Introduction
Urothelial carcinoma is the second most common
urological malignancy worldwide, after prostate cancer (1). A distinguishing feature of urothelial
carcinomas is their multiple foci, which cause the tumors to appear
synchronously or sequentially throughout the urinary tract,
including the upper urinary tracts (renal pelvis or ureter),
bladder and urethra (2). Upper tract
urothelial carcinomas (UTUC) are uncommon and account for only
5–10% of urothelial carcinomas (3).
At present, tobacco exposure is considered the most important risk
factor for urothelial carcinoma (4,5). Gross or
microscopic hematuria is the presenting symptom in 70–80% of UTUC
patients (6). Furthermore, at the
time of diagnosis 40–50% of patients exhibit in situ [pTa to
pT1 (7)] disease, 50–60% of patients
exhibit invasive or advanced disease [p≥T2 (7)], and ~25% patients already exhibit
regional metastasis (8,9). Radical nephroureterectomy (RNU) with
excision of the bladder cuff is the gold-standard treatment for
UTUC (3). However, alternative
treatments include ureteroscopic ablation, percutaneous resection
and segmental resection (10). The
oncological outcomes for patients with high-grade or
non-organ-confined disease remain poor, with 5-year cancer-specific
survival rates of <60%; while for patients with
non-muscle-invasive lesions, the 5-year recurrence-free survival
rate is 88.0–91.8% (11). For
patients with low-grade carcinomas, conservative strategies,
including segmental ureterectomy or endoscopic management, provide
cancer-specific survival (CCS) and overall survival (OS) rates
equivalent to that achieved using RNU (12,13), with
a 5-year cancer-specific survival rate of >93% (14), whereas patients at high-risk (pT3 or
N+) may benefit from neoadjuvant chemotherapy (15,16). The
ability to accurately predict pathological outcomes prior to
initiating therapy may aid in clinical risk stratification and the
selection of therapeutic strategies.
Ipsilateral hydronephrosis (HN) is common in UTUC
patients, and may be attributed to one of several factors,
including luminal obstruction, intramural invasion or extrinsic
compression (17). The presence of
ipsilateral HN in patients with bladder cancer is a predictive
factor for poor pathological outcome and poor prognosis (18–20);
however, at present, no consensus has been reached regarding the
predictive role of the presence of HN in UTUC patients. Although HN
has been reported to be associated with advanced disease (17,21–24), only
two studies demonstrated a correlation between HN and poor
prognosis based on small sample (17,25).
Our previous work has revealed associations between
HN and muscle-invasive and grade 3 diseases (21). In the present study, after revising
our database to include the follow-up information of patients
treated between 2000 and 2010, we sought to validate the predictive
value of preoperative HN on clinicopathological outcome and
prognosis with the aim of improving clinical risk stratification
and, thus, the ability to provide more optimal and personalized
risk-informed therapeutic options.
Materials and methods
Patient selection
The clinicopathological data of consecutive UTUC
patients treated between 2000 and 2010 at Peking University First
Hospital (Beijing, China) were collected. Among the 631 patients
with complete follow-up, 111 were excluded from the analysis: 25
with bilateral synchronous UTUCs, 54 who underwent alternative
surgeries rather than RNU, 28 with a follow-up period of <12
months, 2 with metastatic disease and 2 with positive surgical
margins. A total of 520 patients were finally enrolled for
evaluation. All patients were diagnosed using computed tomography
(CT) or magnetic resonance imaging (MRI), urological ultrasound and
ureteroscopy with or without biopsy. None of these patients
received neoadjuvant chemotherapy, however, for certain patients,
adjuvant chemotherapy or radiotherapy was administered when
evidence of distant metastasis or retroperitoneal recurrence was
documented. All patients underwent surgery within two months after
the occurrence of symptoms. Ethical approval was obtained from
Peking University Institutional Review Board
(IRB00001052-13057).
Ipsilateral HN status
Ipsilateral HN was assessed by upper urinary tract
imaging, including CT with or without intravenous contrast in 510
patients, and MRI with or without intravenous contrast in 10
patients. Only imaging studies performed within 6 weeks of RNU and
which were evaluated for HN by radiologists blinded to clinical
outcomes were considered. As ~100 CT films were not available for
re-evaluation, two authors (Dr Xuesong Li and Dr Gengyan Xiong)
blinded to the radiology reports reviewed the 10 MRI films and 100
CT films independently. The concordance between the two observers
in assessing presence or absence of HN was 95.5% and, by consensus
decision, the concordance between re-evaluation and primary reports
was 97.3%.
The evaluation criteria for assessing the presence
or absence of HN were similar to those of a previous study
(23). For renal pelvic lesions,
patients with hydrocalycosis were included in the cohort of
patients considered to have HN. A hydrocalyx was defined as any
degree of dilation within a focal calyx, with or without the
presence of obvious obstruction at the draining infundibulum. For
ureteral tumors, any degree of dilation in any component of the
ureter or associated renal unit was classified as HN. To avoid
small subgroups and heterogeneity with respect to the grading of
HN, the status of HN was evaluated strictly as present or absent in
the current analysis.
Patients evaluation
All pathological specimens were re-reviewed by a
dedicated genitourinary pathologist (Dr Qun He) to unify the
reproducibility of the diagnosis. Tumor stage was assessed
according to the 2002 Union for International Cancer Control TNM
classification of malignant tumors (10). Tumor grade was assessed according to
the World Health Organization classification of 1973 (10). Tumor architecture was defined as
papillary or sessile by examination of the final specimen. Tumor
location was divided into two areas (renal pelvis and ureter) based
on the site of the dominant lesion. Tumor multifocality was defined
as the synchronous presence of two or more pathologically confirmed
macroscopic tumors in any location. The estimated glomerular
filtration rate was calculated using the modified glomerular
filtration rate estimating equation for Chinese patients (26).
Follow-up schedule
Of the total cohort (n=631), 520 patients were
included in the current analyses. For patients who were followed-up
at our institute, the follow-up regimen of the affected patients
included cystoscopy every 3 months for the first 3 years;
cystoscopy intervals were extended to 1 year thereafter. Chest
X-ray, serum creatinine level and abdominal ultrasound or CT were
examined concurrently. The impact of preoperative HN on CSS, OS,
bladder recurrence-free survival and contralateral carcinoma-free
survival times was determined. Bladder recurrence was defined as
the detection of a subsequent bladder tumor during cystoscopy and
confirmation by pathology, while contralateral carcinoma was
defined as urothelial carcinoma in the contralateral upper urinary
tract. The causes of patient mortality were determined by the
treating physicians.
Statistical analysis
All statistical tests were performed using SPSS
software version 20.0 (IBM SPSS, Armonk, NY, USA), and the
threshold for statistical significance was set at P<0.05. The
Pearson test and χ2 test were used to assess the
distribution of categorical variables, and the Mann-Whitney U test
was used for continuous variables. Univariate analysis using the
log-rank test and multivariate analysis using Cox's proportional
hazards regression model were also conducted. Only variables that
were indicated to be significant upon univariate analysis were
considered for the multivariate analysis.
Results
Patient clinical and pathological
characteristics and HN
The clinical and pathological data of the included
patients and their association with HN are shown in Table I. Of the 520 patients enrolled,
ipsilateral HN was present in 271 patients (52.1%). There were 340
patients with muscle-invasive disease (T stage ≥2), and 191
patients were diagnosed with histological grade 3 disease by final
pathology. Preoperative HN was associated with advanced age
(P=0.007), sessile tumor architecture (P<0.001), ureteral
location (P<0.001), high tumor stage (P<0.001) and higher
histological grade (P=0.002). No distribution differences in terms
of gender, preoperative kidney function, multifocality, presence of
carcinoma in situ (CIS) or tumor size were identified.
 | Table I.Patient clinical and pathological
characteristics and ipsilateral hydronephrosis. |
Table I.
Patient clinical and pathological
characteristics and ipsilateral hydronephrosis.
|
| Ipsilateral
hydronephrosis, n (%) |
|
|
|---|
|
|
|
|
|
|---|
| Characteristic | Absent | Present | χa | P-value |
|---|
| Gender |
|
| 0.004 | 0.951 |
|
Male | 110 (21.15) | 119 (22.88) |
|
|
|
Female | 139 (26.73) | 152 (29.23) |
|
|
| Age, years |
|
| 7.320 | 0.007a |
|
<70 | 157 (30.19) | 139 (26.73) |
|
|
|
≥70 | 92
(17.69) | 132 (25.38) |
|
|
| Preoperative renal
function |
|
| 3.221 | 0.522 |
| CKD1
(eGFR≥90) | 26
(5.00) | 18
(3.46) |
|
|
| CKD2
(90>eGFR≥60) | 88
(16.92) | 96
(18.46) |
|
|
| CKD3
(60>eGFR≥30) | 106 (20.38) | 117 (22.50) |
|
|
| CKD4
(30>eGFR≥15) | 13
(2.50) | 19
(3.65) |
|
|
| CKD5
(eGFR<15) | 16
(3.08) | 21
(4.04) |
|
|
| Urinary
cytology |
|
| 0.675 | 0.714 |
|
Negative | 57
(10.96) | 58
(11.15) |
|
|
|
Positive | 122 (23.46) | 128 (24.62) |
|
|
| Missing
data | 70
(13.46) | 85
(16.35) |
|
|
| Tumor
architecture |
|
| 16.604 | 0.000a |
|
Papillary | 219 (42.12) | 200 (38.46) |
|
|
|
Sessile | 30
(5.77) | 71
(13.65) |
|
|
| Multifocality |
|
| 2.788 | 0.095 |
| No | 205 (39.42) | 207 (39.81) |
|
|
|
Yes | 44
(8.46) | 64
(12.31) |
|
|
| Location |
|
| 145.028 | 0.000a |
|
Ureter | 54
(10.38) | 202 (38.85) |
|
|
|
Pelvis | 195 (37.50) | 69
(13.27) |
|
|
| Tumor stage |
|
|
24.199 | 0.000a |
| Ta | 22
(4.23) | 8
(1.54) |
|
|
| T1 | 76
(14.62) | 74
(14.23) |
|
|
| T2 | 75
(14.42) | 127 (24.42) |
|
|
| T3 | 76
(14.62) | 59
(11.35) |
|
|
| T4 | 0
(0.00) | 3
(0.58) |
|
|
| Node stage |
|
| 2.066 | 0.356 |
| N0 | 20
(3.85) | 31
(5.96) |
|
|
| Nx | 225 (43.27) | 233 (42.88) |
|
|
| N+ | 4
(0.77) | 6
(1.15) |
|
|
| Tumor grade |
|
| 12.568 | 0.002a |
| G1 | 10
(1.92) | 9
(1.73) |
|
|
| G2 | 167 (32.12) | 143 (27.50) |
|
|
| G3 | 72
(13.85) | 119 (22.88) |
|
|
| Tumor necrosis |
|
| 0.286 | 0.593 |
| No | 225 (43.27) | 241 (46.35) |
|
|
|
Yes | 24
(4.62) | 30
(5.77) |
|
|
| Tumor size, cm |
|
| 1.205 | 0.272 |
| ≤3 | 156 (30.00) | 157 (30.19) |
|
|
|
>3 | 93
(17.88) | 114 (21.92) |
|
|
| CIS |
|
| 0.030 | 0.863 |
|
Absent | 241 (46.35) | 263 (50.58) |
|
|
|
Present | 8
(1.54) | 8
(1.54) |
|
|
Survival outcomes and HN
During a median follow-up duration of 54 months
(range, 12–151 months), 120 patients (23.1%) died, including 105
patients (20.2%) who succumbed to urothelial cancer. Of these
patients, 78 (65.0%) had preoperative HN. The 5-year CSS and OS
rates for patients with HN were 86.9 and 86.2%, respectively:
Markedly lower than for patients without HN (93.3 and 91.9%,
respectively).
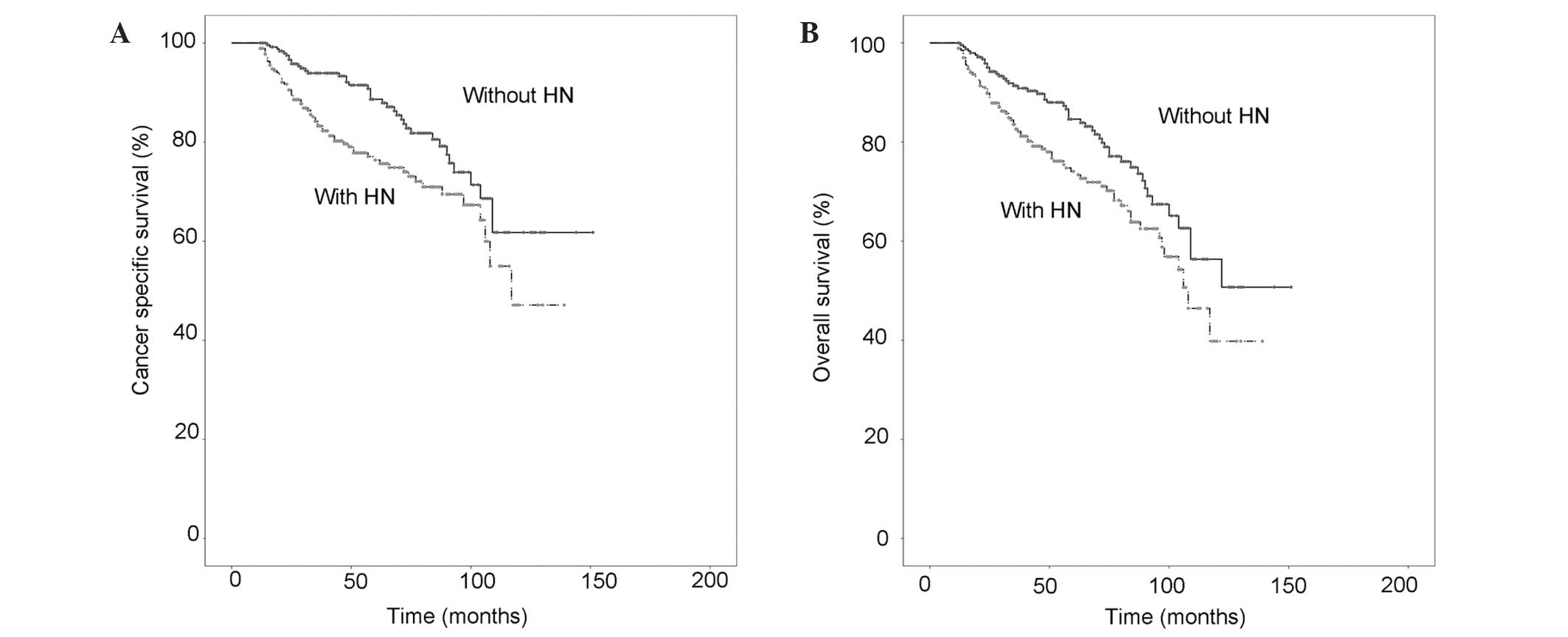
Kaplan-Meier estimated CSS and OS curves are shown
in Fig. 1A and B. The presence of HN
was a significant risk factor for poorer CSS and OS times according
to univariate analysis (P=0.004 and P=0.009, respectively). In the
multivariate analysis, the presence of HN remained a significant
predictive factor for CSS and OS (P=0.001 and P=0.011,
respectively). The multivariate analysis also confirmed male
gender, advanced age and higher tumor stage as risk factors for
reduced survival (Tables II and
III).
 | Table II.Univariate and multivariate analysis
of risk factors for cancer-specific survival. |
Table II.
Univariate and multivariate analysis
of risk factors for cancer-specific survival.
|
|
|
|
| Multivariate
analysis
|
|---|
|
| Patients | Recurrence | Univariate
analysis |
|
|
|
|---|
| Variable | n | n | P-value | Hazard ratio | 95% CI | P-value |
|---|
| Presence of
hydronephrosis |
|
| 0.004a | 1.932 | 1.294–2.883 | 0.001a |
|
Absence | 249 | 39 |
|
|
|
|
|
Presence | 271 | 66 |
|
|
|
|
| Gender |
|
| 0.000a | 0.491 | 0.327–0.738 | 0.001a |
|
Female | 291 | 43 |
|
|
|
|
|
Male | 229 | 62 |
|
|
|
|
| Age, years |
|
| 0.028a |
|
| 0.437 |
|
<50 | 28 | 7 |
|
|
|
|
|
50–60 | 92 | 15 |
|
|
|
|
|
60–70 | 175 | 31 |
|
|
|
|
|
70–80 | 187 | 40 |
|
|
|
|
|
≥80 | 38 | 12 |
|
|
|
|
| Preoperative renal
function |
|
| 0.940 |
|
|
|
| No CKD
(eGFR≥60) | 228 | 44 |
|
|
|
|
| Early
CKD (60>eGFR≥15) | 255 | 52 |
|
|
|
|
|
End-stage CKD
(eGFR<15) | 37 | 9 |
|
|
|
|
| Urinary
cytology |
|
| 0.648 |
|
|
|
|
Negative | 115 | 22 |
|
|
|
|
|
Positive | 250 | 57 |
|
|
|
|
| Missing
data | 155 | 26 |
|
|
|
|
| Surgical
approach |
|
| 0.743 |
|
|
|
|
Open | 348 | 76 |
|
|
|
|
|
Laparoscopic | 172 | 29 |
|
|
|
|
| Tumor
architecture |
|
| 0.008a |
|
| 0.364 |
|
Papillary | 419 | 79 |
|
|
|
|
|
Sessile | 101 | 26 |
|
|
|
|
| Multifocality |
|
| 0.560 |
|
|
|
| No | 412 | 84 |
|
|
|
|
|
Yes | 108 | 21 |
|
|
|
|
| Location |
|
| 0.060 |
|
|
|
|
Ureter | 256 | 58 |
|
|
|
|
|
Pelvis | 264 | 47 |
|
|
|
|
| Tumor stage |
|
| 0.000a | 1.663 | 1.289–2.145 | 0.000a |
| Ta | 30 | 0 |
|
|
|
|
| T1 | 150 | 13 |
|
|
|
|
| T2 | 202 | 46 |
|
|
|
|
| T3 | 135 | 41 |
|
|
|
|
| T4 | 3 | 2 |
|
|
|
|
| Node stage |
|
| 0.284 |
|
|
|
| N0 | 51 | 10 |
|
|
|
|
| Nx | 458 | 91 |
|
|
|
|
| N+ | 10 | 3 |
|
|
|
|
| Tumor grade |
|
| 0.010a |
|
| 0.172 |
| G1 | 19 | 0 |
|
|
|
|
| G2 | 310 | 56 |
|
|
|
|
| G3 | 191 | 49 |
|
|
|
|
| Tumor necrosis |
|
| 0.021a | 2.069 | 1.162–3.686 | 0.014a |
| No | 466 | 90 |
|
|
|
|
|
Yes | 54 | 15 |
|
|
|
|
| Tumor size, cm |
|
| 0.165 |
|
|
|
| ≤3 | 313 | 59 |
|
|
|
|
|
>3 | 207 | 46 |
|
|
|
|
| CIS |
|
| 0.318 |
|
|
|
|
Absent | 504 | 103 |
|
|
|
|
|
Present | 16 | 2 |
|
|
|
|
| Adjuvant
therapy |
|
| 0.470 |
|
|
|
| No | 485 | 95 |
|
|
|
|
|
Yes | 30 | 8 |
|
|
|
|
 | Table III.Univariate and multivariate analysis
of risk factors for overall survival. |
Table III.
Univariate and multivariate analysis
of risk factors for overall survival.
|
|
|
|
| Multivariate
analysis |
|---|
|
|
|
|
|
|
|---|
| Variable | Patients, n | Recurrence, n | Univariate analysis
P-value | Hazard ratio | 95% CI | P-value |
|---|
| Presence of
hydronephrosis |
|
| 0.009a | 1.587 | 1.111–2.265 | 0.011a |
|
Absence | 249 | 52 |
|
|
|
|
|
Presence | 271 | 78 |
|
|
|
|
| Gender |
|
| 0.000a | 0.604 | 0.426–0.858 | 0.005a |
|
Female | 291 | 57 |
|
|
|
|
|
Male | 229 | 73 |
|
|
|
|
| Age, years |
|
| 0.005a | 1.284 | 1.068–1.542 | 0.008a |
|
<50 | 28 | 8 |
|
|
|
|
|
50–60 | 92 | 17 |
|
|
|
|
|
60–70 | 175 | 38 |
|
|
|
|
|
70–80 | 187 | 53 |
|
|
|
|
|
≥80 | 38 | 14 |
|
|
|
|
| Preoperative renal
function |
|
| 0.260 |
|
|
|
| No CKD
(eGFR≥60) | 228 | 48 |
|
|
|
|
| Early
CKD (60>eGFR≥15) | 255 | 68 |
|
|
|
|
|
End-stage CKD
(eGFR<15) | 37 | 14 |
|
|
|
|
| Urinary
cytology |
|
| 0.385 |
|
|
|
|
Negative | 116 | 26 |
|
|
|
|
|
Positive | 250 | 73 |
|
|
|
|
| Missing
data | 155 | 31 |
|
|
|
|
| Surgical
approach |
|
| 0.503 |
|
|
|
|
Open | 348 | 96 |
|
|
|
|
|
Laparoscopic | 172 | 34 |
|
|
|
|
| Tumor
architecture |
|
| 0.015a |
|
| 0.262 |
|
Papillary | 419 | 100 |
|
|
|
|
|
Sessile | 101 | 30 |
|
|
|
|
| Multifocality |
|
| 0.271 |
|
|
|
| No | 412 | 106 |
|
|
|
|
|
Yes | 108 | 24 |
|
|
|
|
| Location |
|
| 0.110 |
|
|
|
|
Ureter | 256 | 69 |
|
|
|
|
|
Pelvis | 264 | 61 |
|
|
|
|
| Tumor stage |
|
| 0.000a | 1.581 | 1.265–1.976 | 0.000a |
| Ta | 30 | 4 |
|
|
|
|
| T1 | 150 | 19 |
|
|
|
|
| T2 | 202 | 57 |
|
|
|
|
| T3 | 135 | 48 |
|
|
|
|
| T4 | 3 | 2 |
|
|
|
|
| Node stage |
|
| 0.519 |
|
|
|
| N0 | 52 | 11 |
|
|
|
|
| Nx | 458 | 116 |
|
|
|
|
| N+ | 10 | 3 |
|
|
|
|
| Tumor grade |
|
| 0.005a |
|
| 0.133 |
| G1 | 19 | 0 |
|
|
|
|
| G2 | 310 | 70 |
|
|
|
|
| G3 | 191 | 60 |
|
|
|
|
| Tumor necrosis |
|
| 0.137 |
|
|
|
| No | 466 | 115 |
|
|
|
|
|
Yes | 54 | 15 |
|
|
|
|
| Tumor size, cm |
|
| 0.306 |
|
|
|
| ≤3 | 313 | 76 |
|
|
|
|
|
>3 | 207 | 54 |
|
|
|
|
| CIS |
|
| 0.344 |
|
|
|
|
Absent | 504 | 127 |
|
|
|
|
|
Present | 16 | 3 |
|
|
|
|
| Adjuvant
therapy |
|
| 0.648 |
|
|
|
| No | 485 | 119 |
|
|
|
|
|
Yes | 30 | 9 |
|
|
|
|
As higher tumor stage is a well-established
predictor of survival (11), the
predictive role of HN was analyzed only in the 340 patients with
muscle-invasive disease (T≥2) (Table
III). Of the 189 patients with HN, there were 68 mortalities,
comprising 58 cancer-specific mortalities, during follow-up. By
contrast, only 39 mortalities, including 31 cancer-specific
mortalities, occurred among the remaining 151 patients without HN.
The differences in CSS and OS times between the two groups were
statistically significant (P=0.009 and P=0.012, respectively;
Fig. 2A and B).
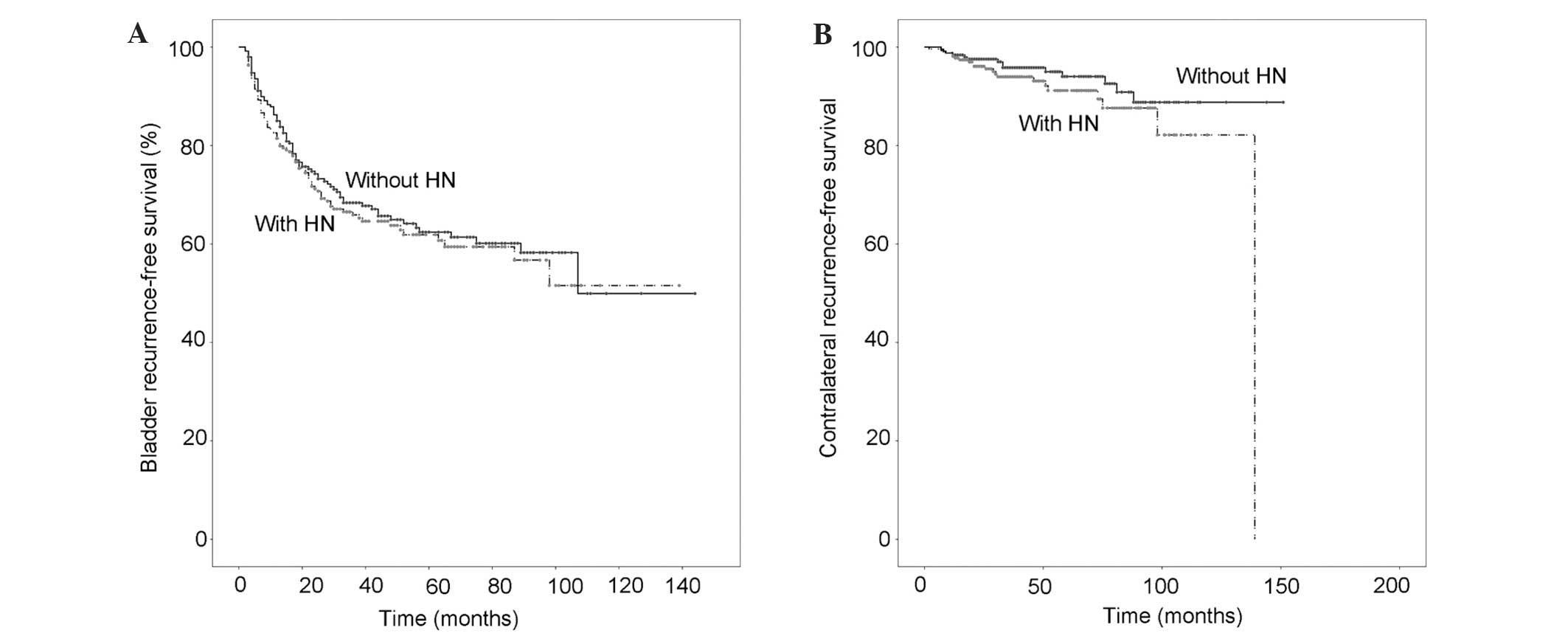
Pathology confirmed that 178 patients (34.2%) had
intravesical recurrence and 35 patients (6.7%) had subsequent
contralateral UTUC. Of the patients with HN, 99 experienced
intravesical recurrence and 22 contralateral disease during
follow-up, and there was no association between the presence of HN
and bladder cancer recurrence-free survival time (P=0.552) or the
development of contralateral carcinoma-free survival time (P=0.164)
(Fig. 3A and B).
Discussion
Preoperative HN can be present in bladder tumors and
UTUCs. The incidence of HN in bladder tumors is reported to be
5.3–22.7%, and the presence of HN has been demonstrated to be
associated with poor pathological outcomes, tumor recurrence and
progression (18–20). The presence of HN is more prevalent in
UTUC than in bladder tumors (52.1% in the current study), which may
be because urinary obstruction is more likely to occur in the
ureter from a small mass.
There have been a number of studies focused on the
association between the presence of HN and clinicopathological
characteristics and prognosis (17,22–25,27).
The majority of these studies have reported a predictive role of HN
in poor pathological outcomes, however, there has been no consensus
on the association between HN and poor prognosis. Ng et al
(17) confirmed that HN was
independently associated with cancer metastasis and cancer-specific
mortality by preoperative multivariable analysis controlling for
preoperative clinical features. However, their research was based
on only 106 patients and HN was no longer an independent risk
factor upon postoperative multivariable analysis. Hwang et
al (25) reported that
preoperative HN predicted poor prognosis in 114 patients; data on
architecture, multifocality and preoperative renal function were
unavailable. Ito et al (22)
reported that 67 patients (73.6%) exhibited HN in a retrospective
study of 91 cases, and validated the correlation between HN and
poor pathological outcomes, whilst a higher HN grade was not
associated with disease-specific or metastasis-free survival.
Bozzini et al (27) conducted
a relatively large-scale study with 401 patients, however, HN was
present in only 18.4% of patients and the median follow-up period
was 26 months. Furthermore, whilst studies by Messer et al
(23) and Brien et al
(24) demonstrated that HN was
associated with muscle-invasive and non-organ confined disease,
these studies were lacking in follow-up data.
The proportions of HN reported in previous studies
may differ due to the lack of clear criteria with which to evaluate
HN. Based on the current analysis, the presence of HN was
associated with a number of poor pathological outcomes, including
high tumor stage, high tumor grade and sessile tumor architecture.
In addition, a greater number of tumors were located in the ureter
in patients with HN, and previous studies have demonstrated that
patients with ureteral tumors have a poorer prognosis compared with
those with renal pelvis tumors, after adjustment for a number of
pathological variables (28,29). A recent study attributed this
difference in prognosis to the fact that ureteral tumors are more
likely to have HN (30). According to
univariate and multivariate survival analyses, the presence of HN
was associated with poorer survival, which confirmed the role of HN
as an independent risk factor for poor prognosis. Preoperative HN
must be carefully evaluated as a significant predictive factor for
prognosis as well as higher tumor stage and tumor grade. The
present study observed no correlation between HN and bladder
recurrence or contralateral UTUC, and no such association has been
reported previously (31).
Using conservative surgeries, including segmental
ureterectomy or endoscopic management, renal function may be
preserved and perioperative complications with RNU avoided
(32). Clinical consideration of
advanced disease based on the presence of HN could allow physicians
to better individually assess treatment options in UTUC; patients
with HN may not be suitable candidates for less invasive surgical
options. In addition, patients with locally advanced UTUC have
significantly higher local recurrence and distant metastasis rates
following RNU, compared with patients exhibiting early stage
disease (11,33). Such findings call for effective
strategies for perioperative systemic therapy to improve survival.
The presence of HN also indicates a need for aggressive treatment,
including lymphadenectomy and systemic chemotherapy.
Neoadjuvant chemotherapy appears to achieve
favorable oncological outcomes in high-risk patients (15,16), while
adjuvant chemotherapy confers minimal impact on OS or CSS (34,35). In
addition, not all patients are able to receive adjuvant treatment
due to comorbidities and impaired renal function following RNU
(36). Hoshino et al (37) found that patients with no HN or a
lower grade of HN have a higher risk of missing the opportunity to
undergo adjuvant chemotherapy for impaired renal function following
RNU. Thus, if patients without HN are evaluated as high-risk (based
on lymph node metastasis or higher biopsy grade) and systemic
chemotherapy is considered, neoadjuvant chemotherapy is recommended
before renal function becomes impaired.
The limitations of the current study include the
retrospective design and data collection, and the lack of
re-evaluation of a number of CT films. Therefore, the study cohort
may be subject to selection and recall bias. The incidence of UTUC
in the Chinese population is markedly higher compared with that of
western populations, and the biology may differ (38). Although the mechanisms related to
these ethnic differences are still not fully known, dietary
exposure to toxins may play a major role (39). In addition, Chinese UTUC patients are
more likely to be female, and females are less likely to be of an
advanced pathological disease stage compared with males (40,41).
Despite its limitations, the present study is currently the largest
to report on the predictive role of HN in UTUC patients, and the
first study confirming an association between HN and poor prognosis
after controlling for other clinical and pathological
characteristics in a large sample.
In conclusion, preoperative HN is prevalent in UTUC.
The presence of preoperative HN predicted poorer pathological
outcomes and was a significant risk factor affecting survival. The
evaluation of HN may therefore be informative for decisions
concerning surgical strategies, and the presence of HN should raise
the possibility of employing an aggressive treatment strategy.
Acknowledgements
The present study was supported by grants from the
Clinical Features Research of Capital (grant nos. Z121107001012154
and Z151100004015173), the Collaborative Research Foundation of
Peking University Health Science Center and the College of
Medicine, National Taiwan University (grant no. BMU20120318), the
Natural Science Foundation of China (grant nos. 81172419 and
81372746), the Natural Science Foundation of Beijing (grant no.
7122183), and the Research Foundation of Peking University First
Hospital (grant no. 2015QN026).
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pérez M Pérez-Utrilla, Bazán A Aguilera,
Dorrego JM Alonso, et al: Simultaneous cystectomy and
nephroureterectomy due to synchronous upper urinary tract tumors
and invasive bladder cancer: Open and laparoscopic approaches. Curr
Urol. 6:76–81. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cummings KB: Nephroureterectomy: Rationale
in the management of transitional cell carcinoma of the upper
urinary tract. Urol Clin North Am. 7:569–578. 1980.PubMed/NCBI
|
|
4
|
McLaughlin JK, Silverman DT, Hsing AW, et
al: Cigarette smoking and cancers of the renal pelvis and ureter.
Cancer Res. 52:254–257. 1992.PubMed/NCBI
|
|
5
|
Colin P, Koenig P, Ouzzane A, et al:
Environmental factors involved in carcinogenesis of urothelial cell
carcinomas of the upper urinary tract. BJU Int. 104:1436–1440.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cowan NC: CT urography for hematuria. Nat
Rev Urol. 9:218–226. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sobin L, Gospodarowicz M and Wittekind C:
Urological tumours. Renal pelvis and ureter. TNM Classification of
Malignant Tumours (7th). Wiley-Blackwell. (Hoboken, NJ). 258–261.
2009.
|
|
8
|
Rink M, Ehdaie B, Cha EK, et al: Bladder
Cancer Research Consortium (BCRC); Upper Tract Urothelial Carcinoma
Collaboration (UTUCC): Stage-specific impact of tumor location on
oncologic outcomes in patients with upper and lower tract
urothelial carcinoma following radical surgery. Eur Urol.
62:677–684. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cha EK, Shariat SF, Kormaksson M, et al:
Predicting clinical outcomes after radical nephroureterectomy for
upper tract urothelial carcinoma. Eur Urol. 61:818–825. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Roupret M, Babjuk M, Comperat E, et al:
European Association of Urology: European guidelines on upper tract
urothelial carcinomas: 2013 update. Eur Urol. 63:1059–1071. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Margulis V, Shariat SF, Matin SF, et al:
Outcomes of radical nephroureterectomy: A series from the upper
tract urothelial carcinoma collaboration. Cancer. 115:1224–1233.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gadzinski AJ, Roberts WW, Faerber GJ and
Wolf JS Jr: Long-term outcomes of nephroureterectomy versus
endoscopic management for upper tract urothelial carcinoma. J Urol.
183:2148–2153. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Colin P, Ouzzane A, Pignot G, et al:
Comparison of oncological outcomes after segmental ureterectomy or
radical nephroureterectomy in urothelial carcinomas of the upper
urinary tract: Results from a large French multicentre study. BJU
Int. 110:1134–1141. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Murphy DM, Zincke H and Furlow WL: Primary
grade 1 transitional cell carcinoma of the renal pelvis and ureter.
J Urol. 123:629–631. 1980.PubMed/NCBI
|
|
15
|
Matin SF, Margulis V, Kamat A, et al:
Incidence of downstaging and complete remission after neoadjuvant
chemotherapy for high-risk upper tract transitional cell carcinoma.
Cancer. 116:3127–3134. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Youssef RF, Shariat SF, Lotan Y, et al:
Upper urinary tract urothelial carcinoma with loco-regional nodal
metastases: Insights from the upper tract urothelial carcinoma
collaboration. BJU Int. 108:1286–1291. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ng CK, Shariat SF, Lucas SM, et al: Does
the presence of hydronephrosis on preoperative axial CT imaging
predict worse outcomes for patients undergoing nephroureterectomy
for upper-tract urothelial carcinoma? Urol Oncol. 29:27–32. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Divrik RT, Sahin A, Altok M, Unlu N and
Zorlu F: The frequency of hydronephrosis at initial diagnosis and
its effect on recurrence and progression in patients with
superficial bladder cancer. J Urol. 178:802–806. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Haleblian GE, Skinner EC, Dickinson MG,
Lieskovsky G, Boyd SD and Skinner DG: Hydronephrosis as a
prognostic indicator in bladder cancer patients. J Urol.
160:2011–2014. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bartsch GC, Kuefer R, Gschwend JE, de
Petriconi R, Hautmann RE and Volkmer BG: Hydronephrosis as a
prognostic marker in bladder cancer in a cystectomy-only series.
Eur Urol. 51:690–697. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen XP, Xiong GY, Li XS, Matin SF, Garcia
M, Fang D, Wang TY, Yu W, Gong K, Song Y, et al: Predictive factors
for worse pathological outcomes of upper tract urothelial
carcinoma: Experience from a nationwide high-volume centre in
China. BJU Int. 112:917–924. 2013.PubMed/NCBI
|
|
22
|
Ito Y, Kikuchi E, Tanaka N, Miyajima A,
Mikami S, Jinzaki M and Oya M: Preoperative hydronephrosis grade
independently predicts worse pathological outcomes in patients
undergoing nephroureterectomy for upper tract urothelial carcinoma.
J Urol. 185:1621–1626. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Messer JC, Terrell JD, Herman MP, Ng CK,
Scherr DS, Scoll B, Boorjian SA, Uzzo RG, Wille M, Eggener SE, et
al: Multi-institutional validation of the ability of preoperative
hydronephrosis to predict advanced pathologic tumor stage in
upper-tract urothelial carcinoma. Urol Oncol. 31:904–908. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Brien JC, Shariat SF, Herman MP, Ng CK,
Scherr DS, Scoll B, Uzzo RG, Wille M, Eggener SE, Terrell JD, et
al: Preoperative hydronephrosis, ureteroscopic biopsy grade and
urinary cytology can improve prediction of advanced upper tract
urothelial carcinoma. J Urol. 184:69–73. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hwang I, Jung SI, Nam DH, Hwang EC, Kang
TW, Kwon DD and Ryu SB: Preoperative hydronephrosis and diabetes
mellitus predict poor prognosis in upper urinary tract urothelial
carcinoma. Can Urol Assoc J. 7:E215–E220. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ma YC, Zuo L, Chen JH, Luo Q, Yu XQ, Li Y,
Xu JS, Huang SM, Wang LN, Huang W, et al: Modified glomerular
filtration rate estimating equation for Chinese patients with
chronic kidney disease. J Am Soc Nephrol. 17:2937–2944. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bozzini G, Nison L, Colin P, Ouzzane A,
Yates DR, Audenet F, Pignot G, Arvin-Berod A, Merigot O, Guy L, et
al: Influence of preoperative hydronephrosis on the outcome of
urothelial carcinoma of the upper urinary tract after
nephroureterectomy: The results from a multi-institutional French
cohort. World J Urol. 31:83–91. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Akdogan B, Dogan HS, Eskicorapci SY, Sahin
A, Erkan I and Ozen H: Prognostic significance of bladder tumor
history and tumor location in upper tract transitional cell
carcinoma. J Urol. 176:48–52. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ouzzane A, Colin P, Xylinas E, Pignot G,
Ariane MM, Saint F, Hoarau N, Adam E, Azemar MD, Bensadoun H, et
al: Ureteral and multifocal tumours have worse prognosis than renal
pelvic tumours in urothelial carcinoma of the upper urinary tract
treated by nephroureterectomy. Eur Urol. 60:1258–1265. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang X, Zhu Z, Zhong S, Xu T and Shen Z:
Ureteral tumours showing a worse prognosis than renal pelvis
tumours may be attributed to ureteral tumours more likely to have
hydronephrosis and less likely to have haematuria. World J Urol.
31:155–160. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Azémar MD, Comperat E, Richard F, Cussenot
O and Rouprêt M: Bladder recurrence after surgery for upper urinary
tract urothelial cell carcinoma: Frequency, risk factors and
surveillance. Urol Oncol. 29:130–136. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Silberstein JL, Power NE, Savage C, Tarin
TV, Favaretto RL, Su D, Kaag MG, Herr HW and Dalbagni G: Renal
function and oncologic outcomes of parenchymal sparing ureteral
resection versus radical nephroureterectomy for upper tract
urothelial carcinoma. J Urol. 187:429–434. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hall MC, Womack S, Sagalowsky AI, Carmody
T, Erickstad MD and Roehrborn CG: Prognostic factors, recurrence
and survival in transitional cell carcinoma of the upper urinary
tract: A 30-year experience in 252 patients. Urology. 52:594–601.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hellenthal NJ, Shariat SF, Margulis V,
Karakiewicz PI, Roscigno M, Bolenz C, Remzi M, Weizer A, Zigeuner
R, Bensalah K, et al: Adjuvant chemotherapy for high risk upper
tract urothelial carcinoma: Results from the upper tract urothelial
carcinoma collaboration. J Urol. 182:900–906. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Audenet F, Yates DR, Cussenot O and
Roupret M: The role of chemotherapy in the treatment of urothelial
cell carcinoma of the upper urinary tract (UUT-UCC). Urol Oncol.
31:407–413. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
O'Donnell PH and Stadler WM: The role of
chemotherapy in upper tract urothelial carcinoma. Adv Urol.
2009:4190282009. View Article : Google Scholar
|
|
37
|
Hoshino K, Kikuchi E, Tanaka N, Akita H,
Ito Y, Miyajima A, Jinzaki M and Oya M: Preoperative
hydronephrosis: Independent predictor for changes in renal function
following nephroureterectomy. Jpn J Clin Oncol. 42:202–207. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wang SM, Lai MN, Chen PC, Pu YS, Lai MK,
Hwang JS and Wang JD: Increased upper and lower tract urothelial
carcinoma in patients with end-stage renal disease: A nationwide
cohort study in Taiwan during 1997–2008. Biomed Res Int.
2014:1497502014.PubMed/NCBI
|
|
39
|
Chen CH, Dickman KG, Moriya M, Zavadil J,
Sidorenko VS, Edwards KL, Gnatenko DV, Wu L, Turesky RJ, Wu XR, et
al: Aristolochic acid-associated urothelial cancer in Taiwan. Proc
Natl Acad Sci USA. 109:8241–8246. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Chou YH and Huang CH: Unusual clinical
presentation of upper urothelial carcinoma in Taiwan. Cancer.
85:1342–1344. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Yu W, Zhao YY, Shen Q, Yang XY, He Q, Song
Y and Jin J: Gender-related differences in pathological
characteristics of upper urinary tract urothelial carcinoma:
Analysis of 597 cases. Beijing Da Xue Xue Bao. 43:522–524. 2011.(In
Chinese). PubMed/NCBI
|