Introduction
Lung cancer is one of the most common causes of
cancer-associated mortality worldwide, with a 5-year survival rate
of only ~15% (1,2). Approximately 80% of lung malignancies
are non-small cell lung cancer (NSCLC) (3), and >50% of these patients have
advanced invasion and/or metastasis, which require further
postoperative treatment, including chemotherapy, radiotherapy and
biotherapy. Although the prognosis remains poor, significant
improvements have been made in the efficacy of lung cancer
treatments, with molecular targeted therapy being one of the most
efficient therapies (4–7). Previous studies have demonstrated that
molecular targeted therapy may enhance the antitumor effect of
radiotherapy by inducing apoptosis or inhibiting proliferation of
tumor cells (8,9). However, the radiosensitivity effect of
molecular targeted therapy remains to be fully elucidated.
Endostar is a recombinant human endostatin, which
was approved by the Food and Drugs Administration of China in 2005
for use in the treatment of non-small-cell lung cancer (10). It specifically induces apoptosis and
potently inhibits endothelial cell proliferation, angiogenesis and
tumor growth (10,11). However, the enhanced antitumor effect
of Endostar in combination with radiotherapy is not completely
understood.
Hypoxia, a pathophysiological characteristic of
solid malignancies, interferes with the fixation of DNA damage and
is, therefore, a major cause of resistance to irradiation (12). A previous study of our group revealed
that Endostar is involved in the radiosensitivity of lung cancer by
inhibiting the expression of hypoxia-inducible factor 1α (HIF-1α)
(13,14). In cancer cells, HIF-1α induces the
expression and enhances the activity of several glycolytic proteins
that differ from those detected in nonmalignant cells, including
transporters (glucose transporters 1 and 3) and enzymes (hexokinase
I and II, and phosphofructokinase-L) (15). Carbonic anhydrase IX (CA IX)
contributes to the acidification of the tumor environment by
efficiently catalyzing the hydration of carbon dioxide to
bicarbonate and protons with its extracellularly situated active
site. CA IX expression is strongly induced by hypoxia, which is
present in numerous tumors and regulated by the transcription
factor, HIF (16).
The present study hypothesized that the antitumor
effect of the antigenic agent, Endostar, in combination with
radiotherapy was associated with glycolysis and changes of tumor
environment pH. In order to investigate the possible mechanism
responsible for the enhanced tumor killing effect of Endostar and
radiotherapy, changes in the metabolism and hypoxic tumor fraction
were determined in a Lewis lung carcinoma (LLC) mouse model. LLC
mouse model is a commonly-used model in lung cancer research, since
it is easy to produce, with low cost and high rate of tumor
formation. This model has favorable conditions for the study of the
pathogenesis of lung cancer, drug treatments and biological
treatments. To the best of our knowledge, the present study is the
first to investigate the combination of Endostar administration and
radiotherapy in a LLC mouse model.
Materials and methods
Tumor model and groups
Inbred C57BL/6 male mice (age, 6–7 weeks; weight,
18–22 g) were purchased from the Experimental Animal Center of
Wuhan University (SYXK2003-0013; Wuhan, China). Animals were bred
in a barrier-free animal house in the First Clinical College of
Wuhan University Laboratory Animal Center (SPFIII; Wuhan, China).
This study was performed in strict accordance with the
recommendations of the Guide for the Care and Use of Laboratory
Animals (8th edition, 2011). The Animal Use Protocol was reviewed
and approved by the Institutional Animal Care and Use Committee at
Renmin Hospital of Wuhan University.
An LLC model was established by adopting a
tumor-bearing tumor cell inoculation in vitro method. LLC
tumor cells (purchased from the Chinese Academy of Medical
Sciences, Beijing, China) were inoculated in C57BL/6 mice. When a
tumor volume of ~0.125×0.125×0.125 cm was developed on the right
shoulder, the mice were sacrificed and the tumor tissue was removed
and dispersed into a cell suspension by the enzymatic hydrolysis
method. Briefly, tumor tissue was hydrolyzed with 1% collagenase VI
(Sigma-Aldrich, St. Louis, MO, USA), incubated at 37° for 50 min,
pipetted and filtrated. Next, single cell suspensions were
collected in centrifuge tubes and centrifuged at 500 × g for 5 min;
then, the supernatant was discarded, the cells were resuspended
with phosphate-buffered saline (Beyotime Institute of
Biotechnology, Shanghai, China), and the cell suspensions were
prepared.
Subsequently, C57BL/6 mice were subcutaneously
injected with 0.2 ml carcinoma cell suspension (2×106
living cells) into the left armpit. When the maximum tumor diameter
reached 10 mm (after 7–10 days), 192 tumor-bearing mice were
randomly divided into four groups (n=48 in each group) as follows:
control; Endostar (ES); radiotherapy (RT); and radiotherapy plus
Endostar (ES + RT) groups. Six subgroups were formed according to
the different time points at which the mice were sacrificed (days
2, 4, 6, 8, 10 and 12). The mice received treatment once per
day.
Mice in the control group were subjected to
subcutaneous injection of 0.2 ml 0.9% normal saline. In the ES
group, the mice were subjected to subcutaneous injection of 0.2 ml
Endostar (2 mg/ml). The mice in the radiotherapy group were
subjected to subcutaneous injection of 0.2 ml 0.9% normal saline on
the aforementioned time points (days 2, 4, 6, 8, 10 and 12),
followed by 2 Gy radiation that was topically used on the tumor
between days 6 and 10. Mice in the ES + RT group were subjected to
subcutaneous injection of 0.2 ml Endostar (2 mg/ml), followed by 2
Gy radiation that was topically used on the tumor between days 6
and 10.
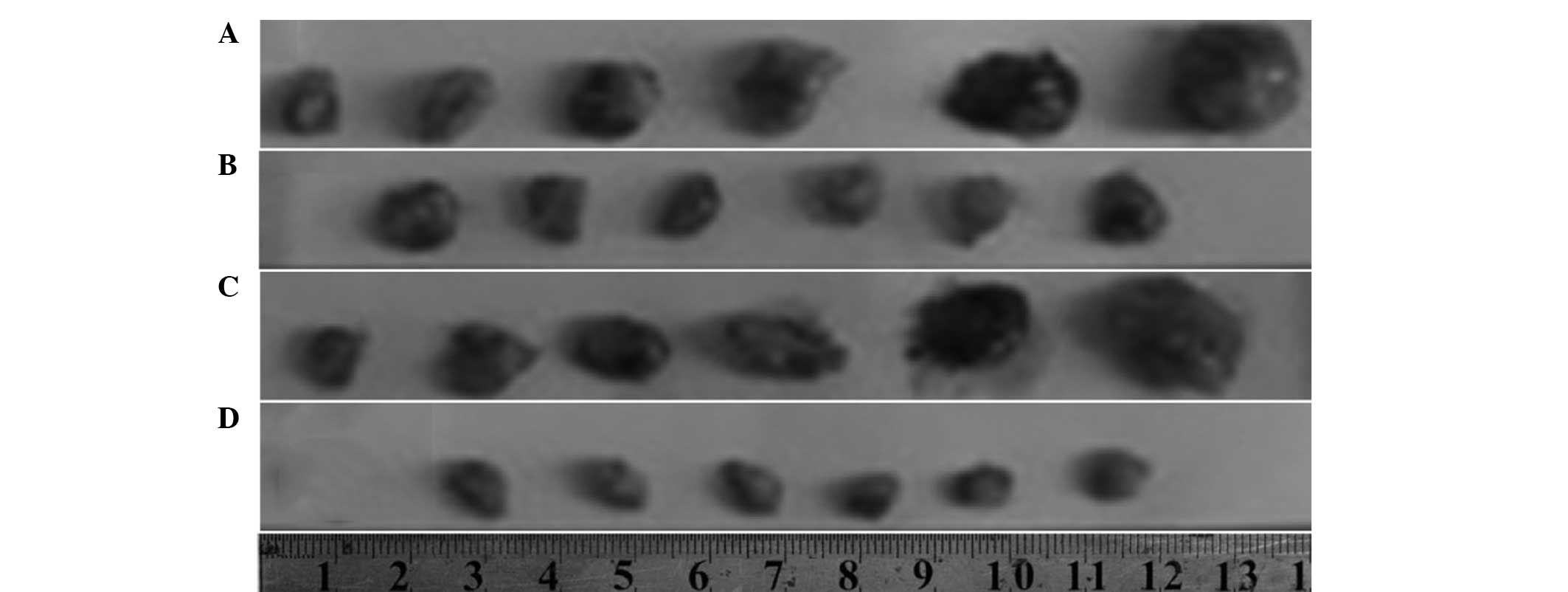
Tumor volume
When the tumor model was established (maximum tumor
diameter, ~10 mm), the tumor length and diameter were determined
with a vernier caliper at aforementioned time points (days 0, 2, 4,
6, 8, 10 and 12). The tumors volume was measured prior to treatment
in each group. Next, 4 mice from each group were sacrificed and
soaked for 3–5 min in 75% ethanol; then, the right shoulder was
cut, and the tumor was removed and washed twice with saline. The
tumor tissue samples were stored at −80°C for follow-up
experiments.
Tumors in different groups and subgroups were
separated following the treatment termination. Tumor volumes were
calculated according to the formula V=a x
b2 × 0.52, where a is the longest diameter
and b is the maximum transverse diameter. Subsequently,
growth curves were constructed using Microsoft Office Excel 2010
(Microsoft Corporation, Redmond, WA, USA) and SPSS 16.0 software
(SPSS, Inc., Chicago, IL, USA).
Ultraviolet (UV) enzymatic
analysis
The tumor tissue samples were ground, homogenized in
cold HClO4 (4°C) and centrifuge at 500 × g for 10 min.
Next, the tissue supernatant was used to measure lactate levels
with a UV spectrophotometer (UV-2450/2550; Shimadzu Corporation,
Tokyo, Japan). The lactate levels were calculated using the optical
density values at 340 nm, which required the use of nicotinamide
adenine dinucleotide, L-lactate dehydrogenase (L-LDH) and alanine
transaminase (Sigma-Aldrich).
Reverse transcription-polymerase chain
reaction (RT-PCR)
Total RNAs were isolated from the tumor tissues
using TRIzol® reagent (Life Technologies, Grand Island, NY, USA),
extracted using chloroform and precipitated using ice-cold
isopropanol. Next, cDNA was synthesized from ~1 µg mRNA using the
ReverTra Ace qPCR RT kit (Toyobo Corporation, Osaka, Japan)
according to the manufacturer's instructions. The primer sequences
used for PCR were as follows: β-actin forward,
5′-CACGATGGAGGGGCCGGACTCATC-3′, and reverse,
5′-TAAAGACCTCTATGCCAACACAGT-3′; and LDH forward,
5′-TGGCAGCCTCTTCCTTAAAA-3′, and reverse,
5′-CAGCTTGCAGTGTGGACTGT-3′. Quantitative RT-PCR was performed using
the Thunderbird SYBR® qPCR Mix (QPS-201, QPS-201T; Toyobo
Corporation). All the reactions were prepared in 10 ml samples
using the standard PCR conditions according to the manufacturer's
instructions. β-actin was used as a control.
pH
The mice were anesthetized by intraperitoneal
injection of 0.15 ml 1.5% sodium pentobarbital (Sigma-Aldrich).
Next, a 1.0 cm incision was made in the tumor. A pH microelectrode
(Meph-3 pH meter, PH-016; Shanghai Sunlight Opto Device Co., Ltd.,
Shanghai, China) was inserted 4.0 mm into the tumor tissue in order
to detect the pH.
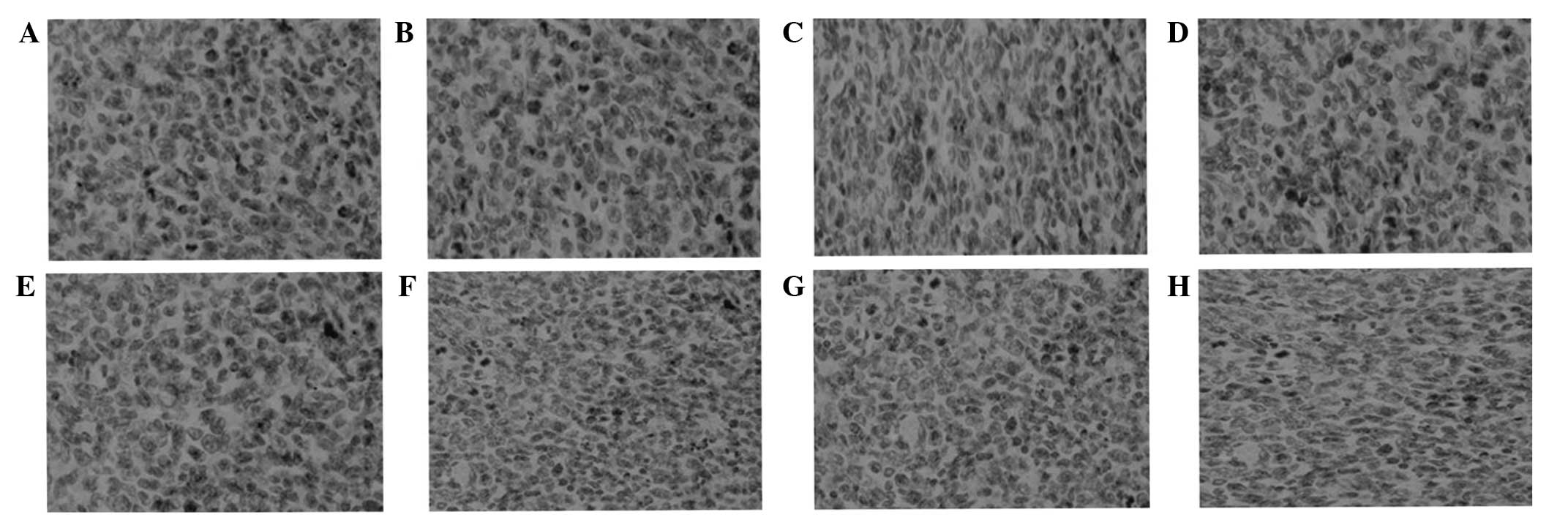
Immunohistochemical analysis
Hypoxyprobe™-1 kit (Hypoxyprobe, Inc., Burlington,
MA, USA), containing the anti-pimonidazole mouse immunoglobulin G1
monoclonal antibody, was used to detect tumor hypoxia. Hematoxylin
and eosin, as well as substance P, were used for
immunohistochemical analysis. Hypoxia in tumors was detected
through the formation of pimonidazole adducts (11) and the Olympus CX21 microscope (Olympus
Corporation, Tokyo, Japan) was used to identify the hypoxic tumor
cells. Next, pimonidazole hydrochloride was intraperitoneally
injected at a dose of 60 mg/kg. At 1 h after injection, the mice
were sacrificed, and the tumor tissue was prepared and detected
following the manufacturer's instructions.
Statistical analysis
All the results are expressed as the mean ± standard
error of mean and were analyzed using SPSS 16.0 software (SPSS,
Inc., Chicago, IL, USA). The F-test was applied to assess
differences between the groups. In all the statistical analyses,
P<0.05 was considered to indicate a statistically significant
difference.
Results
Tumor growth
Compared with the control group, treatment with
Endostar alone was found to only slightly inhibit the tumor
proliferation (ES group; P>0.05). However, treatment with
Endostar in combination with 2 Gy radiation significantly
suppressed increases in tumor volume between days 6 and 12 (ES +RT
group; P<0.05). Differences in tumor growth inhibition between
the ES + RT and RT groups were also statistically significant
between days 6 and 10 (P<0.05). These results indicated that the
ES + RT group exhibited increased tumor growth inhibition when
compared with the other three groups (Fig. 1).
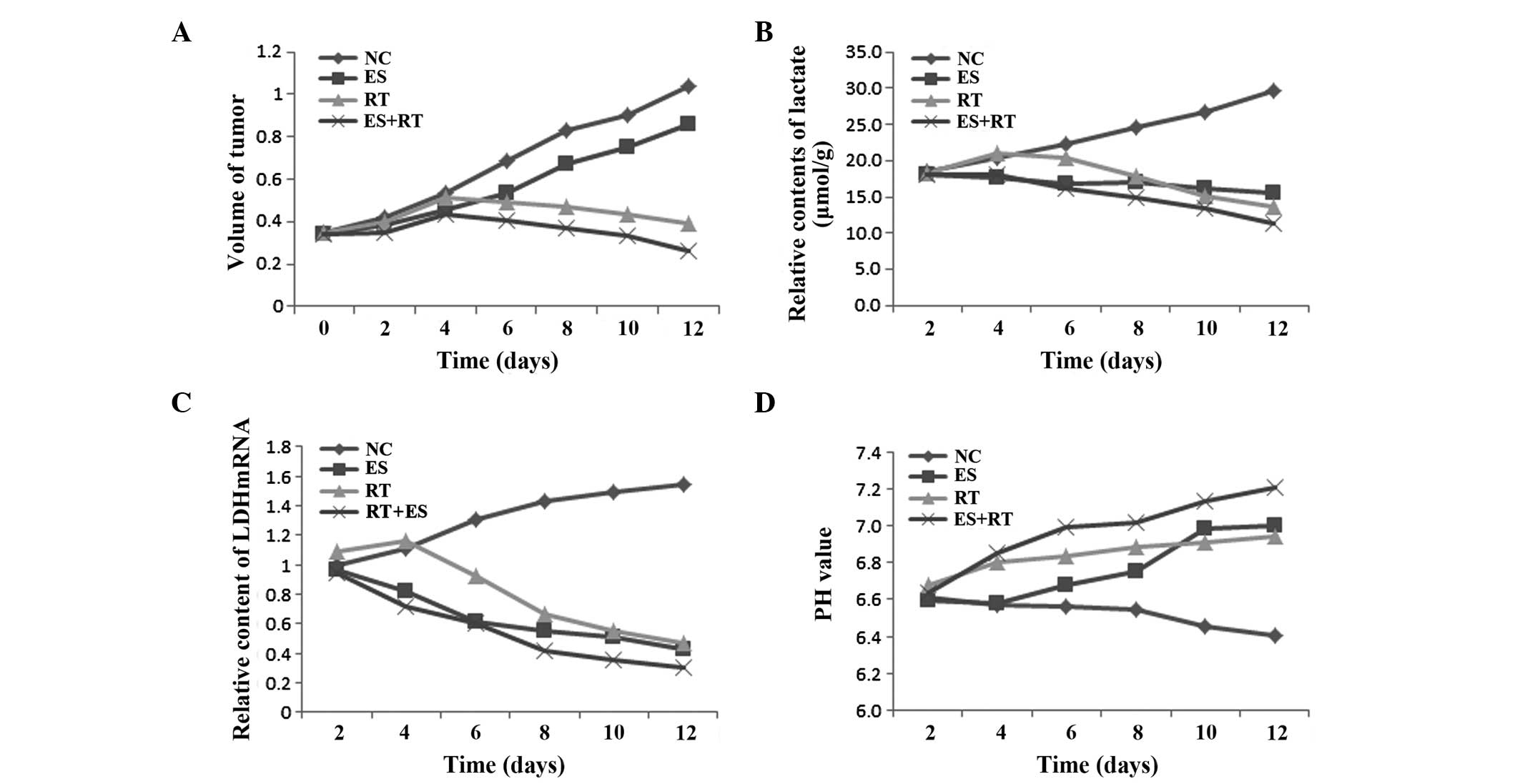
Lactate levels
Lactate is considered a dead-end product of
glycolysis, and its generation and accumulation promote tumor
growth and metastasis. With the recent advances in tumor metabolism
and gene therapy, lactate was identified as a potential therapeutic
target in tumors (17–20). Tumor cells can also uptake and utilize
lactate, and a high concentration of lactate is a sign or marker of
tumor metabolic adaptation, suggesting a poor prognosis
(17–20).
As shown in Fig. 2,
the lactate levels in the ES, RT and ES+RT groups were
significantly lower compared with the control group between days 6
and 10 (P<0.05). In addition, the lactate level in the ES + RT
group was reduced the most, when compared with the ES and RT groups
(P<0.05). The decreased lactate levels show that the treatments
reduced tumor metabolism and inhibited tumor growth.
LDH mRNA
As shown in Fig. 2C,
the LDH mRNA expression in each group significantly decreased
between days 6 and 10, when compared with the control group
(P<0.05). The expression of LDH mRNA in the ES + RT group was
lower compared with the ES and RT groups, and the difference was
statistically significant (P<0.05). These results show that the
RT, ES and ES + RT treatments reduced the in vivo expression
levels of LDH in the tumor tissues. Thus, ES+RT treatment with
radiotherapy may exhibit a synergistic effect in the regulation of
tumor metabolism.
pH
All the groups exhibited an increasing trend in the
tumor pH values between days 6 and 10, when compared with the
control group (P<0.05). As shown in Fig. 4, the changes in pH between days 6 and
10 also indicated that the ES + RT group presented a significantly
increased pH when compared with the ES and RT groups (P<0.001).
The increased pH value shows that the treatments reduced the
acidification of the tumors.
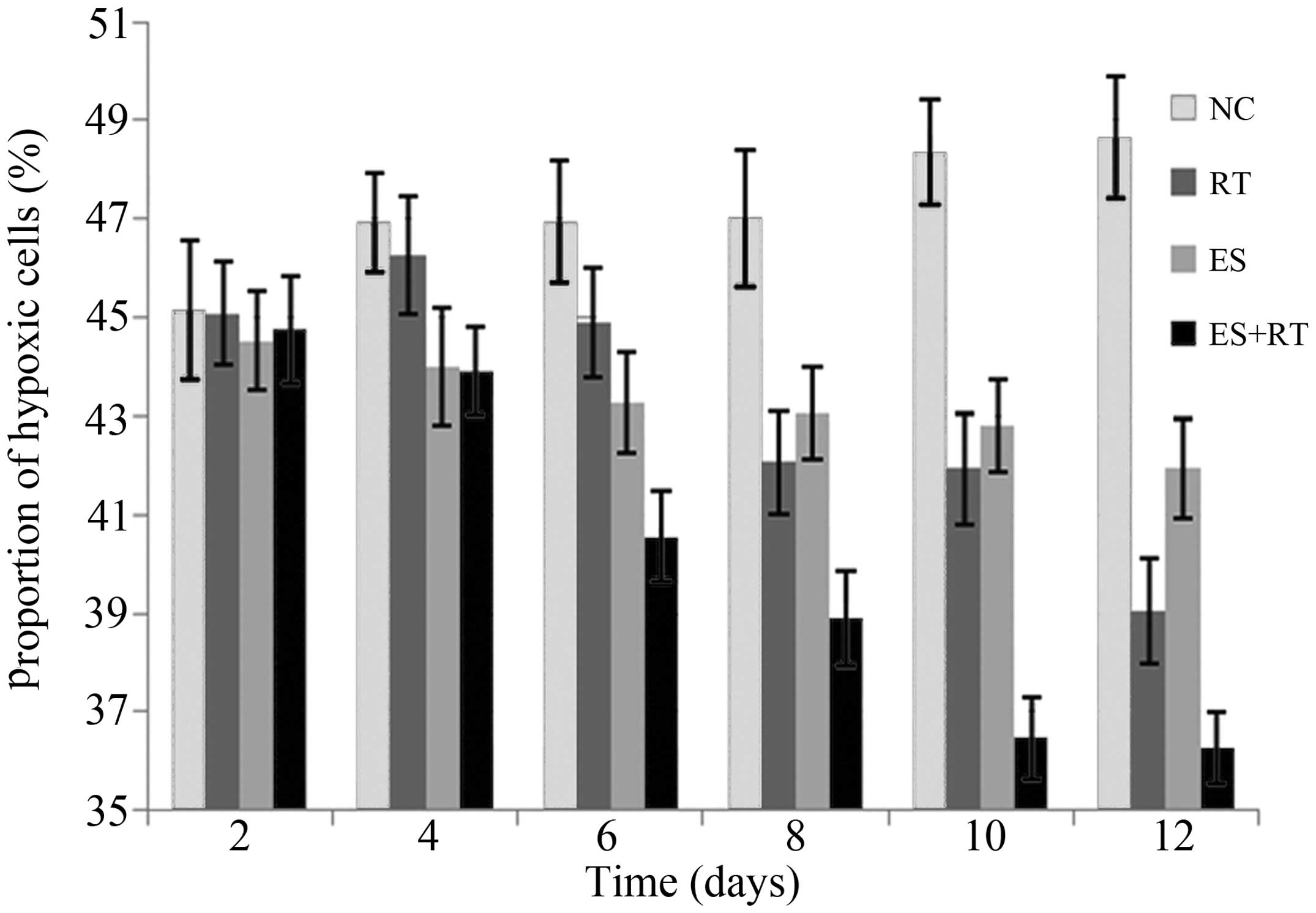
Hypoxic cell fraction
Following immunohistochemical analysis, tumor cells
exhibiting brown particles in the cytoplasm were identified as
hypoxic tumor cells and assessed under a microscope (Figs. 3 and 4).
The results indicated that the hypoxic cell fractions in the
treatment groups were significantly decreased when compared with
the control group after five days of treatment (P<0.05),
particularly in the ES+RT group. The hypoxic cell fraction in the
ES+RT group was markedly lower compared with the ES and RT groups,
and the difference was statistically significant (P<0.001). The
decreased hypoxic cell fraction indicates that the treatments
reduced hypoxia in the tumor micro-environment.
Discussion
The cellular metabolism of lung cancer tissues is
markedly different from that of normal tissues. Major metabolic
changes are known as ‘aerobic glycolysis’, and are accompanied with
hypoxia and an acidulated tumor microenvironment (21,22).
Micro-environmental hypoxia in tumor cells is one of the causes of
resistance to chemotherapy and/or radiation in solid tumors
(23–25). For instance, hypoxia induces the
expression of multidrug resistance gene 1 in the tumor, increasing
the resistance of tumor cells to chemotherapeutic drugs. In
addition, hypoxia and HIF-1 are able to induce the expression of
various tumor genes, resulting in tumor cells tolerance to
radiotherapy. Consequently, the antitumor effects of radiotherapy
and chemotherapy may be hampered (23–25). In
the present study, an LLC mouse model was used to investigate the
effect of Endostar treatment and radiotherapy. The results
indicated that Endostar may enhance the efficacy of radiation by
reducing hypoxia and acidification in the tumor microenvironment,
finally resulting in suppression of tumor growth.
Treatment with radiation causes generation of
reactive oxygen species, including superoxide radical anions and
hydroxyl radicals (26,27). Thus, an accumulation of antioxidants,
such as lactate, may induce or enhance resistance to radiation
(28). The results of a previous
study that included >1,000 individual xenografts of human head
and neck cancer demonstrated that lactate concentrations are
positively correlated with radioresistance (29). This lactate-associated radioresistance
was hypoxia-independent, indicating that well-oxygenated
high-lactate tumors are radioresistant (30). Tumor cells ensure sufficient oxygen
and nutrient supply for proliferation through lactate-induced
secretion of vascular endothelial growth factor (VEGF), which
results in the formation of new vessels (31).
In the present study, the levels of lactate and LDH
mRNA in the ES+RT group were significantly decreased compared with
the levels in the RT group between days 6 and 10. This result
indicated that Endostar treatment combined with radiotherapy
exhibited a synergistic effect on glycolysis inhibition. Therefore,
Endostar may suppress glycolysis, leading to reduced lactate
production and thereby increased radiosensitivity of the tumor. In
addition, the reduced level of lactate may be responsible for the
decreased expression of VEGF (32).
An acidic microenvironment (decreased pH) may also
result from overgeneration of lactate. Cancer cells produce a large
amount of lactic acid, which is generated through glucose
metabolism and inefficient vascular clearing, resulting in an
acidic microenvironment within solid tumors (33). In tumor cells, the lactate generated
by glycolysis and the carbonic acid catalyzed by CA IX are the
major sources of hydrogen ions (H+) in the extracellular
fluid, reducing the tumor extracellular pH. In the present study,
the pH value in the ES + RT group was significantly improved when
compared with the RT and ES groups between days 6 and 10. This
observation is consistent with the changes in the lactate levels.
Therefore, the status of acidic environment is hypothesized to be
positively associated with glycolysis activity in tumor.
Hypoxic tumor cells are more resistant to
radiotherapy as a consequence of the interference of hypoxia with
the fixation of free radical-induced DNA damage (6). In the present study, immunohistochemical
analysis to detect tumor hypoxia indicated that the hypoxic cell
fraction in the ES+RT group was significantly decreased after day
6. This observation is attributed to the continuous intervention of
anti-angiogenic agents, which weaken glycolysis and improve the
acidic microenvironment. These changes possibly increased the
temporary blood and oxygen supplies to meet the increased tumor
cell metabolism, thereby indirectly increasing the sensitivity of
hypoxic cells to radiation.
Since Jain first proposed the normalization of tumor
vasculature (34), several studies on
Endostar in chemotherapy and radiotherapy sensitivity have been
conducted, which determined the ‘normalization window’ (35–37).
Rh-endostatin may normalize the tumor vasculature and
microenvironment in LLCs, possibly through modulation of the
balance between VEGF-A and thrombospondin-1 (38). During vascular normalization,
treatment with paclitaxel was identified to exhibit a maximal
effect on tumor growth inhibition (38). In addition, Endostar was demonstrated
to normalize tumor vasculature, which alleviated hypoxia and
significantly sensitized the antitumor function of radiation in
human nasopharyngeal cancer (39).
Based on energy metabolism, the present study investigated the
underlying mechanism through which Endostar exhibits a
radiosensitization effect. Further studies are required to
determine whether normalization of tumor vasculature is associated
with changes in the metabolism and microenvironment of tumors.
In conclusion, Endostar may enhance the antitumor
effect of radiation by reducing glycolysis, hypoxia and
acidification of the tumor microenvironment. These results provide
an important experimental basis for the combination of Endostar
with radiotherapy in the treatment of lung cancer.
Acknowledgements
This study was supported by grants from the Key
Program of National Natural Science Foundation of China (nos.
30970860 and 81272500) and the China International Medical
Foundation (no. CIMF-F-H001-001).
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jemal A, Tiwari RC, Murray T, Ghafoor A,
et al: Cancer statistics, 2004. CA Cancer J Clin. 54:8–29. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
No authors listed: Cancer facts and
figures 2004. American Cancer Society. Atlanta, GA: 2004.
|
|
4
|
Reungwetwattana T, Weroha SJ and Molina
JR: Oncogenic pathways, molecularly targeted therapies, and
highlighted clinical trials in non-small-cell lung cancer (NSCLC).
Clin Lung Cancer. 13:252–266. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kobayashi K and Hagiwara K: Epidermal
growth factor receptor (EGFR) mutation and personalized therapy in
advanced nonsmall cell lung cancer (NSCLC). Target Oncol. 8:27–33.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bria E, Bonomi M, Pilotto S, et al:
Clinical meta-analyses of targeted therapies in adenocarcinoma.
Target Oncol. 8:35–45. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ge W, Cao DD, Wang HM, Jie FF, Zheng YF
and Chen Y: Endostar combined with chemotherapy versus chemotherapy
alone for advanced NSCLCs: a meta-analysis. Asian Pac J Cancer
Prev. 12:2705–2711. 2011.PubMed/NCBI
|
|
8
|
Du Y, Peyser ND and Grandis JR:
Integration of molecular targeted therapy with radiation in head
and neck cancer. Pharmacol Ther. 142:88–98. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Koh PK, Faivre-Finn C, Blackhall FH and De
Ruysscher D: Targeted agents in non-small cell lung cancer (NSCLC):
clinical developments and rationale for the combination with
thoracic radiotherapy. Cancer Treat Rev. 38:626–640. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Folkman J: Antiangiogenesis in cancer
therapy - endostatin and its mechanisms of action. Exp Cell Res.
312:594–607. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
O'Reilly MS, Boehm T, Shing Y, et al:
Endostatin: an endogenous inhibitor of angiogenesis and tumor
growth. Cell. 88:277–285. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Meijer TW, Kaanders JH, Span PN and
Bussink J: Targeting hypoxia, HIF-1, and tumor glucose metabolism
to improve radiotherapy efficacy. Clin Cancer Res. 18:5585–5594.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang L, Ge W, Hu K, et al: Endostar
down-regulates HIF-1 and VEGF expression and enhances the
radioresponse to human lung adenocarcinoma cancer cells. Mol Biol
Rep. 39:89–95. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ge W, Zheng Y, Zhang L, et al: Endostar
enhances the radioresponse on Lewis lung carcinoma by regulating
HIF-1α. Biomedical Engineering and Informatics (BMEI), 2011. 4th
International Conference. Ding YS, Peng YH, Shi R, et al: 3:(IEEE,
Shanghai). 1486–1490. 2011. View Article : Google Scholar
|
|
15
|
Marín-Hernández A, Gallardo-Pérez JC,
Ralph SJ, Rodríguez-Enríquez S and Moreno-Sánchez R: HIF-1alpha
modulates energy metabolism in cancer cells by inducing
over-expression of specific glycolytic isoforms. Mini Rev Med Chem.
9:1084–1101. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Winum JY, Rami M, Scozzafava A, Montero JL
and Supuran C: Carbonic anhydrase IX: a new druggable target for
the design of antitumor agents. Med Res Rev. 28:445–463. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yokota H, Guo J, Matoba M, et al: Lactate,
choline, and creatine levels measured by vitro 1H-MRS as prognostic
parameters in patients with non-small-cell lung cancer. J Magn
Reson Imaging. 25:992–999. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bonuccelli G, Tsirigos A, Whitaker-Menezes
D, et al: Ketones and lactate ‘fuel’ tumor growth and metastasis:
Evidence that epithelial cancer cells use oxidative mitochondrial
metabolism. Cell Cycle. 9:3506–3514. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Goetze K, Walenta S, Ksiazkiewicz M, et
al: Lactate enhances motility of tumor cells and inhibits monocyte
migration and cytokine release. Int J Oncol. 39:453–463.
2011.PubMed/NCBI
|
|
20
|
Yaligar J, Thakur SB, Bokacheva L, et al:
Lactate MRSI and DCE MRI as surrogate markers of prostate tumor
aggressiveness. NMR Biomed. 25:113–122. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Warburg O: On respiratory impairment in
cancer cells. Science. 124:269–270. 1956.PubMed/NCBI
|
|
22
|
Denko NC: Hypoxia, HIF1 and glucose
metabolism in the solid tumour. Nat Rev Cancer. 8:705–713. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Comerford KM, Wallace TJ, Karhausen J,
Louis NA, Montalto MC and Colgan SP: Hypoxia-inducible factor-1
dependent regulation of the multidrug resistance (MDR1) gene.
Cancer Res. 62:3387–3394. 2002.PubMed/NCBI
|
|
24
|
Semenza GL: Targeting HIF-1 for cancer
therapy. Nat Rev Cancer. 3:721–732. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Williams KJ, Telfer BA, Xenaki D, et al:
Enhanced response to radiotherapy in tumours deficient in the
function of hypoxia inducible factor-1. Radiother Oncol. 75:89–98.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Masaki H, Okano Y and Sakurai H:
Generation of active oxygen species from advanced glycation
end-products (AGEs) during ultraviolet light A (UVA) irradiation
and a possible mechanism for cell damaging. Biochim Biophys Acta.
1428:45–56. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jagetia GC, Shetty PC and Vidyasagar MS:
Inhibition of radiation-induced DNA damage by jamun, Syzygium
cumini, in the cultured splenocytes of mice exposed to different
doses of γ-radiation. Integr Cancer Ther. 11:141–153. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sattler UG and Mueller-Klieser W: The
anti-oxidant capacity of tumour glycolysis. Int J Radiat Biol.
85:963–971. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sattler UG, Meyer SS, Quennet V, et al:
Glycolytic metabolism and tumour response to fractionated
irradiation. Radiother Oncol. 94:102–109. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Quennet V, Yaromina A, Zips D, et al:
Tumor lactate content predicts for response to fractionated
irradiation of human squamous cell carcinomas in nude mice.
Radiother Oncol. 81:130–135. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hirschhaeuser F, Sattler UG and
Mueller-Klieser W: Lactate: a metabolic key player in cancer.
Cancer Res. 71:6921–6925. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kwasiborski PJ, Kowalczyk P, Mrówka P, et
al: Selected, biochemical markers of hypoxia. Przegl Lek.
69:115–119. 2012.(In Polish). PubMed/NCBI
|
|
33
|
Gatenby RA and Gillies RJ: Why do cancers
have high aerobic glycolysis? Nat Rev Cancer. 4:891–899. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Jain RK: Normalization of tumor
vasculature: An emerging concept of anti-angiogenic therapy.
Science. 307:58–62. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wen QL, Meng MB, Yang B, et al: Endostar,
a recombined humanized endostatin, enhances the radioresponse for
human nasopharyngeal carcinoma and human lung adenocarcinoma
xenografts in mice. Cancer Sci. 100:1510–1519. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Du H, Ge W, Li C, Zhao Z, Xu X and Yang F:
Effects of rh-endostar in combination with radiotherapy on rats
with lung cancer. Zhongguo Fei Ai Za Zhi. 13:386–390. 2010.(In
Chinese). PubMed/NCBI
|
|
37
|
Xu M, Huang H, Xiong Y, Peng B, et al:
Combined chemotherapy plus endostar with sequential stereotactic
radiotherapy as salvage treatment for recurrent esophageal cancer
with severe dyspnea: A case report and review of the literature.
Oncol Lett. 8:291–294. 2014.PubMed/NCBI
|
|
38
|
Huang G and Chen L: Recombinant human
endostatin improves anti-tumor efficacy of paclitaxel by
normalizing tumor vasculature in Lewis lung carcinoma. J Cancer Res
Clin Oncol. 136:1201–1211. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Peng F, Xu Z, Wang J, et al: Recombinant
human endostatin normalizes tumor vasculature and enhances
radiation response in xenografted human nasopharyngeal carcinoma
models. PLoS One. 7:e346462012. View Article : Google Scholar : PubMed/NCBI
|