| <5
cm vs. ≥5 cm | 0.426 | 0.263–0.689 | 0.000 |
| T
classification |
|
|
|
| T1-2
vs. T3-4 | 0.235 | 0.126–0.440 | 0.000 |
| TNM |
|
|
|
| I/II
vs. III/IV | 0.179 | 0.088–0.362 | 0.000 |
| Lymph node
status |
|
|
|
| Yes vs.
no | 0.194 | 0.084–0.448 | 0.000 |
| Distant
metastasis |
|
|
|
| Yes vs.
no | 0.189 | 0.111–0.324 | 0.000 |
| Peritoneal
seeding |
|
|
|
| Yes vs.
no | 0.352 | 0.195–0.635 | 0.001 |
| Snail
expression |
|
|
|
| High
vs. low | 0.403 | 0.251–0.646 | 0.000 |
| CXCL1
expression |
|
|
|
| High
vs. low | 0.371 | 0.215–0.641 | 0.000 |
 | Table III.Multivariate analysis of CXCL1
expression associated with the overall survival rate. |
Table III.
Multivariate analysis of CXCL1
expression associated with the overall survival rate.
|
| Overall
survival |
|
|---|
|
|
|
|
|---|
| Variables | HR | 95% CI | P-value |
|---|
| Age |
|
|
|
| <60
years vs. ≥60 years | 0.863 | 0.519–1.433 | 0.569 |
| Gender |
|
|
|
| Male
vs. female | 1.208 | 0.692–2.110 | 0.506 |
| T
classification |
|
|
|
| T1-2
vs. T3-4 | 0.518 | 0.261–1.027 | 0.060 |
| Lymph node
status |
|
|
|
| Yes vs.
no | 0.267 | 0.102–0.701 | 0.007 |
| Distant
metastasis |
|
|
|
| Yes vs.
no | 0.255 | 0.137–0.476 | 0.000 |
| Peritoneal
seeding |
|
|
|
| Yes vs.
no | 0.718 | 0.366–1.406 | 0.334 |
| Differention |
|
|
|
|
Well/moderately vs.
poorly | 1.045 | 0.579–1.887 | 0.884 |
| CXCL1
expression |
|
|
|
| High
vs. low | 0.473 | 0.266–0.842 | 0.011 |
Association between the expression
levels of CXCL1 and Snail and the clinical characteristics of
patients
The association between the expression of CXCL1 and
Snail and the clinical characteristics of the 127 patients with GC
is illustrated in Table VI. The
expression levels of CXCL1 and Snail were each significantly
associated with tumor size (P=0.013 and P=0.026, respectively),
depth of invasion (P=0.003 and P=0.001, respectively), lymph node
metastasis (P=0.022 and P=0.014, respectively) and TNM staging
(P=0.001 and P=0.005, respectively). The overexpression of Snail
was also associated with peritoneal seeding (P=0.009).
 | Table VI.Association between CXCL1 and Snail
expression levels and clinical characteristics of patients with
gastric cancer. |
Table VI.
Association between CXCL1 and Snail
expression levels and clinical characteristics of patients with
gastric cancer.
|
| CXCL1
expression |
| Snail
expression |
|
|---|
|
|
|
|
|
|
|---|
|
Characteristics | Present | Absent | P-value | Present | Absent | P-value |
|---|
| Age |
|
| <60
years | 43 | 34 | 0.067 | 34 | 43 | 0.986 |
| ≥60
years | 36 | 14 | | 22 | 28 |
|
| Gender |
|
|
Male | 56 | 36 | 0.614 | 44 | 48 | 0.170 |
|
Female | 23 | 12 |
| 12 | 23 |
|
| Location |
|
| Upper
third | 18 | 24 | 0.513 | 17 | 25 | 0.309 |
| Middle
third | 11 | 17 |
| 16 | 12 |
|
| Lower
third | 17 | 29 |
| 17 | 29 |
|
|
Whole | 2 | 9 |
| 6 | 5 |
|
| Maximal tumor
diameter |
|
|
| <5
cm | 31 | 33 | 0.013 | 22 | 42 | 0.026 |
| ≥5
cm | 17 | 46 |
| 34 | 29 |
|
| pT |
|
|
T1-2 | 18 | 23 | 0.003 | 9 | 32 | 0.001 |
|
T3-4 | 61 | 25 |
| 47 | 39 |
|
| pN |
|
|
N0+N1 | 47 | 38 | 0.022 | 31 | 54 | 0.014 |
|
N2+N3 | 32 | 10 |
| 25 | 17 |
|
| TNM staging |
|
|
I/II | 16 | 23 | 0.001 | 29 | 10 | 0.005 |
|
III/IV | 63 | 25 |
| 42 | 46 |
|
| Distant
metastasis |
|
|
Negative | 39 | 61 | 0.590 | 41 | 59 | 0.091 |
|
Positive | 9 | 18 |
| 16 | 11 |
|
| Peritoneal
seeding |
|
|
Negative | 66 | 43 | 0.344 | 43 | 66 | 0.009 |
|
Positive | 13 | 5 |
| 13 | 5 |
|
| Histology type |
|
|
Well | 2 | 2 | 0.597 | 3 | 1 | 0.443 |
|
Moderately | 21 | 16 |
| 23 | 14 |
|
|
Poorly | 56 | 30 |
| 45 | 41 |
|
Correlation between CXCL1 and Snail
overexpression
The association between CXCL1 and Snail expression
was evaluated using Spearman's rank correlation and Fisher's exact
test. The results revealed that there was a significantly positive
correlation between the expression levels of CXCL1 and Snail in GC
tissue (r=0.431, P<0.001; Fig.
5).
Discussion
In the present study, CXCL1 and Snail were found to
frequently be overexpressed in GC tissues, and the overexpression
was associated with a worse prognosis. Patients with combined CXCL1
and Snail overexpression exhibited a significantly worse prognosis
compared to patients with either CXCL1 or Snail overexpression
alone or decreased expression of these proteins. Correlation
analysis revealed that CXCL1 expression was significantly
associated with Snail expression in GC tissue. These results
demonstrated that CXCL1 and Snail expression may act as a
prognostic biomarker in GC, and CXCL1 may promote tumor metastasis
by regulating EMT through Snail.
CXCL1-mediated melanocyte transformation is involved
in the induction of Ras expression, and RAS-induced CXCL1
facilitates the transformation of human ovarian epithelial cell
(4,18). The present data revealed that CXCL1
was highly expressed in GC (62.2%) and may be critical to
tumorigenesis and tumor metastasis. CXCL1 was highly expressed in
breast cancer metastases and CXCL1 regulated the expression of
matrix metalloproteinase (MMP)-13 and promoted the invasion of the
tumor into the bladder (19,20). CXCL1 also mediated the metastasis of
squamous cell carcinoma (21). In GC,
CXCL1/CXCR2 upregulates MMP2, MMP9, N-Ras, K-Ras, signal transducer
and activator of transcription 3 and promote the migratory and
invasive abilities of tumor cells (8). The results of the present analysis
revealed that high expression of CXCL1 is associated with tumor
invasion and tumor metastasis and may act as an independent
prognosis biomarker in GC. Subsequently, Snail expression was
detected by IHC and the association between Snail and the prognosis
of patients with GC and clinicopathological characteristics was
analyzed. The results indicated that Snail was associated with a
worse prognosis and clinicopathological characteristics.
Snail-mediated downregulation of E-cadherin expression has been
reported to perform a critical role in tumor invasion and
metastasis, including in hepatocellular carcinoma and breast cancer
(22,23). In GC, NF-κB activation increases Snail
expression. Snail then downregulates E-cadherin expression and
promotes EMT. Finally, the activation of EMT leads to tumor
metastasis (12–14,24).
Analysis of the prognosis of patients with combined CXCL1 and Snail
expression, the CXCL1+/Snail+ group exhibited
a worse prognosis compared with the other groups, particularly the
CXCL1−/Snail− group. Furthermore, the
difference between the CXCL1+/Snail+ and
CXCL1−/Snail− groups was more significant
compared with that of the CXCL1+ or Snail+
groups.
Finally, Spearman's rank correlation and Fisher's
exact test were performed to analyze the association between CXCL1
expression and Snail expression. Notably, the expression of Snail
was significantly associated with the expression of CXCL1. CXCL1
combines with CXCR2 and may regulate cellular apoptosis through
NF-κB in ovarian cancer (25).
Augmentation of the proinflammatory chemokine CXCL1 may also
activate NF-κB and promote tumor cell migration and invasion, such
as in ovarian and prostate cancer (6,7). As
aforementioned, NF-κB activation may upregulate Snail and Snail
overexpression results in EMT and leads to tumor metastasis.
Therefore, CXCL1 may upregulate Snail in GC. In order to further
clarify the association between CXCL1 and Snail, the GC AGS cell
line was stimulated by CXCL1 (48 h) and western blot was performed
to detect Snail expression. As a result, Snail was upregulated
(data not shown). Thus, CXCL1 may bind to CXCR2, activate NF-κB and
upregulate Snail. The upregulation of Snail downregulates
E-cadherin and promotes EMT, leading to tumor invasion and
metastasis. However, additional studies are required to explore
this hypothesis.
The present results demonstrated that CXCL1 and
Snail are highly expressed in GC and are associated with tumor
progression and a worse prognosis. The combined expression of CXCL1
and Snail may be more effective to predict the prognosis of
patients with GC, and the expression of Snail and CXCL1 are
positively correlated with each other. Therefore, additional
studies are required to confirm whether CXCL1 may upregulate Snail
expression and lead to tumor progression and clarify the underlying
mechanisms.
Acknowledgements
This study was supported by the National Nature
Science Research Fund of China (grant nos. 30700805 and
81272643).
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Okholm C, Svendsen LB and Achiam MP:
Status and prognosis of lymph node metastasis in patients with
cardia cancer-A systematic review. Surg Oncol. 23:140–146. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Deng JY and Liang H: Clinical significance
of lymph node metastasis in gastric cancer. World J Gastroenterol.
20:3967–3975. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang D, Yang W, Du J, Devalaraja MN, Liang
P, Matsumoto K, Tsubakimoto K, Endo T and Richmond A:
MGSA/GRO-mediated melanocyte transformation involves induction of
Ras expression. Oncogene. 19:4647–4659. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dhawan P and Richmond A: Role of CXCL1 in
tumorigenesis of melanoma. J Leukoc Biol. 72:9–18. 2002.PubMed/NCBI
|
|
6
|
Dong YL, Kabir SM, Lee ES and Son DS:
CXCR2-driven ovarian cancer progression involves upregulation of
proinflammatory chemokines by potentiating NF-κB activation via
EGFR-transactivated Akt signaling. PLoS One. 8:e837892013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kuo PL, Shen KH, Hung SH and Hsu YL:
CXCL1/GROα increases cell migration and invasion of prostate cancer
by decreasing fibulin-1 expression through NF-κB/HDAC1 epigenetic
regulation. Carcinogenesis. 33:2477–2487. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cheng WL, Wang CS, Huang YH, et al:
Overexpression of CXCL1 and its receptor CXCR2 promote tumor
invasion in gastric cancer. Ann Oncol. 22:2267–2276. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Thompson EW, Newgreen DF and Tarin D:
Carcinoma invasion and metastasis: A role for
epithelial-mesenchymal transition? Cancer Res. 65:5991–5995;
discussion 5995. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shin NR, Jeong EH, Choi CI, et al:
Overexpression of Snail is associated with lymph node metastasis
and poor prognosis in patients with gastric cancer. BMC Cancer.
12:5212012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
He H, Chen W, Wang X, et al: Snail is an
independent prognostic predictor for progression and patient
survival of gastric cancer. Cancer Sci. 103:1296–1303. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cho HJ, Park SM, Kim IK, et al: RhoGDI2
promotes epithelial-mesenchymal transition via induction of Snail
in gastric cancer cells. Oncotarget. 5:1554–1564. 2014.PubMed/NCBI
|
|
13
|
Chen Z, Liu M, Liu X, et al: COX-2
regulates E-cadherin expression through the NF-κB/Snail signaling
pathway in gastric cancer. Int J Mol Med. 32:93–100.
2013.PubMed/NCBI
|
|
14
|
Zheng H, Li W, Wang Y, et al: Glycogen
synthase kinase-3 beta regulates Snail and β-catenin expression
during Fas-induced epithelial-mesenchymal transition in
gastrointestinal cancer. Eur J Cancer. 49:2734–2746. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Washington K: 7th edition of the AJCC
cancer staging manual: Stomach. Ann Surg Oncol. 17:3077–3079. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ding T, Xu J, Wang F, et al: High
tumor-infiltrating macrophage density predicts poor prognosis in
patients with primary hepatocellular carcinoma after resection. Hum
Pathol. 40:381–389. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Goulding H, Pinder S, Cannon P, et al: A
new immunohistochemical antibody for the assessment of estrogen
receptor status on routine formalin-fixed tissue samples. Hum
Pathol. 26:291–294. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yang G, Rosen DG, Zhang Z, et al: The
chemokine growth-regulated oncogene 1 (Gro-1) links RAS signaling
to the senescence of stromal fibroblasts and ovarian tumorigenesis.
Proc Natl Acad Sci USA. 103:16472–16477. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kawanishi H, Matsui Y, Ito M, et al:
Secreted CXCL1 is a potential mediator and marker of the tumor
invasion of bladder cancer. Clin Cancer Res. 14:2579–2587. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lerebours F, Vacher S, Andrieu C, et al:
NF-kappa B genes have a major role in inflammatory breast cancer.
BMC Cancer. 8:412008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Loukinova E, Dong G, EnamoradoAyalya I, et
al: Growth regulated oncogene-alpha expression by murine squamous
cell carcinoma promotes tumor growth, metastasis, leukocyte
infiltration and angiogenesis by a host CXC receptor-2 dependent
mechanism. Oncogene. 19:3477–3486. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhou L, Wang DS, Li QJ, Sun W, Zhang Y and
Dou KF: The down-regulation of Notch1 inhibits the invasion and
migration of hepatocellular carcinoma cells by inactivating the
cyclooxygenase-2/Snail/E-cadherin pathway in vitro. Dig Dis Sci.
58:1016–1025. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dong CF, Wu Y, Yao J, et al: G9a interacts
with Snail and is critical for Snail-mediated E-cadherin repression
in human breast cancer. J Clin Invest. 122:1469–1486. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yoo YA, Kang MH, Lee HJ, et al: Sonic
hedgehog pathway promotes metastasis and lymphangiogenesis via
activation of Akt, EMT and MMP-9 pathway in gastric cancer. Cancer
Res. 71:7061–7070. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yang G, Rosen DG, Liu GZ, et al: CXCR2
promotes ovarian cancer growth through dysregulated cell cycle,
diminished apoptosis and enhanced angiogenesis. Clin Cancer Res.
16:3875–3886. 2010. View Article : Google Scholar : PubMed/NCBI
|