Introduction
Lung cancer is the most common type of cancer
worldwide (1,2). Non-small cell lung cancer (NSCLC)
accounts for 80–85% of all lung cancers, and although the surgical
resection of early-stage tumors confers the greatest potential for
long-term survival, 30–60% of patients with disease stages IB to
IIIA succumb within 5 years of surgery (3,4).
Therefore, more useful prognostic factors for those patients who
have undergone a resection may enable a more accurate prediction of
the outcome and could identify those patient groups with poor
survival who may benefit from a more precise indication of the
efficacy of treatment.
In hematological malignancies, the role for Notch is
well established, while more recent studies have demonstrated the
significance of Notch activity in the initiation and progression of
solid tumors (5–9). The mammalian Notch receptor family
consists of four type I transmembrane receptors (known as Notch
1–4), all of which have been implicated in human cancer. There are
also five known Notch ligands in mammals, namely Jagged 1 (JAG1),
JAG2, Delta-like 1 (DLL1), DLL3 and DLL4, which undergo processing
that is similar to Notch processing. The Notch receptor undergoes
multiple proteolytic cleavages upon ligand binding. The final
cleavage (S3 cleavage) by the γ-secretase complex results in the
release of the active Notch intracellular domain from the plasma
membrane and its subsequent translocation into the nucleus
(10). It is the S3 cleavage that is
targeted by a class of compounds known as the γ-secretase
inhibitors (GSIs). Therefore, treatment with GSIs blocks the
terminal cleavage and release from the plasma membrane, preventing
Notch signaling.
It has been demonstrated that Notch 1, Notch 3 and
their ligands, JAG1 and DLL4, may be involved in malignant
transformation. The activation of Notch 1 signaling appears to
sustain the motility, migration and invasion of tumor cells in
esophagus squamous cell carcinomas, lingual squamous cell
carcinoma, endometrial carcinoma and breast cancer (11–15).
Almost all cases of T cell acute lymphoblastic leukemia (T-ALL),
and colorectal, pancreatic and ovarian cancer have been reported to
exhibit aberrant Notch 3 expression (6,8,16,17). Our
previous study also reported that Notch 3 overexpression was
associated with a poor prognosis in NSCLC patients (18). However, the prognostic role of Notch 3
compared with other Notch family members and its association with
Notch 1 in human NSCLC remains unclear. In the present study, the
expression of Notch 1, Notch 3, JAG1 and DLL4 was investigated in
101 NSCLC tissue samples and association between the expression of
the four Notch family members and the clinicopathological variables
and prognosis in NSCLC patients was further assessed.
Materials and methods
Lung cancer specimens
Paraffin-embedded sections were acquired from 101
patients with NSCLC who underwent surgical resection at the
Department of Thoracic Surgery, The First Affiliated Hospital of
Anhui Medical University (Heifei, Anhui, China) between January
2007 and December 2007. The criteria for study enrollment were as
follows: Patients with histopathologically diagnosed NSCLC, no
receipt of radiotherapy or chemotherapy prior to surgery, and no
history of other tumors. Prior consent and approval was obtained
from all NSCLC patients prior to surgery for the use of cancer
tissues and adjacent non-cancerous lung tissues for research
purposes, and all experiments were conducted adhering to the
bioethics rules issued by the Medical Ethics Committee of The First
Affiliated Hospital of Anhui Medical University.
The follow-up period ranged from 1 to 60 months,
with a median time of 36 months. Informed consent was obtained from
all patients for publication of this study. The age of the patients
ranged from 32 to 80 years (median, 62 years), and the cohort
included 78 males (77.2%) and 23 females (22.7%). The histological
diagnosis was determined by hematoxylin and eosin staining
according to the new pathological classification of lung cancer
(19). Tumor grading and staging were
classified according to the new lung cancer staging system
developed by the International Association for the Study of Lung
Cancer (2009) (20). The results
revealed 49 adenocarcinomas (including 9 mucinous adenocarcinomas),
51 squamous cell carcinomas and 1 large cell carcinoma.
Furthermore, 18 tumors were well-differentiated, 53 were
moderately-differentiated and 30 were poorly-differentiated.
Tumor-node-metastasis (TNM) staging revealed that 20 patients were
at stage I, 48 were at stage II, 32 were at stage III and 1 was at
stage IV.
Ventilatory function and small airway function were
detected by the Jaeger Masterscope computer system (Jaeger-Toennies
GmbH, Hoechberg, Germany). A predicted forced vital capacity (FVC)
of <80% or a forced expiratory volume in 1 sec/FVC of <70%
was defined as abnormal ventilatory function. A predicted maximal
mid-expiratory flow curve (75/25%) value of <65% was defined as
abnormal small airway function. Overall survival (OS) was defined
as the interval between the date of surgery and the date of
mortality. OS was censored at the date of the patient's last tumor
assessment, at the date of mortality from other causes or at 5
years post-surgery. Other patient information is summarized in
Table I.
 | Table I.Correlations between the expression of
Notch 1, Notch 3, Notch 1 plus Notch 3, JAG1 and DLL4 proteins and
clinicopathological parameters in patients with non-small cell lung
cancer. |
Table I.
Correlations between the expression of
Notch 1, Notch 3, Notch 1 plus Notch 3, JAG1 and DLL4 proteins and
clinicopathological parameters in patients with non-small cell lung
cancer.
|
|
| Notch 1 expression,
n |
| Notch 3 expression,
n |
| Notch 1 and Notch 3
expression, n |
| JAG1 expression,
n |
| DLL4 expression,
n |
|
|---|
|
|
|
|
|
|
|
|
|
|
|
|
|
|---|
| Characteristic | Cases, n | Low | High | P-value | Low | High | P-value | Both low | Both high | P-value | Low | High | P-value | Low | High | P-value |
|---|
| Gender |
|
|
| 0.4041 |
|
| 1.0000 |
|
| 0.3953 |
|
| 0.3203 |
|
| 0.9401 |
| Male | 78 | 37 | 41 |
| 36 | 42 |
| 22 | 27 |
| 43 | 35 |
| 30 | 48 |
|
Female | 23 | 8 | 15 |
| 11 | 12 |
| 5 | 9 |
| 16 | 7 |
| 8 | 15 |
|
| Age, years |
|
|
| 1.0000 |
|
| 0.8433 |
|
| 0.9131 |
|
| 0.8005 |
|
| 0.8937 |
|
<60 | 43 | 19 | 24 |
| 21 | 22 |
| 14 | 17 |
| 24 | 19 |
| 17 | 26 |
|
|
≥60 | 58 | 26 | 32 |
| 26 | 32 |
| 13 | 19 |
| 35 | 23 |
| 21 | 37 |
|
| Tumor
histology |
|
|
| 0.2661 |
|
| 0.2695 |
|
| 0.1347 |
|
| 0.7750 |
|
| 1.0000 |
|
Squamous cell carcinoma | 51 | 26 | 25 |
| 27 | 24 |
| 18 | 16 |
| 31 | 20 |
| 19 | 32 |
|
|
Adenocarcinoma + large | 50 | 19 | 31 |
| 20 | 30 |
| 9 | 20 |
| 28 | 22 |
| 19 | 31 |
|
| cell
carcinoma |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| T status |
|
|
| 0.3023 |
|
| 0.0856 |
|
| 0.0800 |
|
| 0.4710 |
|
| 0.4604 |
|
T1+T2 | 77 | 37 | 40 |
| 40 | 37 |
| 24 | 24 |
| 47 | 30 |
| 31 | 46 |
|
|
T3+T4 | 24 | 8 | 16 |
| 7 | 17 |
| 3 | 12 |
| 12 | 12 |
| 7 | 17 |
|
| Lymph node
status |
|
|
| 0.1875 |
|
| 0.0026a |
|
| 0.0056a |
|
| 0.8213 |
|
| 0.9752 |
| N0 | 41 | 22 | 19 |
| 27 | 14 |
| 17 | 9 |
| 25 | 16 |
| 16 | 25 |
|
| N+ | 60 | 23 | 37 |
| 20 | 40 |
| 10 | 27 |
| 34 | 26 |
| 22 | 38 |
|
| TNM stage |
|
|
|
<0.0001a |
|
|
<0.0001a |
|
| 0.0001a |
|
| 0.2319 |
|
| 0.0313a |
|
I+II | 68 | 36 | 32 |
| 42 | 26 |
| 25 | 15 |
| 43 | 25 |
| 31 | 37 |
|
|
III+IV | 33 | 9 | 24 |
| 5 | 28 |
| 2 | 21 |
| 16 | 17 |
| 7 | 26 |
|
| Grading |
|
|
| 0.0330a |
|
| 0.1309 |
|
| 0.0359a |
|
| 0.0754 |
|
| 0.4218 |
|
G1+G2 | 71 | 37 | 34 |
| 37 | 34 |
| 22 | 19 |
| 46 | 25 |
| 29 | 42 |
|
| G3 | 30 | 8 | 22 |
| 10 | 20 |
| 5 | 17 |
| 13 | 17 |
| 9 | 21 |
|
| Smoking
history |
|
|
| 0.4404 |
|
| 0.8855 |
|
| 0.9707 |
|
| 1.0000 |
|
| 0.7273 |
| No | 39 | 15 | 24 |
| 19 | 20 |
| 11 | 16 |
| 23 | 16 |
| 16 | 23 |
|
|
Yes | 62 | 30 | 32 |
| 28 | 34 |
| 16 | 20 |
| 36 | 26 |
| 22 | 40 |
|
| Ventilatory
function |
|
|
| 1.0000 |
|
| 0.2931 |
|
| 0.5363 |
|
| 1.0000 |
|
| 0.9399 |
|
Normal | 54 | 24 | 30 |
| 22 | 32 |
| 12 | 20 |
| 32 | 22 |
| 21 | 33 |
|
|
Abnormal | 47 | 21 | 26 |
| 25 | 22 |
| 15 | 16 |
| 27 | 20 |
| 17 | 30 |
|
| Small airway
function |
|
|
| 0.5277 |
|
| 0.8991 |
|
| 0.9642 |
|
| 1.0000 |
|
| 0.9117 |
|
Normal | 22 | 8 | 14 |
| 11 | 11 |
| 5 | 8 |
| 13 | 9 |
| 9 | 13 |
|
|
Abnormal | 79 | 37 | 42 |
| 36 | 43 |
| 22 | 28 |
| 46 | 33 |
| 29 | 50 |
|
| Anatomical
classification |
|
|
| 1.0000 |
|
| 0.9041 |
|
| 1.0000 |
|
| 0.9601 |
|
| 1.0000 |
|
Peripheral | 52 | 23 | 29 |
| 25 | 27 |
| 14 | 18 |
| 31 | 21 |
| 20 | 32 |
|
|
Central | 49 | 22 | 27 |
| 22 | 27 |
| 13 | 18 |
| 28 | 21 |
| 18 | 31 |
|
Immunohistochemistry
Each tissue was fixed in formalin and embedded in
paraffin, then sectioned to a thickness of 3 µm and mounted on
glass slides. The sections were dewaxed in xylene and dehydrated in
graded alcohol, and endogenous peroxidase activity was blocked with
3% hydrogen peroxide for 10 min. Next, the sections were subjected
to antigen retrieval with 10 mmol/l citrate buffer solution (pH 0)
for 20 min in a microwave oven at 700 W. Subsequently, the sections
were incubated with 10% goat serum albumin to eliminate
non-specific binding and then they were incubated overnight at 4°C
with the following primary antibodies: Rabbit polyclonal Notch 1
(1:100 dilution; Santa Cruz Biotechnology, Inc., Dallas, TX, USA),
rabbit polyclonal Notch 3 (1:50 dilution; Santa Cruz Biotechnology,
Inc.,), rabbit polyclonal JAG1 (1:50 dilution; Santa Cruz
Biotechnology, Inc.) and rabbit polyclonal Delta-4 (1:100 dilution;
Rockland Immunochemicals, Gilbertsville, PA, USA). Sections were
then incubated with the appropriate secondary antibodies (goat
anti-rabbit immunoglobulin G; 1:50 dilution; ZSGB-BIO, Beijing,
China) at room temperature for 60 min. After sufficient
phosphate-buffered saline (PBS) rinses, diaminobenzidine was used
as chromogen and the sections were counterstained with hematoxylin.
The slides were counterstained with hematoxylin, then dehydrated
and coverslipped. Samples incubated with PBS instead of primary
antibodies were used as negative controls.
Evaluation of immunohistochemical
staining
All stained slides were independently evaluated and
scored by three pathologists who had no knowledge of the patients'
clinical information. If a disagreement occurred, the slides were
re-examined to obtain a final consensus. Positive staining was
evaluated in at least five areas at ×400 magnification. The mean
percentage of positive cells were scored as follows: 0, 0%; 1,
1–25%; 2, 26–50%; 3, 51–75%; and 4, 76–100%. The staining intensity
was scored as follows: 0, negative; 1, weak; 2, moderate; and 3,
strong. A final histological score was obtained for each case by
multiplying the percentage staining and intensity scores. Protein
expression levels were further analyzed by classifying histological
scores as low expression (histological score <5) and as high
expression (histological score ≥5).
Statistical analysis
All statistical analyses were performed with R
software for Windows (version 2.15.3; http://cran.r-project.org/web/packages/dlnm/vignettes/dlnmOverview.pdf).
The associations between various clinicopathological parameters and
the expression of Notch 1, Notch 3, JAG1 or DLL4 was evaluated
using χ2 or Fisher's exact tests. Kaplan-Meier and
log-rank methods were used to draw and evaluate the significance of
survival curves. Multivariate Cox proportional hazards regression
analysis was used to identify independent prognostic factors for
survival rates following univariate survival analysis. P<0.05
was used to indicate a statistically significant difference.
Results
Notch 1 expression and the association
with clinicopathological parameters
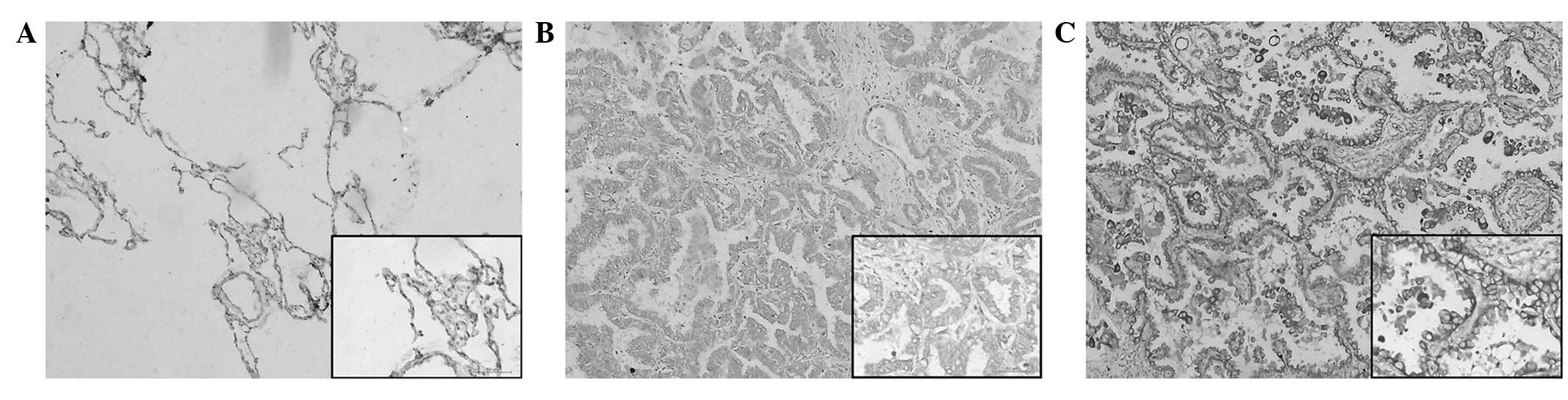
Notch 1 immunoreactivity is shown in Fig. 1. Notch 1 protein expression was
localized mainly in the cytoplasm, with certain tumor cells showing
membranous expression. The staining was absent in the adjacent
non-cancerous normal lung tissues (Fig.
1A). High Notch 1 protein expression was observed in 55.4%
(56/101) of the NSCLC samples (Fig.
1C) and low expression levels were detected in 44.6% (45/101)
of the tumor sections (Fig. 1B). As
summarized in Table I, the high
expression of Notch 1 was significantly correlated with TNM stage
(I+II vs. III+IV) (P=0.0264) and histological grading (G1+G2 vs.
G3) (P=0.0330) in the NSCLC tissues.
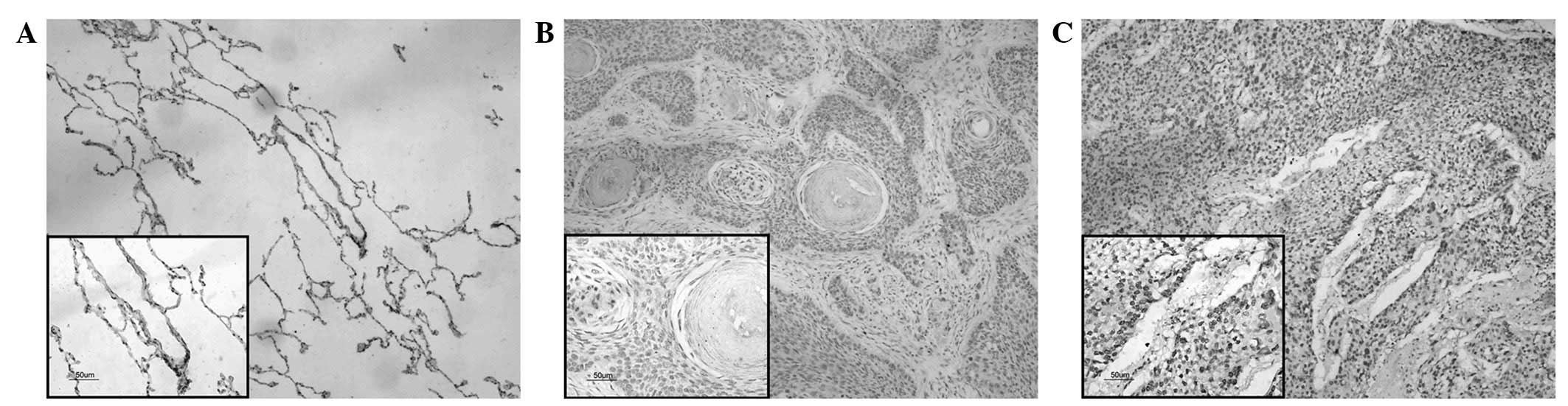
Notch 3 expression and the association
with clinicopathological parameters
Notch 3 immunoreactivity is shown in Fig. 2. Notch 3 was predominantly localized
in the nucleus of the tumor cells. The staining was absent in the
adjacent non-cancerous normal lung tissues (Fig. 2A). High Notch 3 protein expression was
observed in 53.5% (54/101) of the NSCLC samples (Fig. 2C) and low expression levels were
detected in 46.5% (47/101) (Fig. 2B).
As summarized in Table I, the high
expression of Notch 3 was significantly correlated with lymph node
status (N0 vs. N+) (P=0.0026) and TNM stage (I+II vs. III+IV)
(P<0.0001) in the NSCLC samples.
Association between Notch 1 plus Notch
3 coexpression and clinicopathological characteristics
High coexpression of Notch 1 and Notch 3 was
detected in 36 (35.6%) samples, and low expression was observed in
27 samples (26.7%). Table I shows
that the high coexpression of Notch 1 plus Notch 3 was
significantly correlated with lymph node status (N0 vs. N+)
(P=0.0056), TNM stage (I+II vs. III+IV) (P=0.0001) and histological
grading (G1+G2 vs. G3) (P=0.0359).
JAG1 and DLL4 expression and the
association with clinicopathological findings
JAG1 and DLL4 immunoreactivity is shown in Fig. 3. JAG1 and DLL4 protein expression was
localized mainly in the cytoplasm, with membranous staining. High
JAG1 protein expression was observed in 41.6% (42/101) (Fig. 3B) and low expression levels were
detected in 58.4% (59/101) (Fig. 3A).
High DLL4 protein expression was observed in 62.4% (63/101)
(Fig. 3D) and low expression levels
were detected in 37.6% (38/101) (Fig.
3C). Table I shows no association
between high JAG1 expression and the clinicopathological features
in the NSCLC patients (P>0.05), while the high expression of
DLL4 was significantly correlated with TNM stage (I+II vs. III+IV)
(P=0.0313).
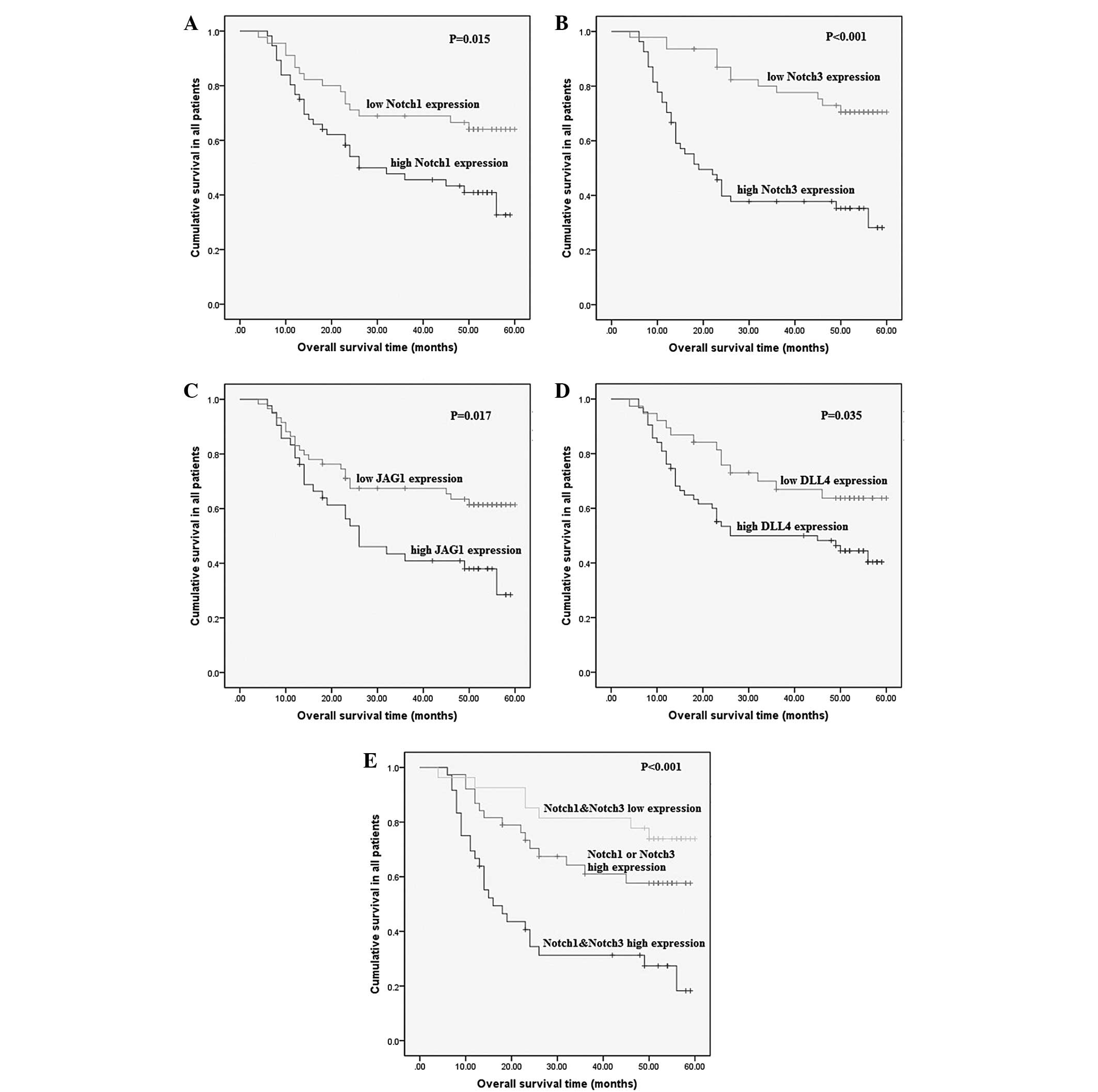
Survival analysis
Follow-up was discontinued for all patients in
December 2012. The median follow-up time was 36 months (range, 1–60
months). The overall 5-year survival rate was 45%, with a median
survival time of 56 months. The data indicated that there was a
significant difference in OS between the patients with high and low
expression of all four Notch family members (P<0.05), which
indicated that the high expression of Notch 1, Notch 3, JAG1 or
DLL4 was correlated with a shorter survival time. Furthermore, the
subtype with high coexpression of Notch 1 and Notch 3 exhibited a
worse outcome than other subtypes (log-rank, 21.227; P<0.001)
(Fig. 4).
Results of the univariate analysis with regard to OS
for the clinicopathological prognostic factors are shown in
Table II. The high expression of the
four Notch proteins was found to be a significant indicator of a
poor OS (P<0.05) (Table II). With
regard to other parameters, TNM stage, lymph node status and
histological grading were determined as positive significant
prognostic factors for OS (P<0.05) (Table II). In the multivariate analysis,
Notch 3 expression was found to be an independent prognostic factor
of OS in patients with NSCLC compared with the other three Notch
family members (P=0.0226) (Table
II).
 | Table II.Univariate and multivariate Cox
proportional hazards analyses for prognostic factors in patients
with non-small cell lung cancer. |
Table II.
Univariate and multivariate Cox
proportional hazards analyses for prognostic factors in patients
with non-small cell lung cancer.
|
| Overall
survival |
|---|
|
|
|
|---|
| Variable | HR | 95% CI | P-value |
|---|
| Univariate
analysis |
|
|
|
| Gender
(female vs. male) | 0.8090 | 0.4026–1.6260 | 0.5508 |
| Age
(≥60 vs. <60 years) | 0.7849 | 0.4453–1.3830 | 0.4011 |
| Tumor
histology (adenocarcinomas vs. quamous cell carcinoma) | 1.0610 | 0.6021–1.8690 | 0.8383 |
| T
status (T3+T4 vs. T1+T2) | 1.7960 | 0.9609–3.3570 | 0.0627 |
| Lymph
node status (N+ vs. N0) | 3.5700 | 1.8140–7.0260 |
<0.0001a |
| TNM
stage (III+IV vs. I+II) | 6.4060 | 3.5500–11.560 |
<0.0001a |
| Grading
(G3 vs. G1+G2) | 1.9020 | 1.0480–3.4490 | 0.0315a |
| Smoking
history (yes vs. no) | 1.3560 | 0.7435–2.4730 | 0.3187 |
|
Ventilatory function (abnormal
vs. normal) | 1.2590 | 0.7149–2.2190 | 0.4237 |
| Small
airway function (abnormal vs. normal) | 1.7240 | 0.8064–3.6840 | 0.1550 |
|
Anatomical classification
(central vs. peripheral) | 1.5630 | 0.8829–2.7680 | 0.1222 |
| Notch 1
expression (high vs. low) | 2.0800 | 1.1370–3.8040 | 0.0151a |
| Notch 3
expression (high vs. low) | 3.7220 | 1.9590–7.0720 |
<0.0001a |
| JAG1
expression (high vs. low) | 1.9760 | 1.1180–3.4940 | 0.0170a |
| DLL4
expression (high vs. low) | 1.9620 | 1.0370–3.7130 | 0.0348a |
| Multivariate
analysis |
|
|
|
| Gender
(female vs. male) | 0.8756 | 0.3004–2.5523 | 0.8076 |
| Age
(≥60 vs. <60 years) | 1.0026 | 0.5048–1.9913 | 0.9940 |
| Tumor
histology (adenocarcinomas vs. quamous cell carcinoma) | 1.0097 | 0.5309–1.9202 | 0.9766 |
| T
status (T3+T4 vs. T1+T2) | 0.8430 | 0.3717–1.9121 | 0.6827 |
| Lymph
node status (N+ vs. N0) | 1.7543 | 0.7431–4.1411 | 0.1997 |
| TNM
stage (III+IV vs. I+II) | 5.1447 | 2.1070–12.5621 | 0.0003a |
| Grading
(G3 vs. G1+G2) | 1.5180 | 0.7615–3.0263 | 0.2357 |
| Smoking
history (yes vs. no) | 0.8977 | 0.3527–2.2850 | 0.8209 |
|
Ventilatory function (abnormal
vs. normal) | 1.4652 | 0.6636–3.2350 | 0.3445 |
| Small
airway function (abnormal vs. normal) | 1.7839 | 0.6867–4.6345 | 0.2347 |
|
Anatomical classification
(central vs. peripheral) | 1.8915 | 0.9343–3.8291 | 0.0765 |
| Notch 1
expression (high vs. low) | 0.9105 | 0.3855–2.1510 | 0.8308 |
| Notch 3
expression (high vs. low) | 2.5126 | 1.1383–5.5460 | 0.0226a |
| JAG1
expression (high vs. low) | 1.1646 | 0.4850–2.7970 | 0.7331 |
| DLL4
expression (high vs. low) | 1.2515 | 0.5477–2.8600 | 0.5947 |
Discussion
In this study, Notch 1, Notch 3, JAG1 and DLL4
protein expression was examined immunohistochemically in a
well-defined cohort of NSCLC patients and the expression levels
were correlated to clinical parameters and patient outcome.
The phenotypic outcome of Notch signaling is often
context-dependent. Different Notch receptors play different, even
opposing, roles in tumor development, showing the complexity of
Notch signaling in cancer. The expression of Notch 3 has been found
to be significantly decreased in human tumor cell lines, and in
primary human breast cancer and melanoma samples compared with
normal control tissues (21).
However, the present data demonstrated that Notch 3 is ubiquitously
expressed in NSCLC. The majority of tumors showed high levels of
nuclear Notch 3 expression. This expression pattern closely
resembles data previously recorded in pancreatic ductal
adenocarcinomas (22), while certain
more recent studies have demonstrated that the immunoreactivity of
Notch 3 is observed mainly in the cytoplasm of tumor cells with or
without nuclei staining (8,9,13). The
mechanism of this phenomenon and how Notch 3 exerts its function in
NSCLC remain unclear and require further research. Most notably, in
the present study, the upregulated expression of Notch 3 was a
predictor of different aggressive tumor behaviors, such as advanced
clinical stage and lymph node metastasis. In lung cancer, Notch 1
is known to suppress tumor proliferation under normoxia, but in
hypoxia, it exhibits a converse role in tumor promotion (23). In the present study, Notch 1
expression, with levels varying from low to high, was demonstrated
in a number of NSCLC patients. Notch 1 expression was localized in
the cytoplasm, with membranous expression, similar to the results
found in studies of other tissue tumors (11–14).
Furthermore, Notch 1 expression was found to be associated with TNM
stage and histological grading, which also indicated the impact of
Notch 1 expression on the progression of NSCLC. The results
strongly suggested that Notch 1 and Notch 3 may play key roles in
the advancement of NSCLC.
As Notch receptor ligands, JAG1 and DLL4 have been
found to function in cancer progression and metastasis (24–28). In
the present study, the expression of the JAG1 and DLL4 notch
ligands was examined in NSCLC tissues and the expression levels
were compared with clinical parameters. However, no correlation was
found between the JAG1 expression levels and clinical parameters in
NSCLC. In addition, it was shown that in tumor tissues, high levels
of DLL4 expression were correlated with TNM stage, which suggested
that DLL4 may be associated with the progression of NSCLC.
Specific foci, including comborbidities, smoking
history, and general clinical and demographic features have been
investigated in previous analyses of prognostic factors in
surgically resected NSCLC. Pathological TNM stage, age and gender
were all determined to be independent prognostic factors for
survival, with pathological TNM stage representing the most
important prognostic factor (29).
However, the outcome may vary depending on several biochemical and
clinical parameters, even among those patients with clinically
localized disease. In the present study, Kaplan-Meier analysis of
the survival curves showed a significantly worse overall survival
rate for patients with tumors that exhibited high Notch 1, Notch 3,
JAG1 or DLL4 protein levels, indicating that high Notch 1, Notch 3,
JAG1 and DLL4 protein levels are markers of a poor prognosis for
patients with NSCLC. Furthermore, the present results showed that
the high coexpression of Notch 1 and Notch 3 predicted a worse
outcome compared with the Notch 1 or Notch 3 high expression
subtypes. These data suggested that the coexpression of Notch 1 and
Notch 3 has additive roles in the biological behavior of NSCLC.
Moreover, univariate analysis showed that high Notch 1, Notch 3,
JAG1 and DLL4 expression, TNM stage and tumor histological grading
were risk factors for a poor prognosis in NSCLC patients, but
multivariate analysis showed that a high level of Notch 3
expression was the only independent risk factor of prognosis for
NSCLC patients, suggesting that the level of Notch 3 expression in
NSCLC tissue samples may be used as a more useful prognostic marker
compared with Notch 1 in NSCLC patients. Previous studies have
demonstrated that cellular proliferation is significantly reduced
by γ-secretase inhibitor (30,31) and
that the apoptosis of Notch 3-expressing cells is induced. In lung
cancer, the inhibition of Notch activation using a γ-secretase
inhibitor is a potential novel approach for targeted therapy
(32). Thus, in NSCLC patients, Notch
3 expression may represent a useful additive prognostic marker to
the TNM staging system and thus, such patients are good candidates
for receiving aggressive adjuvant targeted therapy.
In summary, the present findings demonstrated that
high levels of Notch 1 and Notch 3 expression were significantly
correlated with NSCLC progression and a poor prognosis.
Furthermore, Notch 3 expression can be used as an adjunct to the
TNM staging system to improve prognostication for individual
patients. Additionally, it can be concluded that Notch 3 may
present as a more attractive prognostic biomarker associated with
NSCLC compared with Notch 1. Therefore, we hypothesize that
targeting Notch 3 in specific cell types may be more useful than
targeting Notch 1. In the near future, targeting the Notch 3
pathway may be used for the formation of novel preventive and
therapeutic strategies for NSCLC.
Acknowledgements
This study was funded by grants from the Key
Programs of the Educational Commission of Anhui Province (no.
KJ2011A178), the Natural Science Foundation of Science and
Technology Department of Anhui Province (no. 1208085MH146), and the
Scientific and Technological Programs of Science and Technology
Department of Anhui Province (no. 1501041144).
Abbreviations:
|
NSCLC
|
non-small cell lung cancer
|
|
JAG1
|
Jagged 1
|
|
DLL4
|
Delta-like 4
|
|
PBS
|
phosphate-buffered saline
|
|
HR
|
hazard ratio
|
|
CI
|
confidence interval
|
|
FVC
|
forced vital capacity
|
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bray F, Jemal A, Grey N, Ferlay J and
Forman D: Global cancer transitions according to the Human
Development Index (2008–2030): A population-based study. Lancet
Oncol. 13:790–801. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sangha R, Price J and Butts CA: Adjuvant
therapy in non-small cell lung cancer: Current and future
directions. Oncologist. 15:862–872. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Raso MG and Wistuba II: Molecular
pathogenesis of early-stage non-small cell lung cancer and a
proposal for tissue banking to facilitate identification of new
biomarkers. J Thorac Oncol. 2(7 Suppl 3): S128–S135. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ranganathan P, Weaver KL and Capobianco
AJ: Notch signalling in solid tumours: A little bit of everything
but not all the time. Nat Rev Cancer. 11:338–351. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rahman MT, Nakayama K, Rahman M, Katagiri
H, Katagiri A, Ishibashi T, Ishikawa M, Iida K, Nakayama S, Otsuki
Y and Miyazaki K: Notch 3 overexpression as potential therapeutic
target in advanced stage chemoresistant ovarian cancer. Am J Clin
Pathol. 138:535–544. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jonusiene V, Sasnauskiene A, Lachej N,
Kanopiene D, Dabkeviciene D, Sasnauskiene S, Kazbariene B and
Didziapetriene J: Down-regulated expression of Notch signaling
molecules in human endometrial cancer. Med Oncol. 30:4382013.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mann CD, Bastianpillai C, Neal CP, Masood
MM, Jones DJ, Teichert F, Singh R, Karpova E, Berry DP and Manson
MM: Notch 3 and HEY-1 as prognostic biomarkers in pancreatic
adenocarcinoma. PLoS One. 7:e511192012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kang H, An HJ, Song JY, Kim TH, Heo JH,
Ahn DH and Kim G: Notch 3 and Jagged2 contribute to gastric cancer
development and to glandular differentiation associated with MUC2
and MUC5AC expression. Histopathology. 61:576–586. 2012.PubMed/NCBI
|
|
10
|
Kopan R and Ilagan MX: The canonical Notch
signaling pathway: Unfolding the activation mechanism. Cell.
137:216–233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu J, Fan H, Ma Y, Liang D, Huang R, Wang
J, Zhou F, Kan Q, Ming L, Li H, et al: Notch 1 is a 5-fluorouracil
resistant and poor survival marker in human esophagus squamous cell
carcinomas. PLoS One. 8:e561412013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yu B, Wei J, Qian X, Lei D, Ma Q and Liu
Y: Notch 1 signaling pathway participates in cancer invasion by
regulating MMPs in lingual squamous cell carcinoma. Oncol Rep.
27:547–552. 2012.PubMed/NCBI
|
|
13
|
Mitsuhashi Y, Horiuchi A, Miyamoto T,
Kashima H, Suzuki A and Shiozawa T: Prognostic significance of
Notch signalling molecules and their involvement in the
invasiveness of endometrial carcinoma cells. Histopathology.
60:826–837. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Reedijk M, Odorcic S, Chang L, et al:
High-level coexpression of JAG1 and Notch 1 is observed in human
breast cancer and is associated with poor overall survival. Cancer
Res. 65:8530–8537. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang J, Fu L, Gu F and Ma YJ: Notch 1 is
involved in migration and invasion of human breast cancer cells.
Oncol Rep. 26:1295–1303. 2011.PubMed/NCBI
|
|
16
|
Bellavia D, Campese AF, Checquolo S, et
al: Combined expression of pTalpha and Notch 3 in T cell leukemia
identifies the requirement of preTCR for leukemogenesis. Proc Natl
Acad Sci U S A. 99:3788–3793. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Serafin V, Persano L, Moserle L, Esposito
G, Ghisi M, Curtarello M, Bonanno L, Masiero M, Ribatti D, Stürzl
M, et al: Notch 3 signalling promotes tumour growth in colorectal
cancer. J Pathol. 224:448–60. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ye YZ, Zhang ZH, Fan XY, Xu XL, Chen ML,
Chang BW and Zhang YB: Notch 3 overexpression associates with poor
prognosis in human non-small-cell lung cancer. Med Oncol.
30:5952013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Travis WD, Brambilla E and Riely GJ: New
pathologic classification of lung cancer: Relevance for clinical
practice and clinical trials. J Clin Oncol. 31:992–1001. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Detterbeck FC, Boffa DJ and Tanoue LT: The
new lung cancer staging system. Chest. 136:260–271. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cui H, Kong Y, Xu M and Zhang H: Notch 3
functions as a tumor suppressor by controlling cellular senescence.
Cancer Res. 73:3451–3459. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Doucas H, Mann CD, Sutton CD, et al:
Expression of nuclear Notch 3 in pancreatic adenocarcinomas is
associated with adverse clinical features and correlates with the
expression of STAT3 and phosphorylated Akt. J Surg Oncol. 97:63–68.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen Y, De Marco MA, Graziani I, et al:
Oxygen concentration determines the biological effects of NOTCH-1
signaling in adenocarcinoma of the lung. Cancer Res. 67:7954–7959.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Simon DP, Giordano TJ and Hammer GD:
Upregulated JAG1 enhances cell proliferation in adrenocortical
carcinoma. Clin Cancer Res. 18:2452–2464. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Steg AD, Katre AA, Goodman B, Han HD, Nick
AM, Stone RL, Coleman RL, Alvarez RD, Lopez-Berestein G, Sood AK
and Landen CN: Targeting the notch ligand JAGGED1 in both tumor
cells and stroma in ovarian cancer. Clin Cancer Res. 17:5674–5685.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Koukourakis MI, Giatromanolaki A, Sivridis
E, Gatter KC and Harris AL: High DLL4 expression in
tumour-associated vessels predicts for favorable radiotherapy
outcome in locally advanced squamous cell head-neck cancer (HNSCC).
Angiogenesis. 16:343–351. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu Z, Fan F, Wang A, Zheng S and Lu Y:
Dll4-Notch signaling in regulation of tumor angiogenesis. J Cancer
Res Clin Oncol. 140:525–536. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ishigami S, Arigami T, Uenosono Y, Okumura
H, Kurahara H, Uchikado Y, Setoyama T, Kita Y, Kijima Y, Nishizono
Y, et al: Clinical implications of DLL4 expression in gastric
cancer. J Exp Clin Cancer Res. 32:462013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chansky K, Sculier JP, Crowley JJ, Giroux
D, Van Meerbeeck J and Goldstraw P: International staging committee
and participating institutions: The international association for
the study of lung cancer staging project: Prognostic factors and
pathologic TNM stage in surgically managed non-small cell lung
cancer. J Thorac Oncol. 4:792–801. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Danza G, Di Serio C, Ambrosio MR, Sturli
N, Lonetto G, Rosati F, Rocca BJ, Ventimiglia G, del Vecchio MT,
Prudovsky I, et al: Notch 3 is activated by chronic hypoxia and
contributes to the progression of human prostate cancer. Int J
Cancer. 133:2577–2586. 2013.PubMed/NCBI
|
|
31
|
Chen X, Thiaville MM, Chen L, Stoeck A,
Xuan J, Gao M, Shih IeM and Wan TL: Defining Notch 3 target genes
in ovarian cancer. Cancer Res. 72:2294–2303. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Konishi J, Kawaguchi KS, Vo H, Haruki N,
Gonzalez A, Carbone DP and Dang TP: Gamma-secretase inhibitor
prevents Notch 3 activation and reduces proliferation in human lung
cancers. Cancer Res. 67:8051–8057. 2007. View Article : Google Scholar : PubMed/NCBI
|