Introduction
Desmoplastic small round cell tumor (DSRCT) is a
rare but aggressive primitive malignant neoplasm that occurs mainly
in adolescents and young adults (1–3). The
abdomen and pelvis are the sites most likely to be involved, while
DSRCT of the pleura is even more rare. Review of the English
literature revealed that, to date, <15 cases of primary DSRCT in
the pleura (including the present case) have been reported
worldwide (PubMed, http://www.ncbi.nlm.nih.gov/pubmed/) (1–9). Among
these cases, there are few computed tomography (CT) findings of
pleural DSRCT, which have been previously described in detail
(2,3,8,9). The present study describes a rare case
of pleural DSRCT with differential contrast CT findings in a
72-year-old female, and reviews the English literature. Written
informed consent was obtained from the patient.
Case report
A 72-year-old female presented with a history of
left-side chest pain, and dyspnea for six months. There were no
serious illnesses in the patient's past medical history. Physical
examination demonstrated inaudible breath sounds in the left thorax
but no other remarkable abnormal findings.
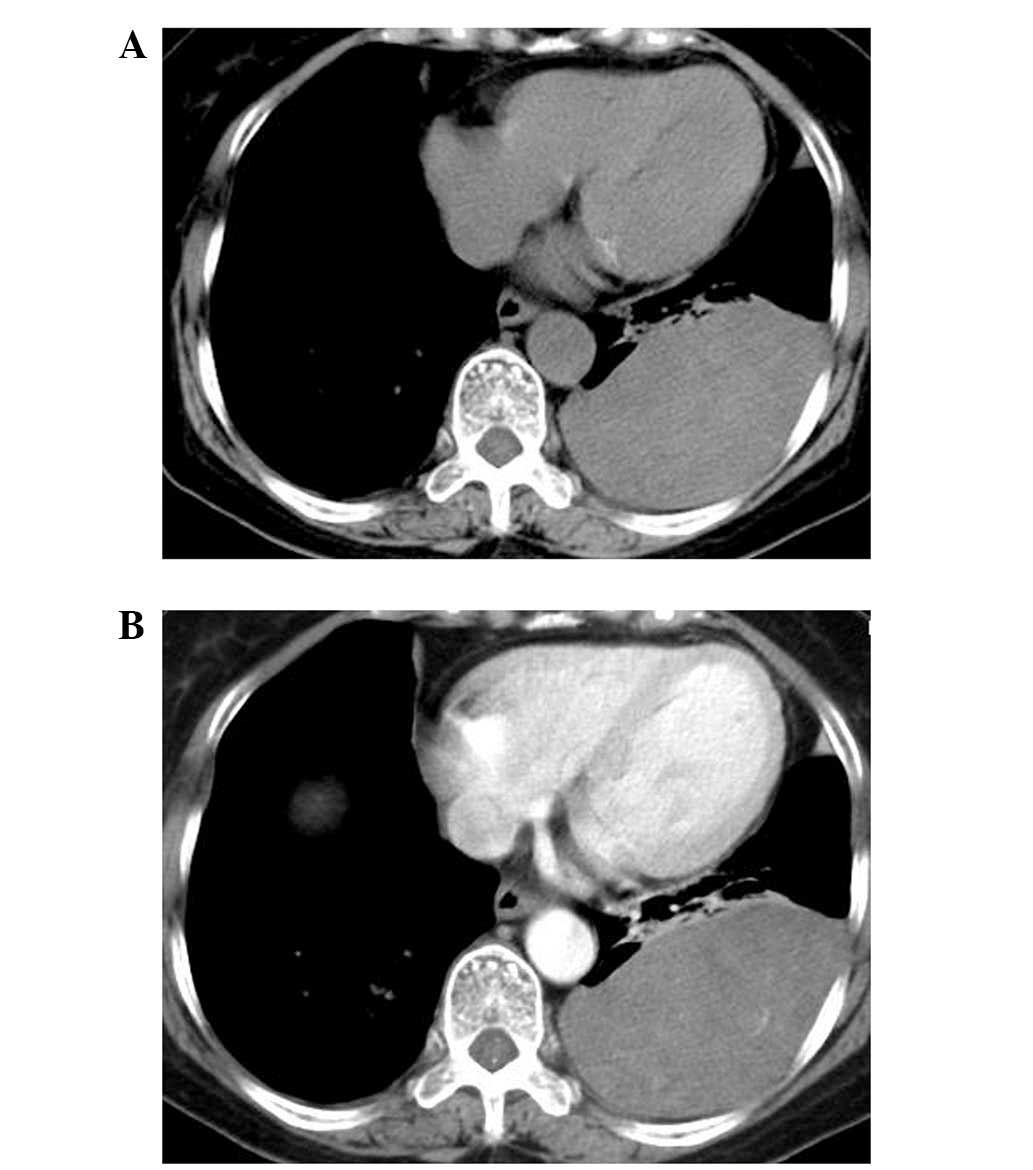
Spiral computed tomography (CT) (Somatom Sensation
16; Siemens, Munich, Germany) findings of the tumor were as follows
(Fig. 1A and B): i) Location, the
tumor was located in the left lower thorax; ii) shape, the tumor
appeared as a large (12.0×10.0×6.5 cm), smooth, oval mass, which
formed obtuse angles with the pleural surface; iii) composition,
the tumor appeared homogenous with low attenuation on plain CT
examination, with a mean CT attenuation value of 28 Hounsfield
units (HU); iv) enhancement, the tumor revealed slight-moderate
unhomogeneous enhancement on contrast-enhanced CT, with a mean CT
attenuation value of 38HU; and v) neighborhood, the adjacent lung
tissues were compressed, and no rib destruction was found, but
several enlarged lymph nodes were identified in the mediastinum.
Based on these findings, localized fibrous tumor of the pleura was
initially considered. Abdominal and pelvic CT scanning identified
no neoplasms.
The patient underwent tumor resection. During the
operation, the mass was not able to be separated from the pleura,
and the basal segment of the left lower lobe was compressed. The
tumor size was ~12.0×10.0×6.0 cm, with a smooth surface, and an
incomplete capsule.
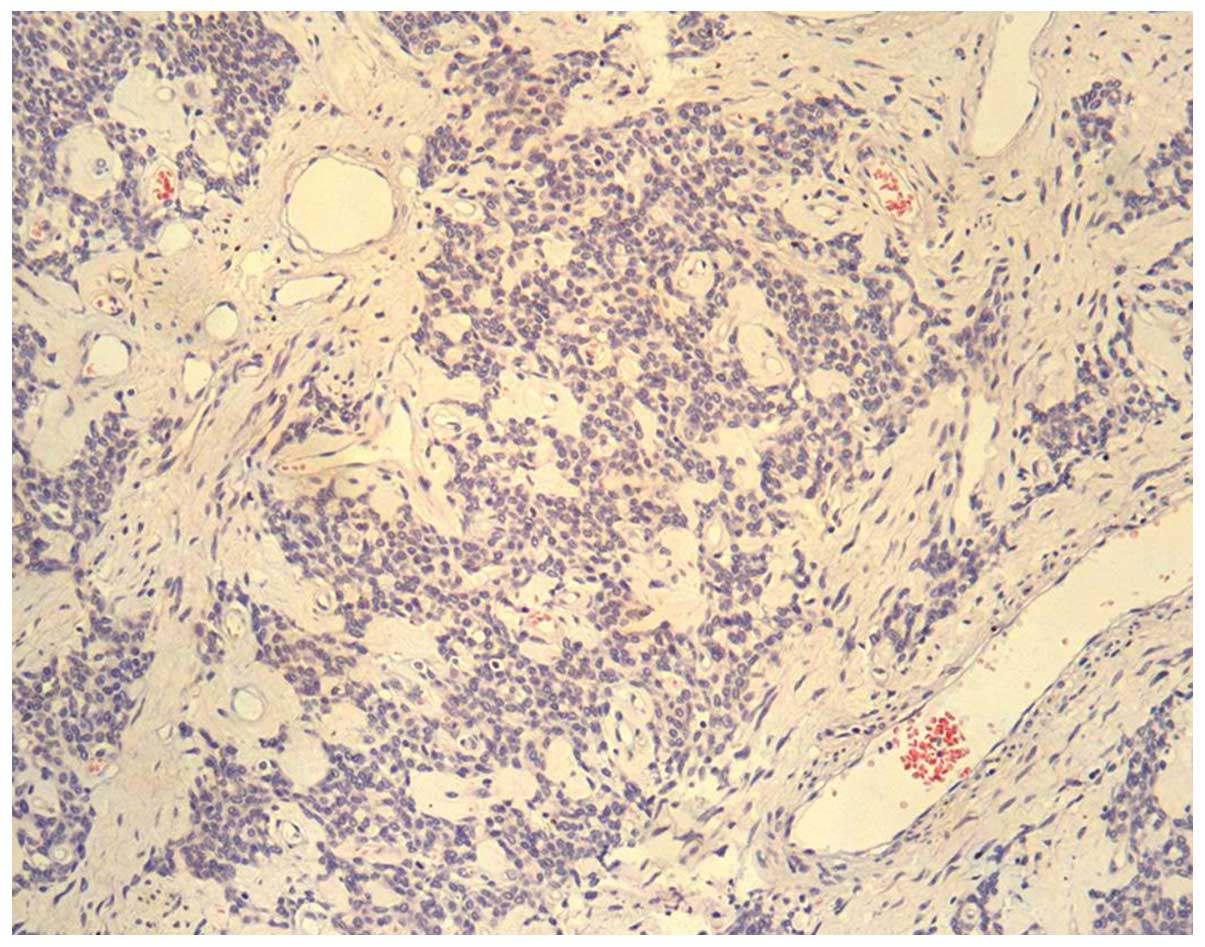
Microscopically, the tumor was composed of small,
round or oval cells, which were generally uniform in size and
shape. Most of the cells were closely packed, with transparent
cytoplasm, pale nuclei and indistinct nucleoli. No mitotic figures
or necrotic cells were detected. The tumor cells were arranged as
beam or nest bulk, surrounded by a dense desmoplastic fibrous
stroma (Fig. 2); the stroma was rich
in vessels, with classic partial thickening of the vascular wall.
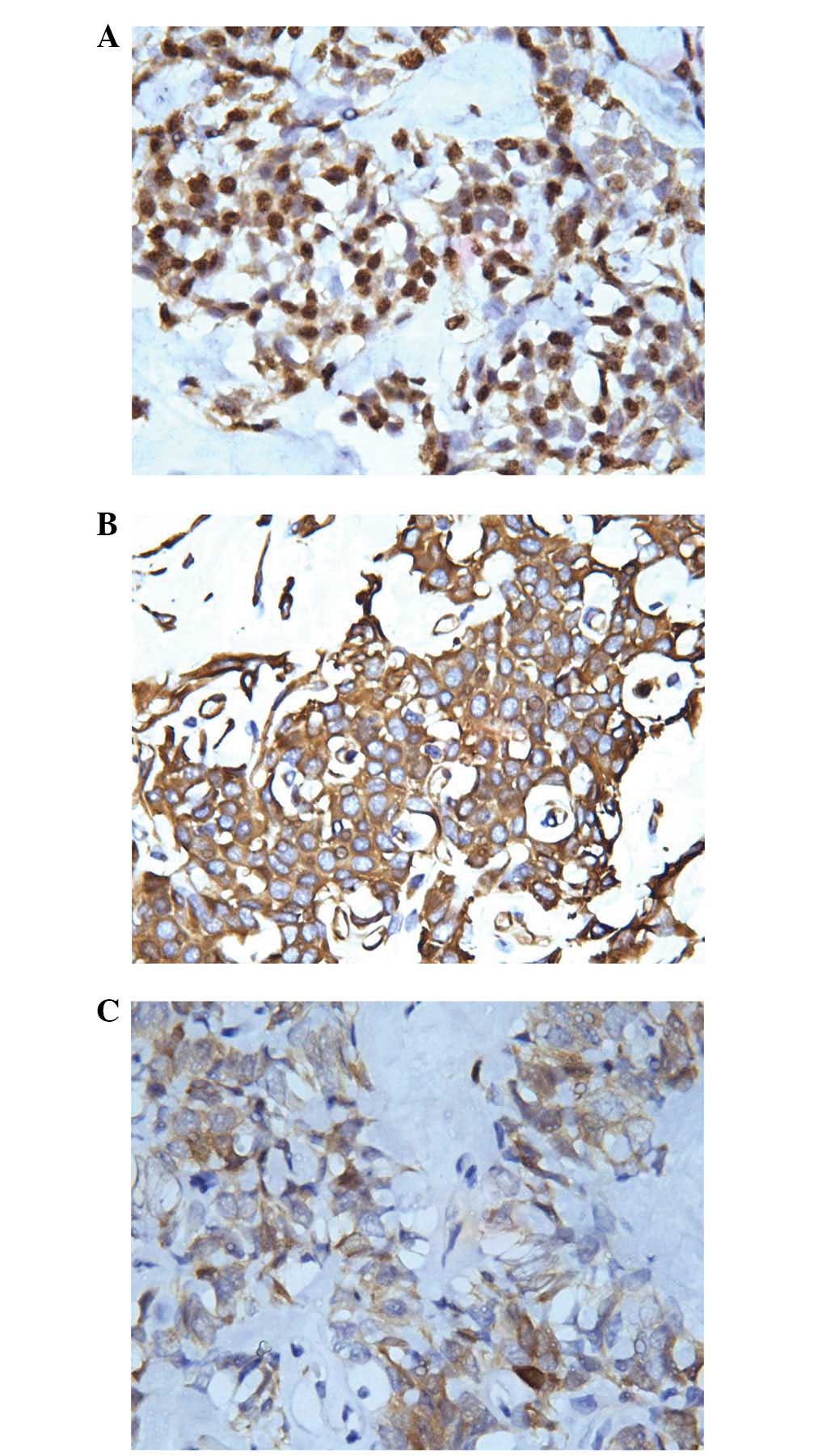
Immunohistochemical study of the tumor revealed: Pan-cytokeratin
(CKpan) (−), CK5/6 (−), epithelial membrane antigen (EMA) (−),
carcinoembryonic antigen (CEA) (−), S100 (−), P63 (−),
neurofilament protein (NF) (−), CD57 (−), CD99 (+), mesothelial
(−), calretinin (+), desmin (−), vimentin (++), smooth muscle actin
(SMA) (+), neuron specific enolase (NSE) (++), CD34 (+), CD15 (−),
B cell lymphoma-2 (Bcl-2) (+), CD56 (+), chromogranin (CgA) (+),
synaptophysin (Syn) (++), adrenocorticotropic hormone (ACTH) (+)
and Wilms' tumor (WT-1) (+). Among these factors, vimentin and NSE
demonstrated dot-like positive positioning in the nucleus adjacent
to the cytoplasm (Fig. 3A–C).
Pathological diagnosis of DSRCT of the pleura was ultimately
ascertained.
Postoperatively, the patient immediately recovered
from her symptoms, refused further chemotherapy or local
radiotherapy and was discharged. There has been no evidence of
recurrence or metastasis during the past 32 months of
follow-up.
Discussion
DSRCT is a rare malignancy first described by Gerald
and Rosai (1) in 1989. DSRCT is
characterized by aggressive behavior and poor prognosis, with a
predominance amongst male patients and increased frequency during
the second and third decades of life (5). Typically, the abdomen and pelvis are the
sites most likely to be involved in DSRCT. DSRCT of the pleura is
extremely rare and, to the best of our knowledge, <15 cases of
primary DSRCT in the pleura (including the present case) have been
reported worldwide (PubMed, http://www.ncbi.nlm.nih.gov/pubmed/) (1–9). The
majority of these cases have been reported in adolescents or young
adults, while the eldest patient in the published literatures was a
29-year-old male. The present article described the case of
72-year-old female with DRSCT in the left pleural cavity, which
therefore represents the eldest patient in the literature to date.
The most common presentation of patients with DSRCT of the pleura
is that of nonspecific chest pain and respiratory symptoms
(2,3).
The primary symptom in the present case was comparable with that of
previous reports. Certain patients may have a history of exposure
to asbestos, smoking and exposure to jute (2). No risk factors or specific causes were
identified in the present case.
Histologically, DRSCTs are characterized by nests of
small round tumor cells embedded in a dense desmoplastic fibrous
stroma. Immunohistochemically, the primary diagnostic feature of
DRSCTs is the coexpression of epithelial, mesenchymal and neural
cell markers, supported by molecular studies which have identified
a specific translocation t (11;22) (p13;q12) unique to this
neoplasm (4,5). Gerald et al (5) indicated that genetic studies are
essential for accurate diagnosis in unclear cases, since these may
identify the characteristic Ewing's sarcoma (EWS)/WT-1 gene fusion
product, which induces transcriptional activation and facilitates
uncontrollable growth of tumor cells (10). The immunohistochemical results of the
present study were mainly consistent with previous observations.
However, the present case of DRSCT was negative for epithelial cell
markers and fewer mitotic figures were identified, compared with
that of previous reports (2,3,8,9). It was hypothesized that these two
factors may be associated with the relatively good prognosis of the
patient in the present case. Unfortunately genetic analysis was not
performed in the present case, so it remains unknown as to whether
the patient possessed the EWS/WT-1 gene fusion.
A number of radiological findings in pleural DSRCT
have previously been described (2,3,8,9); however,
contrast-enhanced CT findings have not been well addressed.
According to the literature on pleural DSRCT, radiological
manifestations are variable (2,3,8,9). Jian
et al (9) and Parkash et
al (2) reported diffuse irregular
or nodular pleural thickening, as well as pleural effusion of
pleural DSRCT on plain CT in adolescents and young adults. Oliveira
et al (8) identified a solid
mass in the superior mediastinum, multiple pulmonary nodules and a
voluminous right pleural effusion, with involvement of the liver
and spleen. Furthermore, Karavitakis et al (3) reported a pediatric case of primary
pleural DSRCT, presenting with a solid paraspinal lesion extending
from vertebrae T-5 to T-12 and invading the 9th and 10th vertebral
bodies, posterior section of the left analogue ribs and epidural
space, which was identified by magnetic resonance imaging. In the
present case, the tumor demonstrated differential imaging findings.
The tumor presented as a large, smooth, oval-shaped solid mass in
the left lower thorax, with slight-moderate unhomogenous
enhancement on contrast-enhanced CT. It was suggested that the
slight-moderate enhancement on the contrast CT scan may be
associated with the dense desmoplastic fibrous stroma around the
tumor cells. To the best of our knowledge, these large solid-mass
radiological manifestations have not previously been described in
English literatures. Unfortunately, no two-phase contrast scan was
used in the present study, so whether the tumor may be further
reinforced in a two-phase contrast scan remains unknown, and
further studies are required in the future. All these imaging
features are summarized in Table
I.
 | Table I.Radiological findings of pleural
desmoplastic small round cell tumor. |
Table I.
Radiological findings of pleural
desmoplastic small round cell tumor.
| Study | Ref | Age, years | Gender | Presentation | Location | Radiology | Outcome |
|---|
| Parkash et al
(1995) | (2) | 24 | M | Chest pain, dyspnea,
left | Left | Tumor
involving left pleura and invading into the | Alive
with disease |
|
|
|
|
| pleural effusion |
|
contiguous mediastinum | at 18
months |
|
|
| 29 | M | Chest pain, loculated
right | Right |
Bilateral nodular masses of tumor invading into the | DOD at 2
years |
|
|
|
|
| pleural effusion |
|
mediastinum |
|
|
| 17 | F | Chest pain,
dysphagia, left | Left | Tumor
masses involving left pleura and invading the | DOD at
15 months |
|
|
|
|
| pleural effusion |
|
mediastinum; second surgery three months later |
|
|
|
|
|
|
| revealed
tumor encasing aorta and extending into abdomen |
| Karavitakis et
al (2007) | (3) | 10 | M | Back pain at T-10
vertebrae | Left | MRI
revealed tumor mass adjacent to the spine | Alive
with disease |
|
|
|
|
|
|
|
| at 34
months |
|
|
|
|
| level, scoliosis |
|
extending from T-5 to T-12 vertebrae, invading |
|
|
|
|
|
|
|
vertebral body and epidural space |
| Oliveira et al
(2013) | (8) | 22 | M | Chest pain, weight
loss | Right | Solid
mass in the superior mediastinum, multiple | DOD at
29 months |
|
|
|
|
|
|
|
pulmonary nodules, volimunous right pleural |
|
|
|
|
|
|
| effusion
with involvement of liver and spleen |
| Jian et al
(2014) | (9) | 15 | F | Right chest pain, low
fever, dyspnea | Right | Diffuse,
irregular pleural thickening | DOD at
22 months |
| Present study |
| 72 | F | Chest pain,
dyspnea | Left | Large,
smooth, solid mass; homogenous low attenuation | No
recurrence at |
|
|
|
|
|
|
| of plain
CT, slight-moderate unhomogenous enhancement | 32
months |
There are numerous differential diagnoses that must
be considered upon detection of a pleural mass. Pleural DSRCT
mainly requires differentiation from pleural malignant mesothelioma
and localized fibrous tumor of the pleura. Previous studies
(11–13) indicated that the typical CT results of
pleural malignant mesothelioma include unilateral pleural effusion
and thickening of the mediastinal pleura, as well as
circumferential and nodular pleural thickening of >1 cm with
mild enhancement, in addition to interlobar fissure thickening.
Localized fibrous tumor of the pleura typically presents as a
smooth, round or oval, homogeneous mass, with intermediate to high
attenuation on unenhanced CT scans. In cases of particularly large
lesions, contrast enhancement may be heterogeneous with central
areas of low attenuation that correspond with myxoid alterations,
hemorrhage, necrosis or cystic degeneration (13–15).
Although the patient in the present study refused
further chemotherapy or local radiotherapy and had no evidence of
recurrence or metastasis during 32 months of follow-up, long-term
survivors have been reported in the literature mainly as a result
of multidisciplinary treatments, including chemotherapy, surgery
and radiotherapy (7,16).
Although pleural DSRCT is rare and the final
diagnosis depends on histopathology or gene analysis, it should be
considered in the differential diagnosis list of large solid masses
of the pleura with slight-moderate enhancement on contrast CT,
particularly when there is insufficient evidence for the diagnosis
of malignant pleural mesothelioma, localized fibrous tumor of the
pleura or other relatively common diseases of the pleura in
adolescents and young adults.
References
|
1
|
Gerald WL and Rosai J: Case 2.
Desmoplastic small cell tumor with divergent differentiation.
Pediatr Pathol. 9:177–183. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Parkash V, Gerald WL, Parma A, Miettinen M
and Rosai J: Desmoplastic small round cell tumor of the pleura. Am
J Surg Pathol. 19:659–665. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Karavitakis EM, Moschovi M, Stefanaki K,
Karamolegou K, Dimitriadis E, Pandis N, Karakousis CP and
Tzortzatou-Stathopoulou F: Desmoplastic small round cell tumor of
the pleura. Pediatr Blood Cancer. 49:335–338. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ordóñez NG: Desmoplastic small round cell
tumor: I: A histopathologic study of 39 cases with emphasis on
unusual histological patterns. Am J Surg Pathol. 22:1303–1313.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gerald WL, Ladanyi M, de Alava E,
Cuatrecasas M, Kushner BH, LaQuaglia MP and Rosai J: Clinical,
pathologic and molecular spectrum of tumors associated with t
(11;22)(p13;q12): Desmoplastic small round-cell tumor and its
variants. J Clin Oncol. 16:3028–3036. 1998.PubMed/NCBI
|
|
6
|
Hayes-Jordan A and Anderson PM: The
diagnosis and management of desmoplastic small round cell tumor: A
review. Curr Opin Oncol. 23:385–389. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kushner BH, LaQuaglia MP, Wollner N,
Meyers PA, Lindsley KL, Ghavimi F, Merchant TE, Boulad F, Cheung
NK, Bonilla MA, et al: Desmoplastic small round-cell tumor:
Prolonged progression-free survival with aggressive multimodality
therapy. J Clin Oncol. 14:1526–1531. 1996.PubMed/NCBI
|
|
8
|
Oliveira MJ, de Almeida LP, Wengerkievicz
AC, Siqueira SA and Antonangelo L: From conventional fluid cytology
to unusual histological diagnosis: Report of four cases. Diagn
Cytopathol. 41:348–353. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jian Z, Shaohong H, Wenzhao Z and Lijia G:
Misdiagnosed desmoplastic small round cell tumor of the pleura:
Case report and literature review. J Formos Med Assoc. 113:60–61.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sandberg AA and Bridge JA: Updates on the
cytogenetics and molecular genetics of bone and soft tissue tumors,
desmoplastic small round-cell tumors. Cancer Genet Cytogenet.
138:1–10. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kawashima A and Libshitz HI: Malignant
pleural mesothelioma: CT manifestations in 50 cases. AJR Am J
Roentgenol. 155:965–969. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang ZJ, Reddy GP, Gotway MB, Higgins CB,
Jablons DM, Ramaswamy M, Hawkins RA and Webb WR: Malignant pleural
mesothelioma: Evaluation with CT, MR imaging and PET. Radio
Graphics. 24:105–119. 2004.
|
|
13
|
Jeong YJ, Kim S, Kwak SW, Lee NK, Lee JW,
Kim KI, Choi KU and Jeon TY: Neoplastic and nonneoplastic
conditions of serosal membrane origin: CT findings. Radiographics.
28:801–817. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mendelson DS, Meary E, Buy JN, Pigeau I
and Kirschner PA: Localized fibrous pleural mesothelioma: CT
findings. Clin Imaging. 15:105–108. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dedrick CG, McLoud TC, Shepard JA and
Shipley RT: Computed tomography of localized pleural mesothelioma.
AJR Am J Roentgenol. 144:275–280. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kurre P, Felgenhauer JL, Miser JS,
Patterson K and Hawkins DS: Successful dose-intensive treatment of
desmoplastic small round cell tumor in three children. J Pediatr
Hematol Oncol. 22:446–450. 2000. View Article : Google Scholar : PubMed/NCBI
|