Introduction
Laryngeal cancer accounts for the majority of head
and neck cancers diagnosed worldwide (1). The spread of laryngeal cancer follows
the anatomical structure of the larynx, which is divided into the
glottis (true vocal cords, and anterior and posterior commissures),
the supraglottis (epiglottis, arytenoid folds and false cords), and
the subglottis (1). The majority of
laryngeal cancers originate in the glottis and are classified as
squamous cell carcinomas (SCCs). Laryngeal cancer commonly spreads
via direct extension to adjacent structures through metastasis to
regional cervical lymph nodes. Distant metastases, however, are not
commonly observed in laryngeal malignancies (2,3).
Leptomeningeal metastasis (LM), a lethal
complication of certain cancers, is the metastatic tumor cell
invasion of the leptomeninges (arachnoid and pia mater) of the
meninx and arachnoid spaces (4). To
date, only a few cases of LM due to head and neck cancer have been
reported (5–8). Moreover, a literature search revealed no
reports of confirmed LM from glottic laryngeal cancer. The present
study reports a case of LM from glottic laryngeal cancer and
reviews the corresponding literature to gain insight into this rare
event.
Case report
A 53-year-old male was admitted to Norman Bethune
First Hospital on February 13th, 2014 with an ongoing
headache that had persisted for 1 month and the occurrence of
regular vomiting for 1 week. The patient had no history of chronic
disease or excessive alcohol use, but reported a 20-year smoking
history. The patient had experienced hoarseness 9 years previously
and had sought medical treatment. Laryngoscopy detected SCC of the
glottic larynx. A laryngeal cleft and right vocal cord resection
was performed. Tumor cells were polygonal, and exhibited scattered
distribution; additionally, keratinization was observed within the
cells. The post-operative pathological examination showed SCC
(moderately-differentiated) of tumor stage T1N0M0 (9). The patient was administered prophylactic
radiotherapy due to tumor invasion into the anterior commissure of
the larynx. Radiotherapy to the tumor bed and preventive
radiotherapy to the cervical lymph node (50 Gy, 25 times/5 weeks)
was administered. The patient experienced no evident side-effects
from this treatment, but exhibited hoarseness post-surgery.
During the present admission period, the physical
examination indicated symptoms of drowsiness, hypologia and simple
communication abilities. In addition, no pyramid sign and no
evident cranial nerve sign were observed. Routine hepatic and renal
function tests, and blood and coagulation tests were normal. The
patient was negative for hepatitis B, hepatitis C, syphilis and
human immunodeficiency virus. Tumor marker levels for cancer
antigen (CA)125, CA15, CA72-4, neuron-specific enolase, cytokeratin
19 fragment 21–1, CA19-9, α-fetoprotein and carcinoembryonic
antigen were all normal. Computed tomography (CT) scans of the head
and neck, and chest and abdomen indicated that no nodular lesions
were present. A magnetic resonance imaging (MRI) scan of the head
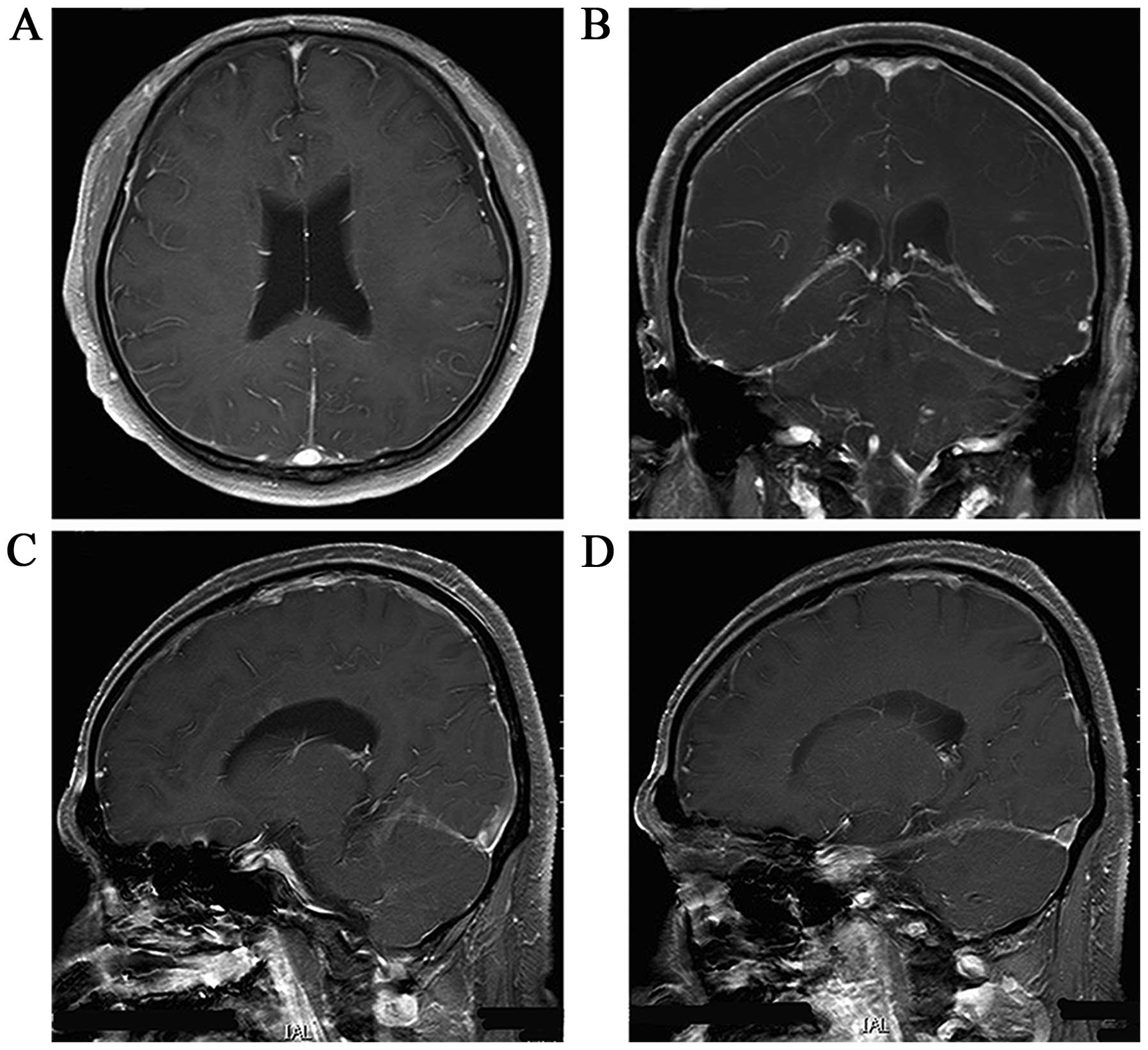
revealed multiple enhancing lesions in the brain and multiple
line-like enhancements in the brain fold (Fig. 1). The patient was therefore diagnosed
with LM from glottic laryngeal cancer.
At 3 days post-admission, the patient exhibited
aconuresis, lethargy and unconsciousness, indicating rapid disease
progression. The Glasgow coma score (GCS) was 11–12 points (normal
score, 15) and the Karnofsky performance status (KPS) score was 20
points (normal score, 100 points) (10,11). A
lumbar puncture was performed, which resulted in the following
(normal ranges presented in brackets following measured value):
Intracranial pressure, 310 mm H2O (80–180 mm
H2O); protein level, 1.57 g/l (0.15–0.45 g/l); glucose
level, 3.91 mmol/l (2.3–4.1 mmol/l); chlorine level, 111.7 mmol/l
(119–129 mmol/l); white blood cell count, 149×106/l
(0–8×106/l); and red blood cell count,
0×106/l (0×106/l). Antibodies against viral
infection of the cerebrospinal fluid (CSF), Microspironema
pallidum or Mycobacterium tuberculosis were not present,
and bacterial smears, Mycobacterium tuberculosis smears, and
Ink staining were all negative. A cytological examination of the
CSF was performed via liquid-based technology (ThinPrep TCT2000;
Hologic, Bedford, MA, USA), and the malignant cells were observed
via Papanicolaou staining (Fig. 2).
The presence of metastatic tumor cells in the CSF confirmed the
diagnosis of LM from glottic laryngeal cancer.
The patient was immediately administered nutritional
support (fat emulsion, 250 ml; amino acids, 250 ml; glucose, 1,000
ml; 10% kalium chloratum, 20 ml; multivitamins; pantoprazole, 40
mg; once per day for 3 weeks), intracranial pressure reduction
therapy (20% mannitol, 250 ml; glycerol fructose, 250 ml; twice per
day for 3 weeks), glucocorticoid therapy (dexamethasone, 10 mg per
day, then 5 mg after 5 days, continued until radiation therapy was
accomplished) and intrathecal chemotherapy (15 mg methotrexate and
5 mg dexamethasone). The patient regained consciousness 5 days
later, with relief from the vomiting and the ability to perform
simple verbal communication. The GCS was 12–14 points. The patient
then underwent radiotherapy of the whole brain and skull base
(linear accelerator 6 MV X-ray, 2 Gy/day) concurrent with
intrathecal chemotherapy once a week (same regimen as
aforementioned). The patient's headache was resolved 2 weeks later
and the vomiting had completely stopped. However, routine blood
tests detected a reduction in the white blood cell count
(2.89×109/l), and lower extremity numbness accompanied
the intrathecal chemotherapy. The myelosuppression and lower
extremity numbness were alleviated once the patient received
recombinant human granulocyte colony-stimulating factor (150 µg per
day, days 1–5) and calcium folinate (100 mg twice per day via
intravenous injection for 1 week). Following 40 Gy (2 Gy/day) of
whole-brain radiotherapy for 4 weeks and 6 intrathecal chemotherapy
treatments (15 mg methotrexate; 5 mg dexamethasone; once a week),
the headache was alleviated and the patient was able to remain
conscious. The GCS was 15 points and the KPS score was 60 points.
An MRI scan of the head showed that the original lesions in the
brain were almost completely gone (Fig.
3). CT scans of the neck, chest and abdomen exhibited no
nodular lesions. Further treatment was suspended. At 3 months
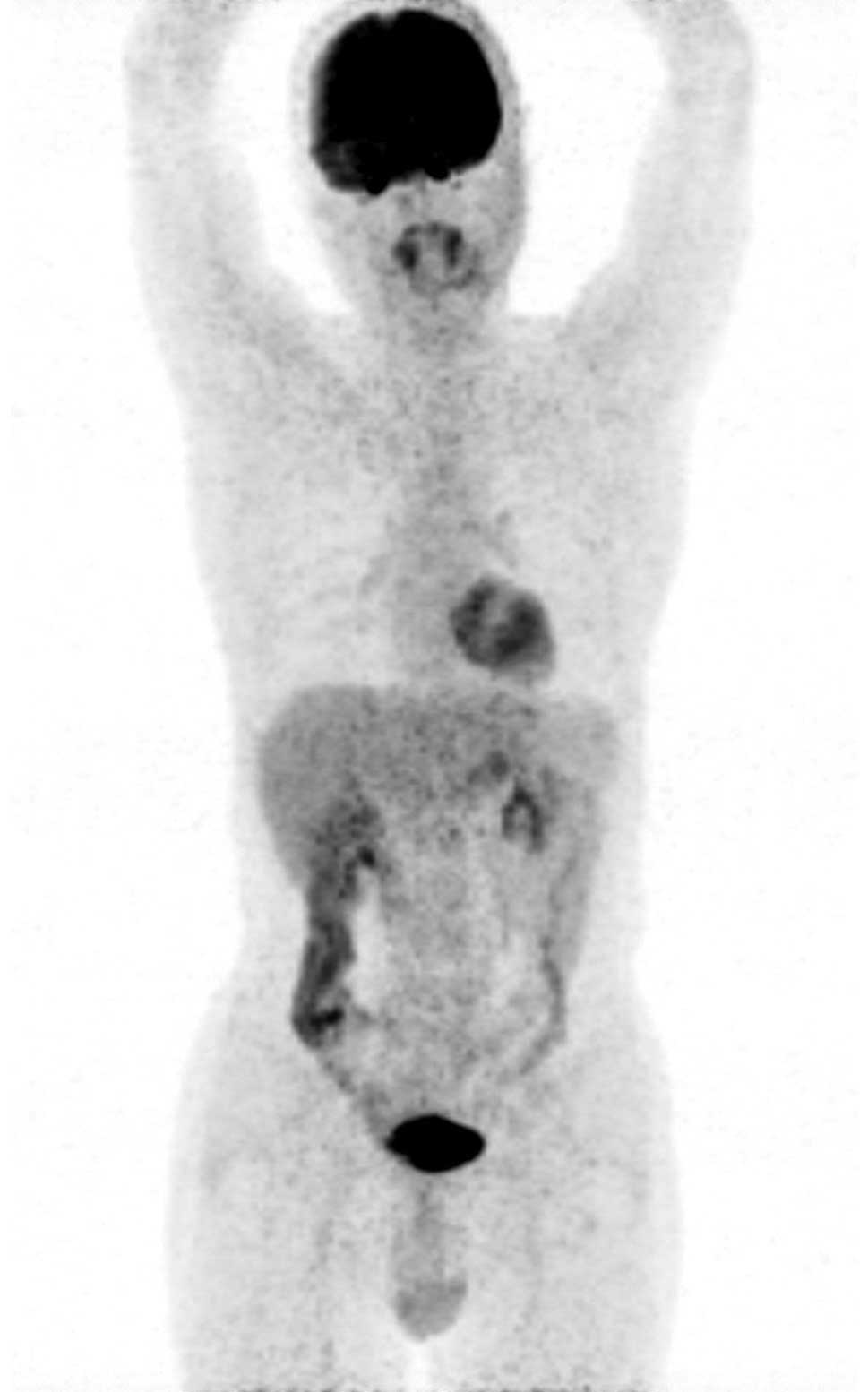
post-treatment, positron emission tomography/CT was performed and
no abnormalities were found (Fig. 4).
It has currently been 9 months since the diagnosis of LM and the
patient is disease-free with ongoing follow-up examinations.
Discussion
Laryngeal cancer is the most common cancer of the
head and neck (12). The incidence of
laryngeal tumors closely correlates with smoking and drinking
(13). A retrospective study
(1) reported that the most frequent
sites of distant metastases in patients with SCC of the head and
neck (HNSCC) were the lungs, bones and liver, while metastasis to
the brain was less common. In addition, 21.8% of patients were
diagnosed with HNSCC at an early stage (T1-2). Relative to other
malignancies, the distant metastases of HNSCC often develop slowly,
with only 16% of patients presenting with distant metastases at the
time of diagnosis. In the remaining 84% of patients, distant
metastases were observed after a median time of 381 days (1). Thus, the time from the diagnosis until
the occurrence of distant metastases of HNSCC is longer than for
other malignancies, and local early lesions also have a high risk
of distant metastasis.
LM is an extremely rare but devastating form of
metastatic spread. Only 6 HNSCC cases (1) with LM have been reported (carcinoma of
paranasal sinuses, carcinoma of ethmoid sinus, carcinoma of tongue,
carcinoma of lip, carcinoma of the palatine tonsil and carcinoma of
the supraglottic larynx), and only 1 case of SCC of the
supraglottic larynx with LM has been described (5–8). However,
the primary tumor site of the previously reported HNSCC cases
differed from that observed in the present study. To the best of
our knowledge, there have been no previous studies in the
literature describing LM secondary to early glottic larynx cancer.
LM can be a fatal complication of malignancies, and results from
metastatic infiltration of the leptomeninges (arachnoid and pia
mater) of the meninx and arachnoid spaces by malignant cells
originating from an extrameningeal primary tumor site (4). The most common methods for diagnosing LM
are neuroimaging and the cytological demonstration of malignant
cells in the CSF, with the latter being the gold standard for the
diagnosis of this condition (4). For
the present case, the MRI scan of the head showed line-like
enhancement in the brain fold of the cerebrum and cerebellum.
Cytological examination of the CSF was also performed and showed a
scattered or clustered distribution of malignant cells. The cells
were irregular in size and pleomorphic, with greatly increased
nuclear-to-cytoplasmic ratios, intensely-stained cytoplasm and
round, red-stained nucleoli in certain nuclei. All of these
features are classic malignant characteristics of metastatic cells
in the CSF. Due to the patient's critical condition, the CSF
examination was limited and other immunocytochemistry examinations
were not performed. However, according to the varied malignant
characteristics of the malignant cells that were detected in the
CSF, the patient could be definitively diagnosed with LM. The
patient had developed glottic laryngeal cancer 9 years previously,
however, CT scans of the neck, chest and abdomen showed no nodular
lesions except those of the nervous system. Furthermore, no lesions
were found on CT scans after 7 months of treatment.
Accordingly, the patient was diagnosed with LM from
glottic laryngeal cancer. LM-directed treatment is currently
palliative and its goal is to improve or stabilize the patient's
neurological status, improve their quality of life and prolonging
their survival time (4). Systemic and
intra-CSF chemotherapy and involved-field radiotherapy are common
and effective treatments for this disease, and methotrexate is the
most commonly utilized intra-CSF chemotherapy drug in the treatment
of LM from solid tumors (4,14). In a previous report of a case in which
the LM arose from a laryngeal SCC, the patient was treated with
intra-CSF methotrexate chemotherapy and palliative radiotherapy.
Although the patient's pain improved, the lower limb weakness
worsened and the patient succumbed 3 weeks after completing the
radiotherapy (6). In the present
case, the patient was administered intra-CSF chemotherapy first.
When the patient's central nervous system symptoms, such as the
unconsciousness state and vomiting, were relieved, the patient
immediately received concurrent whole-brain radiotherapy and
intra-CSF chemotherapy. The patient's symptoms were rapidly
alleviated, and the GCS and KPS scores increased. An MRI scan of
the head demonstrated that the original lesions in the brain were
almost completely gone, while CT scans of the chest and abdomen
showed no nodular lesions. Furthermore, no severe adverse reactions
of the central nervous system occurred during or after treatment,
indicating the effectiveness of the therapy.
In conclusion, this is the first report of a rare
case of LM from early glottic laryngeal cancer. Clinicians should
be aware of the possibility of LM in patients presenting with an
appropriate constellation of signs and symptoms. Moreover, it is
important to report that, in the present study, the treatment of LM
from early glottic laryngeal cancer with intra-CSF methotrexate
chemotherapy concurrent with whole-brain radiotherapy was
effective.
Acknowledgements
The authors would like to thank Medjaden Bioscience
Ltd., (Hong Kong, China) for assisting in the preparation of this
manuscript.
References
|
1
|
Spector ME, Chinn SB, Rosko AJ, Worden FP,
Ward PD, Divi V, McLean SA, Moyer JS, Prince ME, Wolf GT, et al:
Diagnostic modalities for distant metastasis in head and neck
squamous cell carcinoma: Are we changing life expectancy?
Laryngoscope. 122:1507–1511. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
de Araújo Neto VJ Furtado, Cernea CR,
Dedivitis R Aparecido, de Araújo Filho VJ Furtado, Palazzo J
Fabiano and Brandão L Garcia: Cervical metastasis on level IV in
laryngeal cancer. Acta Otorhinolaryngol Ital. 34:15–18.
2014.PubMed/NCBI
|
|
3
|
Gallegos JF, Fuentes A, Arroyo C, Minauro
G, Hernández M and Arias H: Laryngeal function as node metastasis
predictor in patients with cancer of the larynx. Gac Med Mex.
146:175–178. 2010.PubMed/NCBI
|
|
4
|
Le Rhun E, Taillibert S and Chamberlain
MC: Carcinomatous meningitis: Leptomeningeal metastases in solid
tumors. Surg Neurol Int. 4(Suppl 4): S265–S288. 2013.PubMed/NCBI
|
|
5
|
Redman BG, Tapazoglou E and Al-Sarraf M:
Meningeal carcinomatosis in head and neck cancer. Report of six
cases and review of the literature. Cancer. 58:2656–2661. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Thompson SR, Veness MJ, Morgan GJ, Shannon
J and Kench JG: Leptomeningeal carcinomatosis from squamous cell
carcinoma of the supraglottic larynx. Australas Radiol. 47:325–330.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Banerjee TK and Gottschalk PG: Unusual
manifestations of multiple cranial nerve palsies and mandibular
metastasis in a patient with squamous cell carcinoma of the lip.
Cancer. 53:346–348. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Biswal BM, Goyal M, Prasad RR, Lal P,
Sharma R, Mohanti BK and Khader J: Leptomeningeal carcinomatosis
from carcinoma of the palatine tonsil. Australas Radiol. 42:66–68.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pfister DG, Spencer S, Brizel DM, Burtness
B, Busse PM, Caudell JJ, Cmelak AJ, Colevas AD, Dunphy F, Eisele
DW, et al: Head and neck cancers, version 2.2014. Clinical practice
guidelines in oncology. J Natl Compr Canc Netw. 12:1454–1487.
2014.PubMed/NCBI
|
|
10
|
Teasdale G and Jennett B: Assessment of
coma and impaired consciousness. A practical scale. Lancet.
2:81–84. 1974. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Karnofsky DA and Burchenal JH: The
clinical evaluation of chemotherapeutic agents in cancer.
Evaluation of Chemotherapeutic Agents. MacLeod CM: Columbia
University Press. (Columbia). 1961949.
|
|
12
|
Wenig BM: Squamous cell carcinoma of the
upper aerodigestive tract: Precursors and problematic variants. Mod
Pathol. 15:229–254. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Igissinov N, Zatoskikh V, Moore MA,
Igissinov S, Toulebaeyev R, Mustafina M, Valieva S, Aldiyarova G,
Bukeyeva Z and Venglovskiy A: Epidemiological evaluation of
laryngeal cancer incidence in Kazakhstan for the years 1999–2009.
Asian Pac J Cancer Prev. 14:3969–3974. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pan Z, Yang G, Yuan T, Pang X, Wang Y, Qu
L and Dong L: Leptomeningeal metastasis from hepatocellular
carcinoma with other unusual metastases: A case report. BMC Cancer.
14:3992014. View Article : Google Scholar : PubMed/NCBI
|