Introduction
Plasma cell proliferative disorders are
characterized by clonal proliferation of plasma cells, which
produce either entire immunoglobulins (Igs) or Ig fragments
(1). Monoclonal gammopathy of
undetermined significance (MGUS) and multiple myeloma (MM) are the
most common plasma cell proliferative disorders (2). Approximately 1% of adults >50 years
old have MGUS, with a 14-fold risk of developing MM. In 2012, there
were 11,425 new patients with myeloma globally, and it is predicted
that MM will be diagnosed in over 2,000 patients in the United
States alone in 2015 (3). It is
currently unknown whether the occurrence of focal segmental
glomerulosclerosis (FSGS) in these patients is associated with the
primary disease (1). Therapy for
secondary FSGS should be directed at the underlying disorder, and
corticosteroids are the mainstay of treatment for idiopathic FSGS
(4). Furthermore, following a
retrospective review of FSGS and MM English-language studies, nine
publications were identified. Of these studies, seven demonstrated
an association between FSGS and plasma cell proliferative disorders
(2,5–10).
However, the other two studies indicated little or no correlation
between the two conditions (11,12). As
FSGS and MM occur infrequently in the same patient and there are
few relevant previous studies, the association between them remains
unclear. The current study describes a confirmed case of MM in a
patient diagnosed with FSGS and renal amyloidosis on two successive
renal biopsies. According to the literature, a correlation between
the two disorders may be present. Written informed consent was
obtained from the patient for the publication of this case
report.
Case report
In April 2012, a 45-year-old male patient presented
to the Chinese People's Liberation Army General Hospital (Beijing,
China) with intermittent edema in the lower extremities, fever and
fatigue. The patient had previously been diagnosed with nephrotic
syndrome (NS) one year earlier at China-Japan Friendship Hospital
(Beijing, China). The pathology report on the renal biopsy
performed in the previous hospital indicated tip variant FSGS
(Fig. 1). Following treatment with
glucocorticoids and tripterygium glycosides for one year (Table I), the patient's urine protein levels
decreased, but the serum creatinine levels increased. Additionally,
the patient's blood pressure and hemoglobin levels progressively
decreased (Table II). Physical
examination revealed the patient had a temperature of 37°C (normal
range, 36–37°C) and a body-mass index of 26.1 kg/m2
(normal range, 18.5–22.9 kg/m2).
 | Table I.Continuous treatments in the previous
hospital. |
Table I.
Continuous treatments in the previous
hospital.
|
| Treatment at
indicated times |
|---|
|
|
|
|---|
| Drug | April 2012 | November 2012 | December 2012 | March 2013 | June 2013 |
|---|
|
Methylprednisolone | 40 mg i.v. 3D |
|
|
|
|
| Prednisone | 60 mg q.d. | 30 mg q.d. | 10 mg q.d. | 25 mg q.d. | 10 mg q.d. |
| TGT |
| 20 mg tid | 40 mg tid | 20 mg tid | 20 mg tid |
| Leflunomide |
|
|
|
| 10 mg q.d. |
 | Table II.Blood pressure and laboratory test
results from the previous hospital treatment. |
Table II.
Blood pressure and laboratory test
results from the previous hospital treatment.
|
| Values at indicated
times |
|---|
|
|
|
|---|
| Parameter | April 2012 | November 2012 | December 2012 | March 2013 | June 2013 | July 2013 |
|---|
| SBP, mmHg | 130 | 111 | 127 | 107 | 87 | 85 |
| DBP, mmHg | 80 | 78 | 72 | 67 | 59 | 55 |
| MBP, mmHg | 123 | 115 | 114 | 103 | 88 | 83 |
| 24-h urine protein,
g | 21.03 | 5.15 | 6.66 | 9.57 | 9.45 | 7.53 |
| WBC, 1×109
cells/l | 8.65 | 13.5 | 15.9 | 14.3 | 12 | 8.97 |
| HGB, g/l | 111 | 147 | 138 | 127 | 82 | 72 |
| ALB, g/l | 16.4 | 23.1 | 22.8 | 20.4 | 23.3 | 15.4 |
| Scr, µmol/l | 70 | 64.52 | 68.07 | 69.84 | 91.05 | 100 |
| BUN, mmol/l | 5.65 | 6.65 | 6.76 | 5.72 | 5.83 | 5.26 |
| UA, µmol/l | 366 | 395 | 464 | 514 | 544 | 419 |
| GFR,
ml/min/1.73m2 | 80.70 | 94.96 | 89.38 | 86.17 | 68.75 | 55.84 |
Laboratory examinations revealed a calcium level of
2.19 mmol/l (normal range, 2.09–2.54 mmol/l). The serum
immunoglobulin G (IgG) level was 2,950.0 mg/dl (normal range,
700–1,600 mg/dl), the κ-light chain level was 16.6 mg/dl (normal
range, 170–370 mg/dl) and the λ-light chain level was 810.0 mg/dl
(normal range, 90–210 mg/dl). Serum immunofixation electrophoresis
revealed IgG λ MGUS. The patient's urinary κ-light chain level was
1.36 mg/dl (normal range, 0–0.79 mg/dl) and the λ-light chain level
was 135.0 mg/dl (normal range, 0–0.43 mg/dl). Ultrasound of the
renal artery indicated an increased resistance index for the
initial segment, and lesions in the renal microvasculature.
Ultrasound revealed that the left and right kidneys measured
12.9×7.8×6.4 cm and 13.7×7.0×5.7 cm, respectively (normal range,
10.0–12.0×5.0–6.0×3.0–4.0 cm). A bone marrow biopsy revealed that
36% of the plasma cells were abnormal and a diagnosis of IgG λ-type
MM was formed.
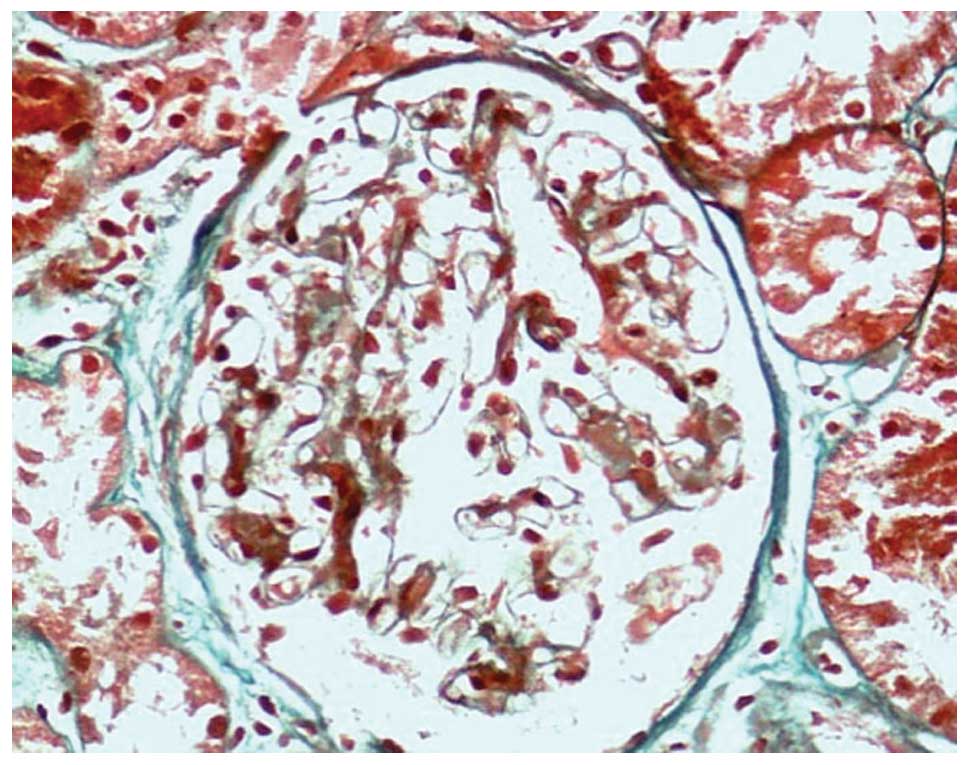
Renal biopsy. Periodic acid-Schiff (PAS) staining
revealed 16 intact glomeruli with normal volumes. Although there
was global sclerosis in one glomerulus (6.3%), there was no
glomerular adhesion to the Bowman's capsule, segmental sclerosis or
glomerular crescent. Furthermore, there was diffuse
mild-to-moderate mesangial broadening, but no mesangial cell
proliferation. The PAS staining was light in the mesangial area and
there was a deposit of a homogeneous, protein-like substance, which
was also observed in the renal interstitium and on the walls of the
renal artery. In addition, a number of capillary loops exhibited
restricted openings (Fig. 2). The
renal tubules were diffuse and severely atrophied, and protein
casts were visible. A few plasma cells were observed to be diffused
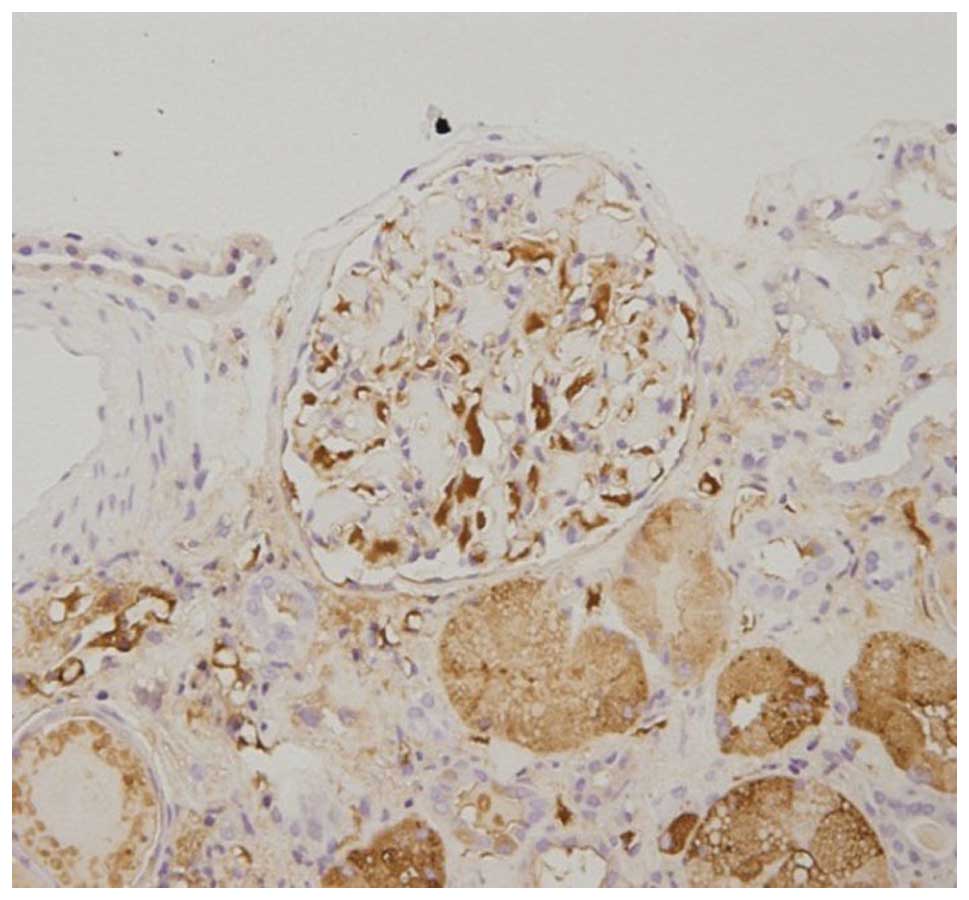
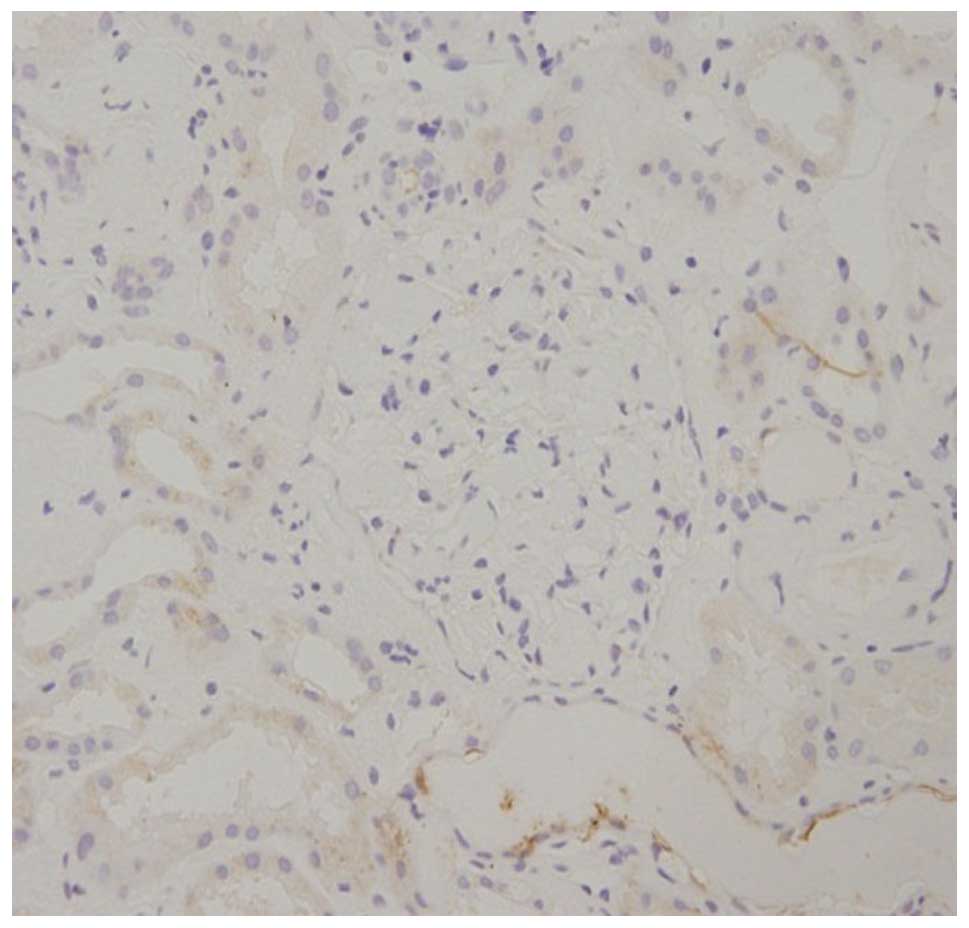
through interstitial spaces. Immunohistochemistry demonstrated that
the glomerular and tubular epithelial cells were λ chain-positive
and κ chain-negative (Figs. 3 and
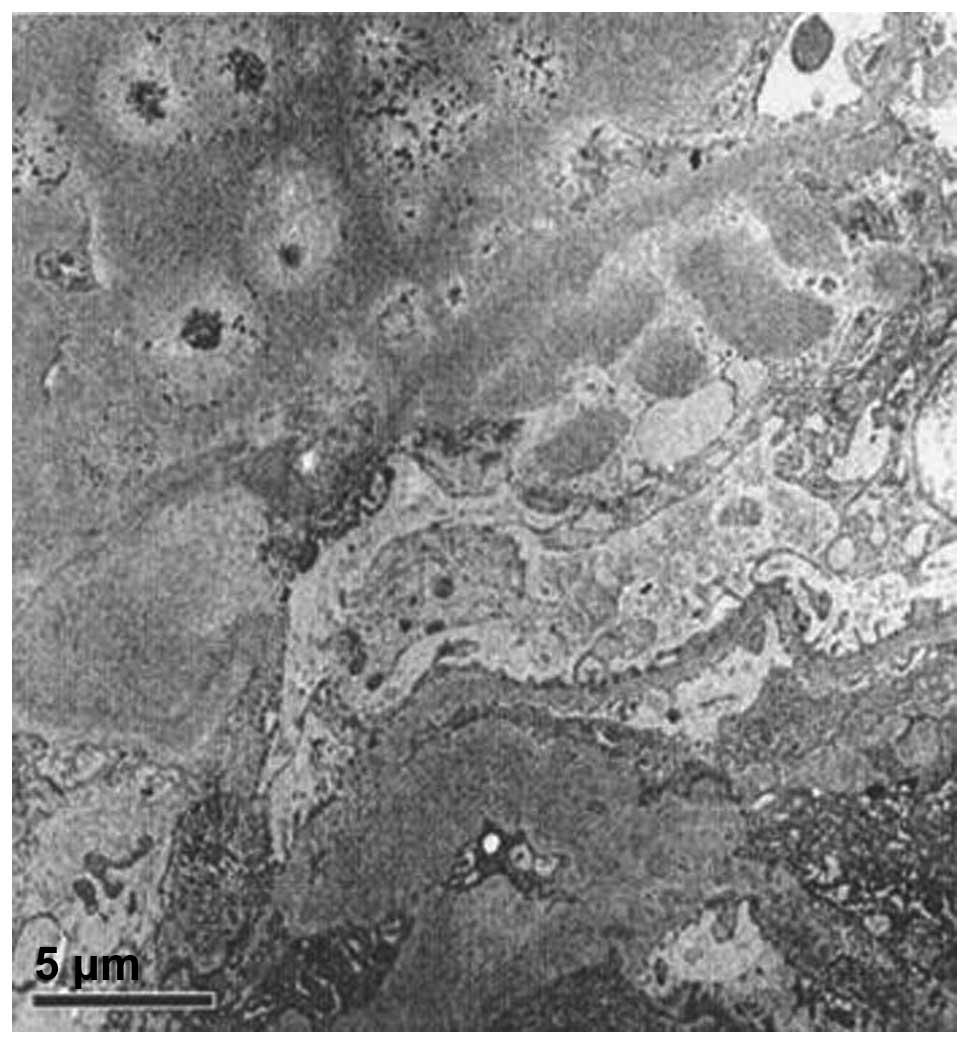
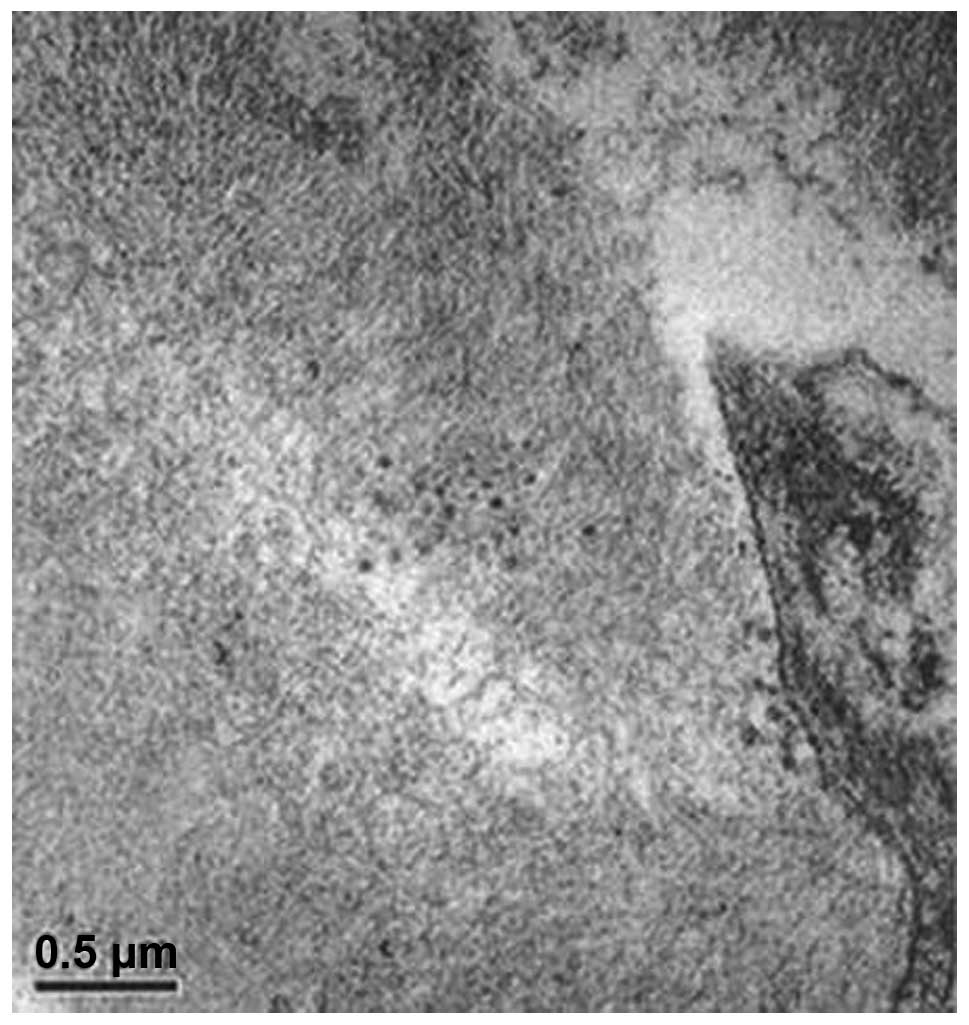
4). Electron microscopy revealed
collagen fiber hyperplasia (Figs. 5
and 6).
Treatment and outcomes. Following discharge, the
patient returned to a local hospital to receive chemotherapy for
the MM. The patient's oncologist commenced treatment for MM with
vincristine, doxorubicin and dexamethasone. Following three cycles
of drug treatment, the NS was resolved and renal function returned
to normal. Upon follow-up examination, the patient's IgG level was
1,480 mg/dl, the κ-light chain level was 316 mg/dl and the λ-light
chain was 91 mg/dl. No antibiotics were administered and the
patient's body temperature returned to a normal level. The patient
has since been lost to follow-up.
Discussion
FSGS is divided into primary and secondary subtypes,
and there are several known causes of the latter. FSGS secondary to
the hematological tumors T-cell lymphoma (13) or Hodgkin's lymphoma (14) are the most common and their
associations are clear. FSGS secondary to a large granular
lymphocytosis (15) is less common,
but their association has also been documented. Cases of FSGS with
MM or MGUS (2) are rare and the link
between them is unclear and speculative.
The patient described in the current study was
pathologically diagnosed with FSGS one year prior to admission.
However, the NS did not resolve following first-line treatment and
the patient's renal function declined. Several phenomena associated
with MM were noted, such as anemia that did not parallel the
decline in renal function, a reduction in blood pressure that was
associated with drug utilization and an increased monoclonal
immunoglobulin level in the serum. Therefore, while forming the
diagnosis of MM via bone marrow biopsy, a repeat renal biopsy was
performed on the patient, which confirmed renal amyloidosis.
Valizadeh et al (10) reported the case of a patient who had
been pathological diagnosed with FSGS and was confirmed with MM
upon follow-up. The study speculated that MM may be a rare
secondary occurrence to FSGS. Although the present patient was
diagnosed with renal amyloidosis after MM, a missed diagnosis of
FSGS-like lesions as a result of not performing serial pathological
sections cannot be excluded. FSGS is a morphological diagnostic
term. Secondary FSGS can be a morphological change in a variety of
diseases that develop to a certain stage rather than being caused
by a single disease. Secondary FSGS has relatively clear risk
factors and FSGS-like changes are usually present during the
development of primary glomerular diseases (4). Electron microscopy (EM) is currently,
the best method for identifying FSGS, and primary FSGS is highly
suggested if it indicates the disappearance of >80% of diffuse
foot processes (16). However, EM for
the present study indicated renal amyloidosis without changes in
the foot processes. On this basis, the possibility of MM combined
with primary FSGS was excluded.
The patient's reduced blood pressure indicated a
decline in vascular elasticity and suggested the deposition of
amyloid substances on the vascular wall. Ultrasound of the renal
artery demonstrated an increased resistance index of the initial
segment. This may have been due to amyloid substances on the walls
of the renal microvasculature narrowing the luminal spaces or to
the deposition of amyloid substances in the mesangial area that may
have restricted the openings of the capillary loops. Furthermore,
the renal pathology results confirmed a similar inference. We
hypothesize that restricted openings of certain capillary loops are
compensated for by the capillary loops with unobstructed openings,
and increased pressure and perfusion inside the glomeruli are
inevitable. This leads to secondary FSGS by compensatory changes,
which negatively affect the structure and function of the
glomeruli. Thus, from the pathophysiological perspective, the renal
pathology of patients may indeed demonstrate FSGS. However, the
study by Valizadeh et al (10)
did not repeat the renal biopsy following the patient's MM
diagnosis and did not perform Congo red staining or light chain
immunological testing on the original pathological sections.
Therefore, the pathological changes in the kidney could not be
confirmed following the MM diagnosis.
Studies on the association between monoclonal
gammopathies and FSGS are rare. Only nine publications were
identified in a retrospective review of the English-language
literature (Table III). Of these
studies, three suggested that there was little or no correlation
between FSGS and plasma cell proliferative disorders. In a study by
Shah et al (12), the NS of
the patient was not resolved by hormone therapy, however, the
patient's smoldering MM did improve. Paueksakon et al
(1) identified that 13 out of 87
(14.9%) patients with MGUS and renal damage had FSGS-like lesions,
and thus, the FSGS was not considered to be primary. Charney and
Wasser (11) demonstrated in their
study population that obesity and sleep apnea were more relevant to
FSGS. These studies did not identify a correlation between these
two diseases, as there was no evidence of MM-induced renal damage,
such as amyloidosis, cast nephropathy or plasma cell infiltration.
However, a secondary FSGS diagnosis does not require renal damage
from the primary disease. In addition, the treatment of MM in the
study by Charney and Wasser (11) was
not continuous, remission rates were poor and renal failure also
progressed to end-stage renal disease. All of these factors support
the notion that the two diseases are associated. While the patients
in a study by Shah et al (12)
exhibited a reduction in serum monoclonal protein and light chain
protein levels following the use of hormones for 3 months, the
proteinuria did not resolve. It is possible that the improvement in
secondary FSGS required a longer time than that required to control
the primary disease. Treatment was subsequently commenced with
cyclophosphamide, which increased the remission rate for MM
compared to cyclosporine, and improved the FSGS. The improved
therapeutic effect of cyclophosphamide on FSGS during MM supports
the association between FSGS and MM. The correlation between FSGS
and MM becomes clearer when patients use pamidronate. Treatment of
osteolytic complications in MM patients using pamidronate can
result in the collapsing variant of FSGS (17). The simultaneous occurrence of FSGS and
MM is not a coincidence; Dingli et al (2) reported the cases of 13 patients who were
diagnosed with syndromes secondary to plasma cell proliferative
disorders (four cases of MM and nine cases of MGUS). For all 13
cases, renal damage improved following treatment of the primary
diseases and recurred with the relapse of the primary diseases.
Therefore, a common pathogenic link between MGUS and MM is
plausible.
 | Table III.Major characteristics of the nine
publications studying MG combined with FSGS. |
Table III.
Major characteristics of the nine
publications studying MG combined with FSGS.
|
|
| Age, years |
|
|
|
|
|
|
|---|
|
|
|
|
|
|
|
|
|
|
|---|
|
|
|
|
|
| Renal feature |
|
|
|
|---|
|
|
|
|
|
|
|
|
|
|
|---|
| First author/s
(ref.) | Gender | Plasma cell
disorder | FSGS/renal
disease | Plasma cell
disorder | Clinical | Pathological | Relevance | Treatment | Prognosis of renal
impairment |
|---|
| Dingli et al
(2) | Male (n=11) Female
(n=2) | 64 | 65 | MGUS (n=9) Myeloma
(n=4) | NS (n=3) Nephrotic
proteinuria >3 g/24 h (n=6) | No collapsing variant
FSGS | Y | Melphalan and
prednisone (n=2) Intermittent dexamethasone then high-dose
melphalan and autologous stem cell transplantation (n=2) | Proteinuria decreased
in 4 patients and stabilized in 3 of the 4; relapse of myeloma was
associated with increasing proteinuria in 1 patient |
| Shah et al
(5) | Female | 51 | 51 | IgAκ MM | Scr 3.5 mg/dl, Ccr
46 ml/min and NS (Alb, 23 g/l; urine protein, 32.6 g/24 h) | Collapsing variant
FSGS | Y | i) Symptomatic
treatment ii) Four cycles of VAD chemotherapy iii) High-dose
melphalan therapy with stem cell rescue six months after VAD
chemotherapy | 14 months after
initial presentation, patient was in remission of NS; renal
function had improved (Scr 1.4 mg/dl) |
| Charney and Wasser
(11) | Male | 36 | 31 | IgGκ MM | Scr 1.7 mg/dl,
urine protein 9.5 g/24 h | 1. FSGS with
hyalinosis, likely secondary, 2. Monoclonal plasma cell
infiltrate | N | Three cycles of
VAD | Renal function
continually deteriorated to the point that vascular access was
instituted for hemodialysis therapy |
| Jain et al
(6) | Male | 70 | 72 | MGUS | Scr increased from
1.1 mg/dl to 5 mg/dl within four months and nephrotic range
proteinuria (PCR 4.8 g/mg) | Collapsing variant
FSGS | Y | Unknown | Scr 6.4 mg/dl on
discharge |
| Ashrafi et
al (7) | Male | 58 (9 months after
FSGS diagnosis) | 58 | κ MM | 1. Scr 1.1 mg/dl,
urine protein 2.2 g/24 h 2. ARF diagnosed 9 months after FSGS | Tip variant
FSGS | Y | Thalidomide,
dexamethasone, pamidronate and autologous bone marrow
transplantation | After one week of
treatment, the patient's Scr had decreased |
| Torun et al
(8) | Male | 44 | 46 | κ light MGUS | Scr 1.7 mg/dl, Ccr
49 ml/min and NS (urine protein 21.3 g/24 h) | FSGS | Y | Immunosuppressive
treatment and autologous bone marrow transplantation | Scr 1.4 mg/dl and
remission of NS |
| Matsuyama et
al (9) | Female | 40 | 40 | IgG κ MGUS | CRF and NS | FSGS with
widespread deposition of large crystalline inclusions in
podocytes | Y | Unknown | Unknown |
| Shah et al
(12) | Male | 62 | 62 | SMM | Scr 0.8 mg/dl and
urine protein 4.3 g/24 h | Collapsing variant
FSGS | N | Glucocorticoids and
cyclophosphamide | Urine protein 3.8
g/24 h |
| Valizadeh et
al (10) | Male | 63 (7 months after
FSGS diagnosis) | 63 | MM | Proteinuria,
hematuria and renal failure | FSGS | Y | Unknown | Unknown |
Vascular endothelial growth factor (VEGF) and
heparanase can change the glomerular permeability and affect the
glomerular basement membrane function to cause proteinuria in FSGS
(18). VEGF and heparanase are
overexpressed in MM patients, and VEGF can induce the growth of
bone marrow blood vessels (19,20)
Heparanase plays important roles in tumor growth, angiogenesis and
metabolism. Furthermore, these two proteins are involved in the
occurrence and development of MGUS and MM (19,20).
Dingli et al (2) used a causal
inference epidemiological method to note the close association
between MGUS, MM and FSGS.
The present study indicates that FSGS patients
>40 years old should undergo serum and urinary immune
electrophoresis, Congo red staining, and κ- and λ-light chain level
analysis routinely to detect plasma cell proliferative disorders
and improve the prognosis of potentially associated FSGS.
Additionally, repeated renal pathological examinations may be
required for a small fraction of patients.
Acknowledgements
This study was supported by the pathologists of the
State Key Laboratory of Kidney Disease in the Chinese People's
Liberation Army General Hospital. Additionally, this study was
partially supported by the Army Medical Science Youth Development
Project (grant no. 13QNP180) and the Project of Chinese PLA 309
Hospital (grant no. 2014MS-006). The authors would like to thank
Professor Zhang Xue-Guang and Mr. Yin Zhong for sharing their
pathological materials.
References
|
1
|
Paueksakon P, Revelo MP, Horn RG, Shappell
S and Fogo AB: Monoclonal gammopathy: Significance and possible
causality in renal disease. Am J Kidney Dis. 42:87–95. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dingli D, Larson DR, Plevak MF, Grande JP
and Kyle RA: Focal and segmental glomerulosclerosis and plasma cell
proliferative disorders. Am J Kidney Dis. 46:278–282. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gertz MA: Utility of the immunoglobulin
free light chain assay for plasma cell disorders 2015. Leuk
Lymphoma. March 27–2015.((Epub ahead of print))
|
|
4
|
Deegens JK, Steenbergen EJ and Wetzels JF:
Review on diagnosis and treatment of focal segmental
glomerulosclerosis. Neth J Med. 66:3–12. 2008.PubMed/NCBI
|
|
5
|
Shah S, Cavenagh J, Sheaf M and
Thuraisingham RC: Remission of collapsing focal segmental
glomerulosclerosis following chemotherapy for myeloma. Am J Kidney
Dis. 43:e10–e12. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jain N, Tantaco MR, Thomas B and Laut J:
Monoclonal gammopathy of uncertain significance (MGUS) and focal
segmental glomerulosclerosis (FSGS) - A case report. Am J Kidney
Dis. 55:B562010. View Article : Google Scholar
|
|
7
|
Ashrafi F, Mortazavi M, Manouchehri N,
Moghaddam NA, Nasri H and Sarrami AH: Focal segmental
glomerulosclerosis, secondary amyloidosis and multiple myeloma. Pak
J Med Sci. 28:345–347. 2012.
|
|
8
|
Torun D, Canpolat T and Özelsancak R:
Focal Segmental Glomerulosclerosis in a patient associated with
kappa-light chain disease. Turk Neph Dial Transpl. 20:96–98. 2011.
View Article : Google Scholar
|
|
9
|
Matsuyama N, Joh K, Yamaguchi Y, et al:
Crystalline inclusions in the glomerular podocytes in a patient
with benign monoclonal gammopathy and focal segmental
glomerulosclerosis. Am J Kidney Dis. 23:859–865. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Valizadeh N, Makhdomi K, Noroozinia F and
Behzadi F: Multiple myeloma presenting with focal segmental
glomerulosclerosis. South Asian J Cancer. 2:1682013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Charney DA and Wasser W: A 36-year-old man
with a monoclonal gammopathy and nephrotic syndrome. Am J Kidney
Dis. 42:1097–1101. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shah R, Shah N, Shah A and Mehta AN:
Steroid-resistant nephrotic syndrome secondary to primary focal
segmental glomerulosclerosis and smoldering multiple myeloma. Proc
(Bayl Univ Med Cent). 27:19–21. 2014.PubMed/NCBI
|
|
13
|
Belghiti D, Vernant JP, Hirbec G, Gubler
MC, Andre C and Sobel A: Nephrotic syndrome associated with T-cell
lymphoma. Cancer. 47:1878–1882. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hyman LR, Burkholder PM, Joo PA and Segar
WE: Malignant lymphoma and nephrotic syndrome. A clinicopathologic
analysis with light, immunofluorescence, and electron microscopy of
the renal lesions. J Pediatr. 82:207–212. 1973. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bassan R, Rambaldi A, Abbate M, et al:
Association of NK-cell lymphoproliferative disease and nephrotic
syndrome. Am J Clin Pathol. 94:334–338. 1990.PubMed/NCBI
|
|
16
|
Bose B and Cattran D: Toronto
Glomerulonephritis Registry: Glomerular diseases: FSGS. Clin J Am
Soc Nephrol. 9:626–632. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nasr SH, Preddie DC, Markowitz GS, Appel
GB and D'Agati VD: Multiple myeloma, nephrotic syndrome and
crystalloid inclusions in podocytes. Kidney Int. 69:616–620. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Brenchley PE: Vascular permeability
factors in steroid-sensitive nephrotic syndrome and focal segmental
glomerulosclerosis. Nephrol Dial Transplant. 18(Suppl 6):
vi21–vi25. 2003.PubMed/NCBI
|
|
19
|
Rajkumar SV and Kyle RA: Angiogenesis in
multiple myeloma. Semin Oncol. 28:560–564. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rajkumar SV, Mesa RA, Fonseca R, et al:
Bone marrow angiogenesis in 400 patients with monoclonal gammopathy
of undetermined significance, multiple myeloma, and primary
amyloidosis. Clin Cancer Res. 8:2210–2216. 2002.PubMed/NCBI
|