Introduction
The incidence of gastric cancer is gradually
declining throughout the world, but it remains the second most
common type of fatal malignancy worldwide (1). Gastric cancer is one of the most
aggressive tumors due to its important pathological features of
easy invasion and metastasis. The key steps of invasion and
metastasis include dissociation from the primary lesions,
degradation and permeation about the extracellular matrix (ECM),
migration in the blood or lymph stream, and adhesion and growth
within secondary organs (2). A number
of pathways and genes have been indicated to be involved in the
metastasis of gastric cancer. Previous studies have demonstrated
that chemokines and their receptors function as essential
regulators of metastatic cancers, including gastric cancer, and are
involved in a number of neoplastic processes (3,4).
Interleukin-8 (IL-8), a member of the
neutrophil-specific CXC subfamily of chemokines with a Glu-Leu-Arg
motif, is important in leukocyte chemotaxis, inflammatory responses
and infectious diseases (5), as well
as in the migration, invasion and proliferation of endothelial
cells via their receptors, and in angiogenesis in vivo
(6,7).
Previous studies have suggested that cancer cells, including those
of pancreatic, colon, ovarian and lung cancer, produce IL-8
(8–15). IL-8, an autocrine growth factor, is
associated with tumor growth, angiogenesis, invasion and metastasis
(8–15). In our previous study, IL-8 ranging
from 0 to 100 ng/ml was shown to interfere with human gastric
cancer SGC-7901 cells in vitro; it was found that high-dose
IL-8 promoted cell adhesion to endothelial cell and ECM components,
while also inducing the migration and invasion capacities of the
SGC-7901 cells (16). However, the
IL-8 level produced by gastric cells is marginal, at even less than
1 ng/ml (17). It remains unclear
whether low-dose IL-8 treatment also induces these capacities in
cells.
The purpose of the present study was to provide
direct information with regard to the role of low-dose IL-8 in
determining the metastasis of gastric cancer. In the current study,
IL-8 ranging from 0 to 1 ng/ml was used to interfere with the
SGC-7901 cells in vitro to investigate the effect of
low-dose IL-8 on the adhesion, migration and invasion capacities of
the cells, and the correlated molecular mechanism of cluster of
differentiation 44 (CD44). The present study was approved by the
ethics committee of the Second Military Medical University
(Shanghai, China).
Materials and methods
Cell culture
The human gastric cancer SGC-7901 cell line was
purchased from the Cell Bank of the Type Culture Collection of the
Chinese Academy of Sciences (Shanghai, China). The SGC-7901 cells
were cultured in RPMI-1640 medium (Wisent, St. Bruno, Quebec,
Canada) supplemented with 10% fetal bovine serum (Zhejiang Tianhang
Biological Technology Co., Ltd., Hangzhou, China), 1%
penicillin/streptomycin and 1% L-glutamine, and maintained at 37°C
in a humidified chamber containing 5% CO2.
Adhesion assay
Fibronectin is an ECM component. To analyze the
attachment of the SGC-7901 cells to fibronectin, 96-well plates
were coated with 100 µg fibronectin (Sigma-Aldrich, St. Louis, MO,
USA) overnight at 4°C. Subsequent to being washed three times with
phosphate-buffered saline (PBS) solution containing 1% bovine serum
albumin (Sigma-Aldrich) to block non-specific cell adhesion, IL-8
(0, 0.2, 0.5, 0.8 and 1.0 ng/ml; Sigma-Aldrich) was added to
1×105 cells/well for 2 h. Thereafter, the non-adherent
cells were washed off with PBS. Cell adhesion was assessed by a
cell counting kit 8 (Dojindo, Kunamoto, Japan) assay, using
cellular DNA labeled with fluorescence reagent. The cells were
cultured for an additional 4 h. Colorimetric absorbance was
measured by a microplate reader (Multiskan MK3; Thermo Fisher
Scientific, Waltham, MA, USA) at 450 nm to obtain an optical
density (OD) value. OD ultimate value = OD measured value - OD
blank value.
Wound scratch assay
Cell migration was monitored in a wound scratch
assay. Briefly, the SGC-7901 cells were seeded on a 6-well plate at
a density of 2×105 cells/well. A scratch was made with a
sterile 10-µl pipette tip in a confluent cell monolayer. Subsequent
to washing twice, IL-8 (dose identical to adhesion assay) was added
in serum-free medium. Images of the wells were captured at the
beginning of the experiment and after 12 and 24 h on an inverted
microscope (CK40-F200; Olympus, Tokyo, Japan). Digital images were
obtained with an Optronics MicroFire digital camera (Optronics,
Goleta, CA, USA) driven by the Picture Frame imaging software
(Optronics). All experiments were repeated three times.
Transwell chamber invasion assay
Transwell chambers (Corning, Tewksbury, MA, USA)
were used to examine the invasion ability of the SGC-7901 cells,
according to the manufacturer's instructions. Briefly, the SGC-7901
cells (8×104) were seeded in the upper chamber
consisting of a thin layer of Matrigel basement membrane matrix (BD
Bioscience, San Jose, CA, USA). Next, 600 µl culture medium and
IL-8 (dose identical to adhesion assay) were added to the lower
chamber. Subsequent to 24 h of incubation, the cells on the upper
surface of the membrane were removed with a cotton swab, and the
cells that had migrated through and attached to the lower surface
of the membrane were fixed with 4% paraformaldehyde for 15 min.
Thereafter, the cells were stained using the crystal violet cell
colony staining kit (Shanghai Genmed Biological Technology Co.,
Ltd., Shanghai, China) according to the manufacturer's
instructions. The results are expressed as the mean number of cells
invading in four random microscopic fields (magnification,
×10).
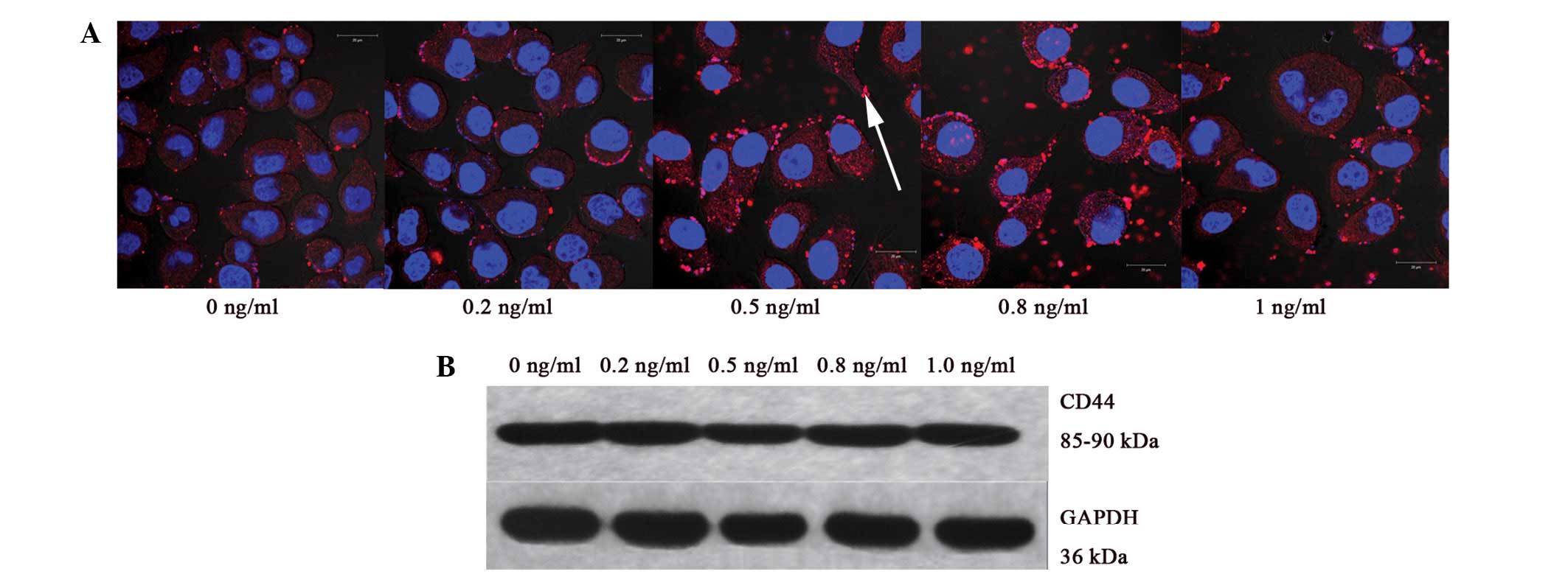
Immunofluorescence staining
A total of 2×105 SGC-7901 cells were
seeded on 6-well plates and cultured with IL-8 (dose identical to
adhesion assay) for 72 h. Subsequently, 7×104 cells were
placed on coverslips and fixed in 4% paraformaldehyde,
permeabilized with 0.5% Triton X-100 (Shanghai Sangon Biotech Co.,
Ltd., Shanghai, China) for 10 min, incubated in blocking buffer,
and then incubated with CD44 rabbit anti-human monoclonal antibody
(1:80; Epitomics, Burlingame, CA, USA) at 4°C overnight.
Cy3-conjugated Affinipure goat polyclonal anti-rabbit
immunoglobulin (Ig)G (H+L; 1:1,000 dilution; Proteintech Group,
Wuhan, Hubei, China) was added for an additional 1-h incubation.
The cell nuclei were labeled with DAPI (Thermo Fisher Scientific).
The coverslips were then mounted on a glass slide and visualized
under a laser confocal scanning microscope (LSM710; Zeiss,
Oberkochen, Germany).
Western blot analyses
A total of 2×105 SGC-7901 cells were
seeded in each well of 6-well plates and incubated for 72 h with
IL-8 (dose identical to adhesion assay). Following stimulation, the
cells were collected and denatured by 150 µl loading buffer.
Proteins in the total cell lysate were separated by SDS-PAGE (10%
separation gel and 5% spacer gel) and electrotransferred to
polyvinylidene difluoride films (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). Blotted films were placed in blocking solution
for 1 h at room temperature. CD44 rabbit anti-human monoclonal
antibody (1:250, Epitomics) was used to probe the blots overnight
at 4°C. The film was washed thoroughly, incubated with goat
polyclonal anti-rabbit IgG horse radish peroxidase secondary
antibody (1:1,000; Santa Cruz Biotechnology, Santa Cruz, CA, USA)
for 1 h, and then visualized using the enhanced chemiluminescence
method (Perkin Elmer Inc., Waltham, MA, USA). Blots were exposed to
plain X-ray film in a darkroom. Grayscale reconstruction was
performed using Image J software 1.48 (http://rsb.info.nih.gov./ij/), and the expression rate
of CD44 versus that of GAPDH protein, which served as an internal
control protein, was calculated. All experiments were repeated
three times.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
The SGC-7901 cells (1×105) were collected
after 72 h of incubation with IL-8 (dose identical to adhesion
assay). Briefly, the total RNA of the cells was extracted using
TRIzol reagent (Takara, Shiga, Japan) according to the
manufacturer's instructions, and reverse transcribed. RT-qPCR was
performed with SYBR Green in a Bio-Rad iQ5 Real-Time PCR system
(Bio-Rad Laboratories Inc.). Cycling conditions consisted of one
cycle of 95°C for 2 min, 95°C for 15 sec, 60°C for 20 sec and 72°C
for 20 sec, and then 71 cycles of 60–95°C for 30 sec (increasing by
0.5°C every other cycles). The primer sequences used for
amplification are shown in Table I.
Based on the 2−ΔΔCt value, the relative levels of CD44
mRNA expression were calculated. The data were normalized using
GADPH as an internal control.
 | Table I.Primer sequences used for quantitative
polymerase chain reaction. |
Table I.
Primer sequences used for quantitative
polymerase chain reaction.
| mRNA | Sense primer
sequence | Size, bp |
|---|
| hGAPDH-F |
5′-GGGTGTGAACCATGAGAAGTATG-3′ | 145 |
| hGAPDH-R |
5′-GATGGCATGGACTGTGGTCAT-3′ |
| CD44-F |
5′-ATGGACAAGTTTTGGTGGCA-3′ | 230 |
| CD44-R |
5′-CAGGTCTCAAATCCGATGCTC-3′ |
Statistical methods
Statistical analysis was performed using SPSS 13.0
software (SPSS, Inc., Chicago, IL, USA). All results are presented
as the mean ± standard deviation. A one-way analysis of variance
was used to assess invasion, adhesion, and protein and mRNA
expression levels. The least significant difference method was used
to analyze multiple post hoc comparisons. P≤0.05 was considered to
indicate a statistically significant difference.
Results
IL-8 promotes SGC-7901 cell
adhesion
The present study investigated the effect of
low-dose IL-8 on the adhesion capacity of SGC-7901 cells to
fibronectin. The data showed that IL-8 at concentrations of ≥0.5
ng/ml significantly promoted cell adhesion (P=0.028, P=0.002 and
P=0.010 for 0.5, 0.8 and 1.0 ng/ml, respectively). The increase in
adhesion peaked at a concentration of 0.8 ng/ml. Notably, this
effect was not in a significant dose-dependent manner. No
significant differences were identified in adhesion between the
groups treated with 0.5, 0.8 and 1 ng/ml IL-8 (P>0.05 between
all three groups; Table II; Fig. 1A).
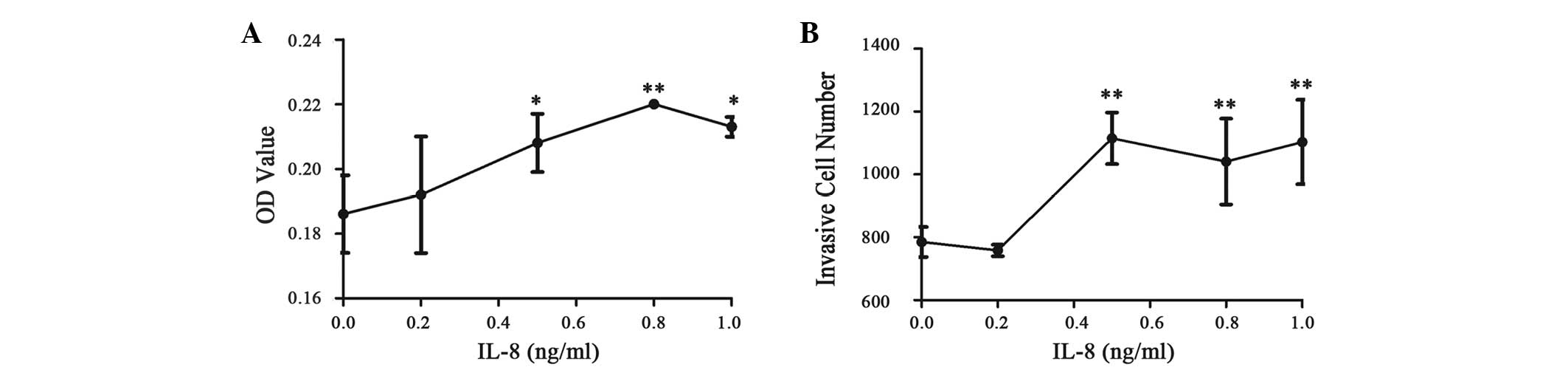 | Figure 1.Effect of interleukin (IL)-8 on
gastric cancer SGC-7901 cell adhesion and invasion. (A) Cell
adhesion was significantly different at various IL-8 concentrations
(P=0.012). Meanwhile, 0.5, 0.8 and 1.0 ng/ml IL-8 all significantly
promoted SGC-7901 cell adhesion compared with 0 ng/ml IL-8
(P=0.028, P=0.002 and P=0.010, respectively). The most significant
adhesion was found following addition of 0.8 ng/ml IL-8. However,
this effect did not occur in a dose-dependent manner (all P>0.05
between the three groups). (B) Similar to the adhesion assay, cell
invasion was significantly different at various IL-8 concentrations
(P<0.001). Moreover, 0.5, 0.8 and 1.0 ng/ml IL-8 all
significantly promoted SGC-7901 cell invasion compared with 0 ng/ml
IL-8 (P<0.001, P=0.002 and P<0.001, respectively), but not in
a dose-dependent manner (all P>0.05 between the three groups).
*P<0.05 and **P<0.01 vs. 0 ng/ml IL-8 group. OD, optical
density. |
 | Table II.Effect of IL-8 on gastric cancer
SGC-7901 cell adhesion and invasion. |
Table II.
Effect of IL-8 on gastric cancer
SGC-7901 cell adhesion and invasion.
| Concentration,
ng/ml | Adhesion (OD
value) | Invasion (invasive
cell number) |
|---|
| 0 | 0.186±0.012 | 785.00±48.13 |
| 0.2 | 0.192±0.018 | 758.00±18.04 |
| 0.5 |
0.208±0.009a |
1115.00±81.85b |
| 0.8 |
0.220±0.001b,c |
1041.00±136.59b |
| 1.0 |
0.213±0.003a,d |
1103.33±134.52b |
IL-8 promotes SGC-7901 cell
invasion
The effect of low-dose IL-8 on SGC-7901 cell
invasion was also investigated. Similar to the adhesion assay, the
invasion of the SGC-7901 cell line was promoted by IL-8 at
concentrations ≥0.5 ng/ml (P<0.001, P=0.002 and P<0.001 for
0.5, 0.8 and 1.0 ng/ml, respectively) and this effect was not in a
significant dose-dependent manner. No significant differences were
identified between the groups treated with 0.5, 0.8 and 1 ng/ml
IL-8 (P>0.05 between all three groups) (Table II; Fig.
1B).
IL-8 promotes SGC-7901 cell
migration
The scratch assay showed IL-8-induced activation of
SGC-7901 cell migration. After 24 h, the scratched area recovered
more rapidly in IL-8-treated cells compared with untreated cells
(P<0.001; Fig. 2). IL-8
significantly promoted the migration of the SGC-7901 cells, however
this effect was not in a significant dose-dependent manner. No
significant differences were identified between the groups treated
with 0.5, 0.8 and 1 ng/ml IL-8 (P>0.05 between all three groups;
Fig. 2).
IL-8 promotes CD44 protein and mRNA
levels in SGC-7901 cells
To investigate the possible mechanism of the
IL-8-induced adhesion and invasion of gastric cancer cell, the
protein and mRNA expression levels of CD44 in SGC-7901 cells
exposed to the various low doses of IL-8 were detected. IL-8 at
concentrations of ≥0.5 ng/ml (0.5, 0.8 and 1.0 ng/ml) significantly
upregulated CD44 protein (P<0.001, P<0.001 and P=0.018) and
mRNA expression (P=0.021, P=0.008 and P=0.012). Corresponding to
the adhesion assay, CD44 protein and mRNA expression levels peaked
following exposure to 0.8 ng/ml IL-8. A dose-dependent effect was
found in CD44 protein expression between the 0.5, 0.8 and 1.0 ng/ml
groups, but not in mRNA expression (P>0.05 between all three
groups; Table III; Figs. 3 and 4).
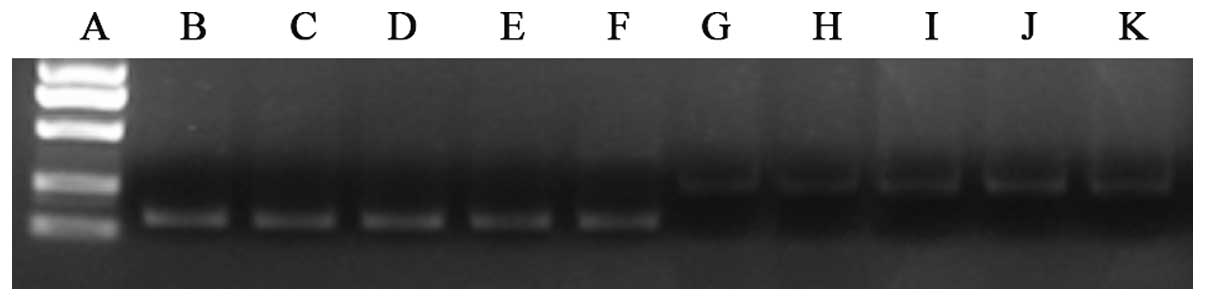 | Figure 4.Effect of interleukin (IL)-8 on
cluster of differentiation (CD)44 mRNA expression levels in
SGC-7901 cells, as determined by reverse transcription-quantitative
polymerase chain reaction. (A) Marker DL2000 (from top to bottom:
1000, 750, 500, 250 and 100 bp). (B-F) hGAPDH at (B) 0, (C) 0.2,
(D) 0.5, (E) 0.8 and (F) 1.0 ng/ml (145 bp). (G-K) CD44 at (G) 0,
(H) 0.2, (I) 0.5, (J) 0.8 and (K) 1.0 ng/ml (230 bp). Similar to
protein expression, CD44 mRNA expression was significantly
different at various IL-8 concentrations (P=0.026). Moreover, 0.5,
0.8 and 1.0 ng/ml IL-8 all significantly upregulated CD44 mRNA
expression compared with 0 ng/ml IL-8 (P=0.021, P=0.008 and
P=0.012, respectively), but not in a dose-dependent manner (all
P>0.05 between the three groups). The mRNA expression level
peaked when the cells were exposed to 0.8 ng/ml IL-8. |
 | Table III.Effect of IL-8 on expression of CD44
protein and mRNA. |
Table III.
Effect of IL-8 on expression of CD44
protein and mRNA.
| Concentration,
ng/ml | CD44 protein | CD44 mRNA |
|---|
| 0 |
0.899±0.006 |
1.00±0.06 |
| 0.2 |
0.894±0.001 |
1.10±0.11 |
| 0.5 |
0.941±0.009ab |
1.33±0.12c |
| 0.8 |
0.964±0.005abd |
1.40±0.22a,e |
| 1.0 |
0.911±0.001b–d,f |
1.37±0.18c,e |
Discussion
Metastasis is one of the most fatal characteristics
of malignancies, accounting for >90% of tumor-related
mortalities worldwide (18). Although
the incidence of gastric cancer has declined, gastric cancer
remains one of the most common malignancies worldwide and
frequently develops lymph node, peritoneal and liver metastases
(19,20). Distant metastasis is an important sign
of a poor prognosis in gastric cancer patients. The metastasis of
tumor cells is a complex, multi-staged process that involves tumor
cell transformation, growth, angiogenesis, invasion, dissemination
and survival in the circulation, followed by adhesion and
colonization of a secondary organ or tissue (18,21).
Therefore, it has been widely accepted that the capacity of cancer
cell adhesion, migration and invasion is one of the most important
prerequisites for cancer metastasis (22). IL-8, a multifunctional
pro-inflammatory cytokine, has been shown to be associated with
infection, inflammation and other disease states, including
tumorigenesis (5,6). As an important regulatory autocrine
factor within the tumor microenvironment (23), IL-8 is considered to be produced by a
range of human cancer cell types, including human melanoma
(24), squamous cell carcinoma
(25), cervical cancer (26), ovarian cancer (27), non-small cell lung cancer (28) and colon cancer (12), and it mediates potential mitogenic,
motogenic and angiogenic effects (29,30). It
has been widely accepted that IL-8 plays a significant role in the
development and metastasis of cancer (12).
Several studies have found an association between
IL-8 and gastric cancer. In one in vitro study, gastric
cancer cells produced IL-8 in response to exposure to the cytotoxic
strain of Helicobacter pylori, which was classified by WHO
as a group I carcinogen (31). IL-8
is involved in the progressive growth of gastric cancer through
autocrine or paracrine mechanisms (32). In vivo, IL-8 has been shown to
be the most markedly upregulated gene in gastric cancer and may
regulate the neovascularization of human gastric cancer (33). A significant correlation has bee found
between IL-8 levels and the depth of invasion, venous invasion and
lymphatic invasion, and IL-8 may be an independent prognostic
factor in human gastric carcinoma (34). In a previous study, using treatment of
IL-8 at a concentration of >1 ng/ml, we reported that IL-8 could
enhance the adhesion, migration and invasion of the human gastric
cancer SGC-7901 cell line in a dose-dependent manner (16). This result was supported by another
similar study by Kuai et al using cDNA and small interfering
RNA transfectants (35). However,
IL-8 production by gastric cells is marginal, at even less than 1
ng/ml. For example, the highest level of IL-8 has been recorded at
only 0.17 ng/ml in IM95 gastric cancer cells cultured for 3 days
in vitro (17). It remains
unclear whether low-dose IL-8 also induces the adhesion, migration
and invasion of gastric cancer cells in a dose-dependent manner. In
the current study, IL-8 ranging from 0.5 to 1 ng/ml promoted the
adhesion, migration and invasion of SGC-7901 gastric cancer cells.
Nevertheless, a dose-dependent effect was not found. These results
suggested that IL-8 may have potential pro-metastatic effects, even
at low doses.
CD44, as a polymorphic integral membrane
glycoprotein expressed by a number of cell types, plays a
significant role in lymph node homing (36), matrix adhesion (37), and T lymphocyte activation (38). Serving as the main transmembrane
hyaluronate receptor, CD44 is regarded not only as a cell adhesion
molecule, but also as a determinant of metastatic and invasive
behavior in a variety of malignancies, including malignant
melanoma, lung carcinoma, breast cancer, leukemia and
gastrointestinal carcinomas (39–42).
Metastasis is characterized by a loss of adhesion,
and is a significant event in human cancer development that allows
cancer cells to leave their original site and subsequently invade
and adhere to other sites, for example, the lymph nodes, liver or
peritoneum (43). In gastric
adenocarcinoma, CD44 is highly expressed and is correlated with a
poor prognosis in patients with the intestinal disease type
(44). In a previous study, we
reported that the regulation of matrix metalloproteinase-9,
intercellular adhesion medlecule-1 and E-Cadherin expression may be
one of the potential molecule mechanisms for IL-8-induced adhesion,
migration and invasion in gastric cancer (16). In the current study, the role of CD44
was further investigated in IL-8-induced adhesion, migration and
invasion as another potential molecule mechanism. Similar to the
results of the adhesion assay, it was found that CD44 protein and
mRNA expression were promoted by low-dose IL-8. An increase in the
CD44 protein and mRNA expression level was induced most
significantly by 0.8 ng/ml IL-8. This result suggests that the
regulation of CD44 expression may be another potential molecule
mechanism involved in IL-8-induced adhesion, migration and invasion
in gastric cancer.
In conclusion, low-dose IL-8 may promote the
adhesion, migration and invasion of the gastric cancer SGC-7901
cell line, but not in a dose-dependent manner, and that this is
correlated with the regulation of CD44 expression.
Acknowledgements
This study was supported by a grant from the
Three-Year Action Plan Fund of Traditional Chinese Medicine,
Shanghai City Health Administration (grant no.
ZYSNXD-CC-ZDYJ024).
References
|
1
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Goubran HA, Kotb RR, Stakiw J, Emara ME
and Burnouf T: Regulation of tumor growth and metastasis: The role
of tumor microenvironment. Cancer Growth Metastasis. 7:9–18. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Araki S, Omori Y, Lyn D, Singh RK,
Meinbach DM, Sandman Y, Lokeshwar VB and Lokeshwar BL:
Interleukin-8 is a molecular determinant of androgen independence
and progression in prostate cancer. Cancer Res. 67:6854–6862. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Waugh DJ and Wilson C: The interleukin-8
pathway in cancer. Clin Cancer Res. 14:6735–6741. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Harada A, Sekido N, Akahoshi T, Wada T,
Mukaida N and Matsushima K: Essential involvement of interleukin-8
(IL-8) in acute inflammation. J Leukoc Biol. 56:559–564.
1994.PubMed/NCBI
|
|
6
|
Raman D, Baugher PJ, Thu YM and Richmond
A: Role of chemokines in tumor growth. Cancer Lett. 256:137–165.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xie K: Interleukin-8 and human cancer
biology. Cytokine Growth Factor Rev. 12:375–391. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gabellini C, Trisciuoglio D, Desideri M,
Candiloro A, Ragazzoni Y, Orlandi A, Zupi G and Del Bufalo D:
Functional activity of CXCL8 receptors, CXCR1 and CXCR2, on human
malignant melanoma progression. Eur J Cancer. 45:2618–2627. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kamohara H, Takahashi M, Ishiko T, Ogawa M
and Baba H: Induction of interleukin-8 (CXCL-8) by tumor necrosis
factor-alpha and leukemia inhibitory factor in pancreatic carcinoma
cells: Impact of CXCL-8 as an autocrine growth factor. Int J Oncol.
31:627–632. 2007.PubMed/NCBI
|
|
10
|
Itoh Y, Joh T, Tanida S, Sasaki M, Kataoka
H, Itoh K, Oshima T, Ogasawara N, Togawa S, Wada T, et al: IL-8
promotes cell proliferation and migration through
metalloproteinase-cleavage proHB-EGF in human colon carcinoma
cells. Cytokine. 29:275–282. 2005.PubMed/NCBI
|
|
11
|
Merritt WM, Lin YG, Spannuth WA, Fletcher
MS, Kamat AA, Han LY, Landen CN, Jennings N, De Geest K, Langley
RR, et al: Effect of interleukin-8 gene silencing with
liposome-encapsulated small interfering RNA on ovarian cancer cell
growth. J Natl Cancer Inst. 100:359–372. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ning Y, Manegold PC, Hong YK, Zhang W,
Pohl A, Lurje G, Winder T, Yang D, Labonte MJ, Wilson PM, et al:
Interleukin-8 is associated with proliferation, migration,
angiogenesis and chemosensitivity in vitro and in vivo in colon
cancer cell line models. Int J Cancer. 128:2038–2049. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Matsuo Y, Ochi N, Sawai H, et al:
CXCL8/IL-8 and CXCL12/SDF-1alpha co-operatively promote
invasiveness and angiogenesis in pancreatic cancer. Int J Cancer.
124:853–861. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kitadai Y, Takahashi Y, Haruma K, Naka K,
Sumii K, Yokozaki H, Yasui W, Mukaida N, Ohmoto Y, Kajiyama G, et
al: Transfection of interleukin-8 increases angiogenesis and
tumorigenesis of human gastric carcinoma cells in nude mice. Br J
Cancer. 81:647–653. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen JJ, Yao PL, Yuan A, Hong TM, Shun CT,
Kuo ML, Lee YC and Yang PC: Up-regulation of tumor interleukin-8
expression by infiltrating macrophages: Its correlation with tumor
angiogenesis and patient survival in non-small cell lung cancer.
Clin Cancer Res. 9:729–737. 2003.PubMed/NCBI
|
|
16
|
Ju D, Sun D, Xiu L, Meng X, Zhang C and
Wei P: Interleukin-8 is associated with adhesion, migration and
invasion in human gastric cancer SCG-7901 cells. Med Oncol.
29:91–99. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Iwai M, Matsuda M and Iwai Y: Cloning of a
cancer cell-producing hepatocyte growth factor, vascular
endothelial growth factor and interleukin-8 from gastric cancer
cells. Vitro Cell Dev Biol Anim. 39:288–290. 2003. View Article : Google Scholar
|
|
18
|
Jin X, Zhu Z and Shi Y: Metastasis
mechanism and gene/protein expression in gastric cancer with
distant organs metastasis. Bull Cancer. 2014.((Epub ahead of
print)). PubMed/NCBI
|
|
19
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Deng JY and Liang H: Clinical significance
of lymph node metastasis in gastric cancer. World J Gastroenterol.
20:3967–3975. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Goubran HA, Kotb RR, Stakiw JI, Emara ME
and Burnouf T: Regulation of tumor growth and metastasis: The role
of tumor microenvironment. Cancer Growth Metastasis. 7:9–18. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Martin TA, Mason MD and Jiang WG: Tight
junctions in cancer metastasis. Front Biosci (Landmark Ed).
16:898–936. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Campbell LM, Maxwell PJ and Waugh DJ:
Rationale and means to target pro-inflammatory interleukin-8
(CXCL8) signaling in cancer. Pharmaceuticals (Basel). 6:929–959.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gabellini C, Trisciuoglio D, Desideri M,
et al: Functional activity of CXCL8 receptors, CXCR1 and CXCR2, on
human malignant melanoma progression. Eur J Cancer. 45:2618–2627.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Christofakis EP, Miyazaki H, Rubink DS and
Yeudall WA: Roles of CXCL8 in squamous cell carcinoma proliferation
and migration. Oral Oncol. 44:920–926. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wu S, Shang H, Cui L, Zhang Z, Zhang Y, Li
Y, Wu J, Li RK and Xie J: Targeted blockade of interleukin-8
abrogates its promotion of cervical cancer growth and metastasis.
Mol Cell Biochem. 375:69–79. 2013.PubMed/NCBI
|
|
27
|
Wang Y, Xu RC, Zhang XL, Niu XL, Qu Y, Li
LZ and Meng XY: Interleukin-8 secretion by ovarian cancer cells
increases anchorage-independent growth, proliferation, angiogenic
potential, adhesion and invasion. Cytokine. 59:145–155. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Luppi F, Longo AM, de Boer WI, Rabe KF and
Hiemstra PS: Interleukin-8 stimulates cell proliferation in
non-small cell lung cancer through epidermal growth factor receptor
transactivation. Lung Cancer. 56:25–33. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Baggiolini M and Clark-Lewis I:
Interleukin-8, a chemotactic and inflammatory cytokine. FEBS Lett.
307:97–101. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhu YM and Woll PJ: Mitogenic effects of
interleukin-8/CXCL8 on cancer cells. Future Oncol. 1:699–704. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Takagi A, Kamiya S, Koga Y, Ohta U,
Kobayashi H, Shirai T, Harasawa S and Miwa T: Analysis of
interleukin-8 secretion induced by Helicobacter pylori from the
gastric epithelial cell line MKN45: A mechanism independent of the
intensity of cytotoxicity. J Gastroenterol Hepatol. 12:368–372.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kitadai Y, Haruma K, Mukaida N, Ohmoto Y,
Matsutani N, Yasui W, Yamamoto S, Sumii K, Kajiyama G, Fidler IJ
and Tahara E: Regulation of disease-progression genes in human
gastric carcinoma cells by interleukin 8. Clin Cancer Res.
6:2735–2740. 2000.PubMed/NCBI
|
|
33
|
Kitadai Y, Haruma K, Sumii K, Yamamoto S,
Ue T, Yokozaki H, Yasui W, Ohmoto Y, Kajiyama G, Fidler IJ and
Tahara E: Expression of interleukin-8 correlates with vascularity
in human gastric carcinomas. Am J Pathol. 152:93–100.
1998.PubMed/NCBI
|
|
34
|
Kido S, Kitadai Y, Hattori N, Haruma K,
Kido T, Ohta M, Tanaka S, Yoshihara M, Sumii K, Ohmoto Y and
Chayama K: Interleukin 8 and vascular endothelial growth
factor-prognostic factors in human gastric carcinomas? Eur J
Cancer. 37:1482–1487. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kuai WX, Wang Q, Yang XZ, Zhao Y, Yu R and
Tang XJ: Interleukin-8 associates with adhesion, migration,
invasion and chemosensitivity of human gastric cancer cells. World
J Gastroenterol. 18:979–985. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Picker LJ, Nakache M and Butcher EC:
Monoclonal antibodies to human lymphocyte homing receptors define a
novel class of adhesion molecules on diverse cell types. J Cell
Biol. 109:927–937. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Aruffo AI, Stamenkovic I, Melnick M,
Underhill CB and Seed B: CD44 is the principal cell surface
receptor for hyaluronate. Cell. 61:1303–1313. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Haynes BF, Telen MJ, Hale LP and Denning
SM: CD44 - a molecule involved in leukocyte adherence and T-cell
activation. Immunol Today. 10:423–428. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hsieh HF, Yu JC, Ho LI, Chiu SC and Harn
HJ: Molecular studies into the role of CD44 variants in metastasis
in gastric cancer. Mol Pathol. 52:25–28. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Abbaszadegan MR, Moaven O, Sima HR,
Ghafarzadegan K, A'rabi A, Forghani MN, Raziee HR, Mashhadinejad A,
Jafarzadeh M, Esmaili-Shandiz E and Dadkhah E: p16 promoter
hypermethylation: A useful serum marker for early detection of
gastric cancer. World J Gastroenterol. 14:2055–2060. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zavrides HN, Zizi-Sermpetzoglou A,
Panousopoulos D, Athanasas G, Elemenoglou I and Peros G: Prognostic
evaluation of CD44 expression in correlation with bcl-2 and p53 in
colorectal cancer. Folia Histochem Cytobiol. 43:31–36.
2005.PubMed/NCBI
|
|
42
|
Wang DR, Chen GY, Liu XL, Miao Y, Xia JG,
Zhu LH and Tang D: CD44v6 in peripheral blood and bone marrow of
patients with gastric cancer as micro-metastasis. World J
Gastroenterol. 12:36–42. 2006.PubMed/NCBI
|
|
43
|
Jothy S: CD44 and its partners in
metastasis. Clin Exp Metastasis. 20:195–201. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ghaffarzadehgan K, Jafarzadeh M, Raziee
HR, Sima HR, Esmaili-Shandiz E, Hosseinnezhad H, Taghizadeh-Kermani
A, Moaven O and Bahrani M: Expression of cell adhesion molecule
CD44 in gastric adenocarcinoma and its prognostic importance. World
J Gastroenterol. 14:6376–6381. 2008. View Article : Google Scholar : PubMed/NCBI
|