Introduction
Prostate cancer is one of the most common types of
tumor and the second highest cause of cancer-related mortality in
males (1). The majority of patients
succumb to tumor recurrence and metastasis. Early diagnosis,
targeted therapy and effective monitoring following radical
prostatectomy may have a significant impact on the prognosis of
patients. The location of the tumor determines the subsequent
treatment. In recent years not only have computed tomography (CT)
and magnetic resonance imaging been used in prostate cancer
diagnosis, single-photon emission computed tomography (SPECT) and
positron emission tomography (PET) also offer new ways of targeting
diagnosis (2,3).
Prostate-specific membrane antigen (PSMA) is a type
2 transmembrane glycoprotein expressed in prostate epithelial
cells. It is shown to be highly expressed in prostate cancer in a
disease progression-dependent manner (4). This study introduces a means of
synthesis of 2-{3-[1-Carboxy-5-(4-[18F]
fluoro-benzoylamino)-pentyl]-ureido}-pentanedioic acid
(18F-Glu-Urea-Lys, [18F]3). This low
molecular weight agent is easily prepared and demonstrates a high
uptake in PSMA+ tumors.
Materials and methods
General procedures
All reagents and solvents were purchased from
Sigma-Aldrich (Milwaukee, WI, USA). 1H NMR spectra were obtained on
an Avance 400 MHz spectrometer (Bruker Corporation, Ettlingen,
Germany). Electrospray ionization (ESI) mass spectra were obtained
on a Bruker Esquire 3000 plus system. High-performance liquid
chromatography (HPLC) purification was performed on a Waters 2998
and Waters 2487 system (Waters Corp., Milford, MA, USA).
[18F]-fluoride was obtained using the M-7 Cyclotron
(Sumitomo Heavy Industries, Ltd., Tokyo, Japan). Solid-phase
extraction cartridges (Sep-Pak C18 Plus) were purchased from Waters
Corp. The precursor 2-[3-(5-amino-1-carboxy-pentyl)-
ureido]-pentanedioic acid 1 was synthesized in Dalian Medical
University, China (5).
This study was approved by the ethics committee of
the First Affiliated Hospital of Dalian Medical University (Dalian,
China).
Cell lines
LNCaP, PC-3, 231 and A549 cells were obtained from
WUXI Molecular Imaging CRO (Wuxi, China). Nude mice were purchased
from JiangNan University, China. Cells (5×106) were
implanted subcutaneously into the right flank of models. Mice were
imaged when the tumor xenografts reached 5–8 mm in diameter.
2-{3-[1-tert-Butoxycarbonyl-5-(4-fluoro-benzoylamino)-pent
yl]-ureido}-pentanedioic acid di-tert-butyl ester 2
N-Hydroxysuccinimidyl-4-[18F]
fluorobenzoate (SFB, 145 mg), hydroxybenzotriazole (HOBt, 165 mg)
and 1-Ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDCI, 235 mg)
were added to a solution of CH2Cl2 (50 ml), then mixed with
Triethylamine (208 mg) and stirred for 1 h. Precursor 1 (500 mg)
was added and stirred at room temperature overnight. The crude
material was purified on a silica column to obtain 550 mg compound
2.
2-{3-[1-Carboxy-5-(4-fluoro-benzoylamino)-pentyl]-ureido}-pe
ntanedioic acid 3
Compound 2 (110 mg) was added to HCl/diethyl ether
solution (20 ml), and stirred overnight at room temperature. The
crude material was purified using HPLC to obtain 24 mg compound
3.
2-[3-[1-Carboxy-5-(4-[18F]fluoro-benzoylamino)-pentyl]-ure
ido]-pentanedioic acid [18F]3
Compound 1 (1 mg) was added to phosphate-buffered
saline (PBS) solution (100 µl). Then [18F]SFB (100 µl)
and Na2CO3 (40 µl) was added, and the mixture
was regulated to pH 7.6, stirred and reacted in an oil bath at 50°C
for 30 min. When the reaction cooled down, trifluoroacetic acid
(100 µl) and benzaldehyde (3 µl) were added, and reacted in an oil
bath at 50°C for 30 min. Water (5 ml) was added, then the mixture
was purified on a silica column, and washed with acetonitrile (0.5
ml). Finally, it was purified using HPLC to obtain
[18F]3.
PET imaging
Small animal PET was used to image the nude mice
implanted with PSMA+ (LNCaP) and PSMA- (PC-3, 231 and A549)
xenografts. The nude mice were anesthetized with diethyl ether and
injected intravenously with 0.2 mCi 18F-Glu-Urea-Lys in
200 µl PBS. The images were obtained at post-injection times of 1,
2 and 4 h.
Results
Synthesis of the compounds 2, 3 and
[18F]3
The final quantity of
2-{3-[1-tert-Butoxycarbonyl-5-(4-flu
oro-benzoylamino)-pentyl]-ureido}-pentanedioic acid di-tert-butyl
ester 2 obtained was 550 mg, with a produce yield of 88%. The
associated parameters are listed as the followings: 1H
NMR (400 MHz, CDCl3) δ7.91–7.96 (m, 2H), 7.26–7.45 (m, 1H),
7.05–7.11 (m, 2H), 5.70–5.72 (m, 1H), 5.40–5.43 (m, 1H), 4.20–4.23
(m, 2H), 3.34–3.51 (m, 2H), 2.24–2.29 (m, 2H), 2.16 (m, 1H),
1.99–2.04 (m, 2H), 1.64–1.77 (m, 32H). The [M+H]+ ESI
mass calculated for
C31H48FN3O8 was
609.7.
The final quantity of
2-{3-[1-Carboxy-5-(4-fluoro-benzoylamino)-pentyl]ureido}-pentanedioic
acid 3 obtained was 24 mg, with a produce yield of ~30%. The
associated parameters are listed as the followings: 1H
NMR (400 MHz, CDCl3) δ8.51 (s, 1H), 7.89–7.92 (m, 2H), 7.27–7.31
(m, 2H), 6.34 (m, 2H), 4.06–4.08 (m, 2H), 3.23–3.55 (m, 3H),
2.25–2.51 (m, 2H), 1.50–1.60 (m, 7H), 1.06–1.35 (m, 3H). The
[M+H]+ ESI mass calculated for
C19H24FN3O8 was
441.4.
The radiochemical yield of [18F]3
achieved was 28.7%. The radiochemical purity was 99.1% and the mean
synthesis time was 168 min (Fig.
1).
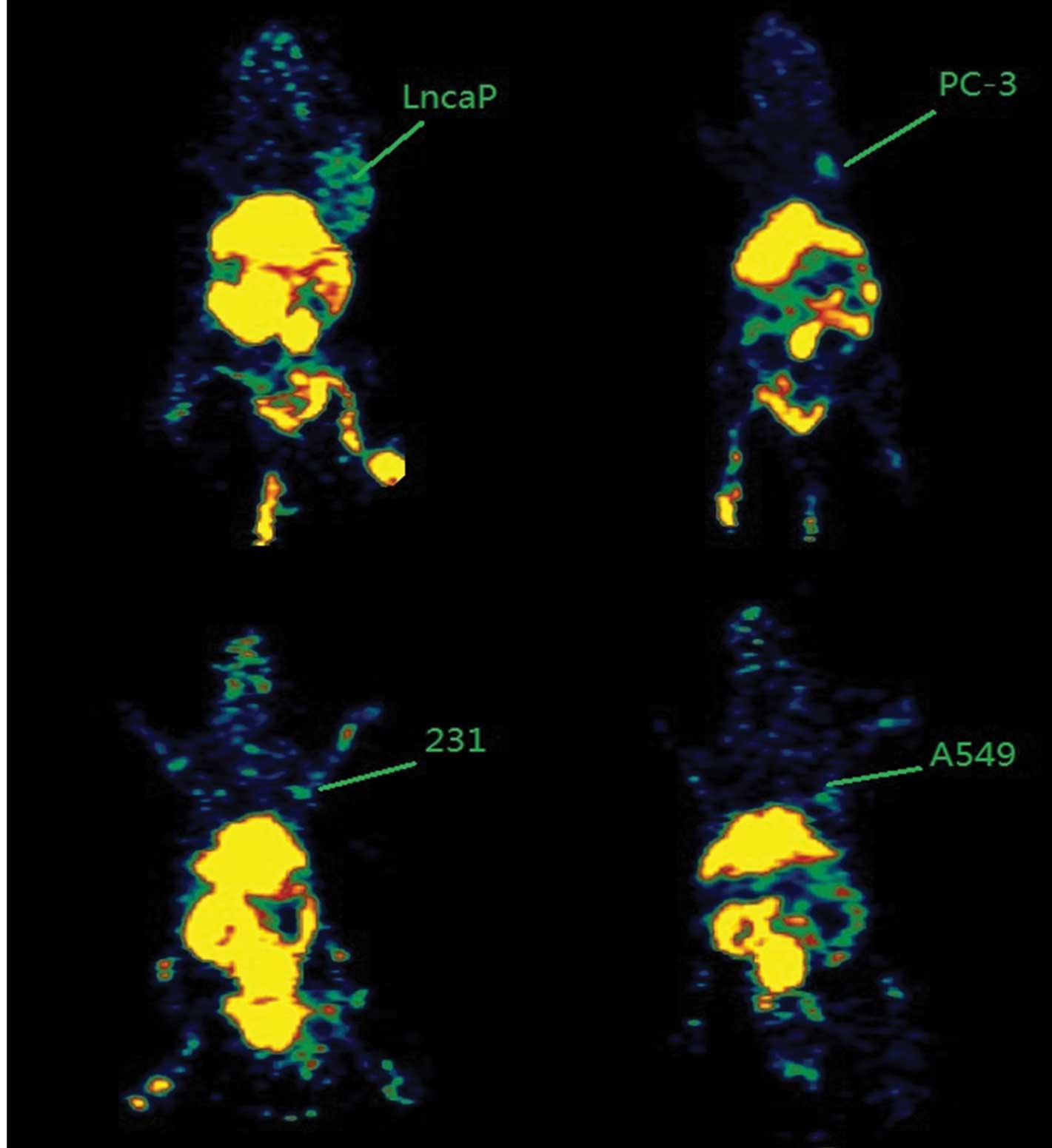
PET imaging
Following the injection, 18F-Glu-Urea-Lys
rapidly and notably delineated PSMA+ LNCaP prostate
tumor xenografts on the PET imaging. At 4 h post-injection, the
contrast was only observed in renal, liver, bladder (the intense
renal uptake was partially due to the specific binding of
18F-Glu-Urea-Lys to proximal renal tubules (6) as well as to the excretion of this
hydrophilic compound) and PSMA+ LNCaP tumors.
PSMA− tumors (PC-3, 231 and A549) were clear according
to the radiotracer (Fig. 2).
Discussion
Due to the relatively low metabolic rate of prostate
cancer, PET with [18F] fluorodeoxy glucose (FDG PET) has
proven ineffective. Other agents for imaging prostate cancer
include the choline series (7),
radiolabeled acetates (8),
[18F] F-FACBC (9),
[18F] FMAU (10) and
[18F] FDHT (11). However,
each has disadvantages, including cost, difficulty to synthesize or
low specificity to prostate cancer.
Overexpressed in prostate cancer, PSMA is becoming
an attractive target for cancer imaging and therapy (12). PSMA has an internalization signal that
allows internalization of the protein on the cell surface into an
endosomal compartment (13). Previous
studies reveal that a type of monoclonal antibody against PSMA is
available for imaging diagnosis and therapy of prostate cancer
(14,15). These agents have long circulation
times, low specificity to target tissue and were expensive to
synthesize, limiting their clinical use in the diagnosis of
prostate cancer.
Maresca et al (16) designed and synthesized a type of
Glu-Urea-R compound which could be marked by 123I and 131I. This
R-group and the substrate coupling with it may notably affect the
affinity of the compounds to PSMA. To improve the diagnosis and
therapy of prostate cancer, in recent years researchers have
developed a series of PSMA-based small molecular agents. This type
of agent was based on various R-groups, including [11C]
DCMC (17), [125I] DCIT
(18) and [18F] DCFBC
(19), each having its own
benefits.
The use of these compounds is not limited to the
area of diagnosis of prostate cancer. Kularatne et al
(20) coupled the chelate
99mTc-Dap-Asp-Cys with Glu-Urea-R for use in SPECT as an imaging
agent. In combination with the chemotherapy drug TubH, this
compound was capable of killing PSMA+ LNCaP cells in
vitro. Zhang et al (21)
coupled dinitrophenyl (DNP) with Glu-Urea-R to target prostate
cancer. The DNP-end increased the immune antibodies and killed the
cancer cells.
These small molecular agents demonstrate high
specificity and affinity with PSMA (22). The use of 18F-Glu-Urea-Lys
provides a new strategy in diagnosis, preoperative or tumor
recurrence staging, and also could be extended from molecular
imaging to the gene target therapy area.
In conclusion, 18F-Glu-Urea-Lys
demonstrated high PSMA+ tumor uptake and low-to-normal
tissue uptake. This radiotracer could be quickly cleared from
non-target tissues and retention may occur in PSMA+
prostate tumor. With its relatively simple and convenient method of
synthesis, this type of PSMA-based small molecular imaging agent
may have a variety of clinical uses to help localize prostate
cancer.
Acknowledgements
The authors are grateful for the financial support
from grants NSFC30670544 and NSFC81271603 from the National Natural
Science Foundation of China. They also thank WUXI Molecular Imaging
CRO for performing the imaging studies and providing excellent
technical support.
References
|
1
|
Chen W, Mao K, Liu Z and Dinh-Xuan AT: The
role of the RhoA/Rho kinase pathway in angiogenesis and its
potential value in prostate cancer (Review). Oncol Lett.
8:1907–1911. 2014.(Review). PubMed/NCBI
|
|
2
|
Geus-Oei LF and Oyen WJ: Predictive and
prognostic value of FDG-PET. Cancer Imaging. 8:70–80. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Liu Y: Diagnostic role of
fluorodeoxyglucose positron emission tomography-computed tomography
in prostate cancer. Oncol Lett. 7:2013–2018. 2014.PubMed/NCBI
|
|
4
|
Risk MC, Knudsen BS, Coleman I, Dumpit RF,
Kristal AR, LeMeur N, Gentleman RC, True LD, Nelson PS and Lin DW:
Differential gene expression in benign prostate epithelium of men
with and without prostate cancer: evidence for a prostate cancer
field effect. Clin Cancer Res. 16:5414–5423. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chen XC, Yang DY and Che XY: Synthesis of
PSMA-targeted small molecule Glu-urea-Lys analogue. J Dalian Med
Univer. 34:13–17. 2012.
|
|
6
|
Silver DA, Pellicer I, Fair WR, Heston WD
and Cordon-Cardo C: Prostate-specific membrane antigen expression
in normal and malignant human tissues. Clin Cancer Res. 3:81–85.
1997.PubMed/NCBI
|
|
7
|
Rinnab L, Mottaghy FM, Blumstein NM, Reske
SN, Hautmann RE, Hohl K, Möller P, Wiegel T, Kuefer R and Gschwend
JE: Evaluation of [11C]-choline positron-emission/computed
tomography in patients with increasing prostate-specific antigen
levels after primary treatment for prostate cancer. BJU Int.
100:786–793. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ponde DE, Dence CS, Oyama N, Kim J, Tai
YC, Laforest R, Siegel BA and Welch MJ: 18F-fluoroacetate: A
potential acetate analog for prostate tumor imaging - in vivo
evaluation of 18F-fluoroacetate versus 11C-acetate. J Nucl Med.
48:420–428. 2007.PubMed/NCBI
|
|
9
|
Oka S, Hattori R, Kurosaki F, Toyama M,
Williams LA, Yu W, Votaw JR, Yoshida Y, Goodman MM and Ito O: A
preliminary study of
anti-1-amino-3-18F-fluorocyclobutyl-1-carboxylic acid for the
detection of prostate cancer. J Nucl Med. 48:46–55. 2007.PubMed/NCBI
|
|
10
|
Tehrani OS, Muzik O, Heilbrun LK, Douglas
KA, Lawhorn-Crews JM, Sun H, Mangner TJ and Shields AF: Tumor
imaging using
1-(2′-deoxy-2′-18F-fluoro-beta-D-arabinofuranosyl)thymine and PET.
J Nucl Med. 48:1436–1441. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Larson SM, Morris M, Gunther I, Beattie B,
Humm JL, Akhurst TA, Finn RD, Erdi Y, Pentlow K, Dyke J, et al:
Tumor localization of 16beta-18F-fluoro-5alpha-dihydrotestosterone
versus 18F-FDG in patients with progressive, metastatic prostate
cancer. J Nucl Med. 45:366–373. 2004.PubMed/NCBI
|
|
12
|
Wang W and Mo ZN: Advances in
prostate-specific membrane antigen targeted therapies for prostate
cancer. Zhonghua Nan Ke Xue. 16:547–551. 2010.(In Chinese).
PubMed/NCBI
|
|
13
|
Rajasekaran SA, Anilkumar G, Oshima E,
Bowie JU, Liu H, Heston W, Bander NH and Rajasekaran AK: A novel
cytoplasmic tail MXXXL motif mediates the internalization of
prostate-specific membrane antigen. Mol Biol Cell. 14:4835–4845.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kim H, Shoji S, Tomonaga T, Shima M,
Terachi T and Uchida T: Prostate cancer with cyst formation
detected by whole body positron emission tomography/computed
tomography: A case report. Oncol Lett. 8:2037–2039. 2014.PubMed/NCBI
|
|
15
|
Tagawa ST, Beltran H, Vallabhajosula S,
Goldsmith SJ, Osborne J, Matulich D, Petrillo K, Parmar S, Nanus DM
and Bander NH: Anti-prostate-specific membrane antigen-based
radioimmunotherapy for prostate cancer. Cancer. 116:(Suppl).
1075–1083. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Maresca KP, Hillier SM, Femia FJ, Keith D,
Barone C, Joyal JL, Zimmerman CN, Kozikowski AP, Barrett JA,
Eckelman WC, et al: A series of halogenated heterodimeric
inhibitors of prostate specific membrane antigen (PSMA) as
radiolabeled probes for targeting prostate cancer. J Med Chem.
52:347–357. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pomper MG, Musachio JL, Zhang J, Scheffel
U, Zhou Y, Hilton J, Maini A, Dannals RF, Wong DF and Kozikowski
AP: 11C-MCG: Synthesis, uptake selectivity, and primate PET of a
probe for glutamate carboxypeptidase II (NAALADase). Mol Imaging.
1:96–101. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Foss CA, Mease RC, Fan H, Wang Y, Ravert
HT, Dannals RF, Olszewski RT, Heston WD, Kozikowski AP and Pomper
MG: Radiolabeled small-molecule ligands for prostate-specific
membrane antigen: In vivo imaging in experimental models of
prostate cancer. Clin Cancer Res. 11:4022–4028. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mease RC, Dusich CL, Foss CA, Ravert HT,
Dannals RF, Seidel J, Prideaux A, Fox JJ, Sgouros G, Kozikowski AP,
et al:
N-[N-[(S)-1,3-Dicarboxypropyl]carbamoyl]-4-[18F]fluorobenzyl-L-cysteine,
[18F]DCFBC: a new imaging probe for prostate cancer. Clin Cancer
Res. 14:3036–3043. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kularatne SA, Wang K, Santhapuram HK and
Low PS: Prostate-specific membrane antigen targeted imaging and
therapy of prostate cancer using a PSMA inhibitor as a homing
ligand. Mol Pharm. 6:780–789. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang AX, Murelli RP, Barinka C, Michel J,
Cocleaza A, Jorgensen WL, Lubkowski J and Spiegel DA: A remote
arene-binding site on prostate specific membrane antigen revealed
by antibody-recruiting small molecules. J Am Chem Soc.
132:12711–12716. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Qi Y, Zhang Q, Huang Y and Wang D:
Manifestations and pathological features of solitary thin-walled
cavity lung cancer observed by CT and PET/CT imaging. Oncol Lett.
8:285–290. 2014.PubMed/NCBI
|