Introduction
Gastric cancer (GC) is one of the most prevalent
types of malignant cancer, and possesses the second highest
cancer-associated mortality rate worldwide (1). Although improvements in hygiene, medical
technologies and food preservation techniques have led to a marked
decline in the incidence and mortality of GC over the past several
decades, the 5-year relative survival rate of patients with GC
remains at only 29%, even in the USA (2). Therefore, novel therapeutic methods for
surgical management and the novel therapeutic targets for GC
treatment remain urgently required.
Although numerous environmental factors, including
Helicobacter pylori infection and dietary habits, have
significant roles in gastric carcinogenesis, the genetic and
epigenetic alterations of multiple genes continue to be considered
to have crucial roles in this process (3). Following developments in molecular
genetics, carboxy-terminal domain phosphatase 1 (CTDP1) has
attracted increasing attention. The CTDP1 gene encodes a
phosphatase, FCP1, which is able to dephosphorylate the serine
residues of the carboxy-terminal domain (CTD) of the largest
subunit of RNA polymerase II, a significant factor involved in gene
transcription in eukaryotic cells (4). The CTD phosphorylation level of RNA
polymerase II is a crucial element in the regulation of gene
expression (5). In addition, FCP1 has
phosphatase-independent functions in transcriptional regulation,
including as an elongation factor and as a splicing associated
factor (6). Although the functions of
CTDP1 indicate that it may have an oncogenic role, the majority of
research regarding CTDP1 has focused on congenital cataracts facial
dysmorphism and neuropathy syndrome, which occurs as a result of
CTDP1 deficiency (7). To the best of
our knowledge, the detailed role of CTDP1 in tumor development has
not previously been studied.
In the present study, the role of CTDP1 in GC cells
was investigated. The expression of CTDP1 was detected in various
human GC cell lines, and subsequently, lentivirus-mediated small
interfering RNA (siRNA) was used to silence the CTDP1 gene in a GC
cell line with high CTDP1 expression. The effects of CTDP1
deficiency on the cell proliferation, cell cycle, cell apoptosis
and tumor formation ability of GC cells were then evaluated.
Materials and methods
Cell culture
Human GC cell lines AGS, BGC-823, SGC-7901, HGC-27
and MGC80-3 were purchased from the American Type Culture
Collection (Manassas, VA, USA). BGC-823, SGC-7901, HGC-27 and
MGC80-3 cells were cultured in RPMI-1640 medium (catalogue no.
11875093; Gibco Life Technologies, Carlsbad, CA, USA) containing
10% fetal bovine serum (FBS; catalogue no. 16000036; Gibco Life
Technologies) and 100 U/ml penicillin-100 µg/ml streptomycin
(catalogue no. 15140148; Gibco Life Technologies) at 37°C under
humidified air containing 5% CO2. AGS cells were
cultured in F-12 medium (catalogue no. 21127022; Gibco Life
Technologies) under identical culture conditions.
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total cell RNA was extracted using TRIzol reagent
(catalogue no. 15596026; Invitrogen Life Technologies, Carlsbad,
CA, USA) according to the manufacturer's instructions.
Subsequently, complementary DNA (cDNA) was synthesized using a
RevertAid First-Strand cDNA Synthesis kit (catalogue no. K1621;
Fermentas; Thermo Fisher Scientific, Pittsburgh, PA, USA). The gene
expression levels were examined by RT-qPCR via One Step SYBR®
PrimeScript™ RT-PCR kit II (catalogue no. RR086A; Takara Bio, Inc,
Otsu, Japan). PCR cycling conditions were initially performed for 4
min at 95°C, followed by 40 cycles at 95°C for 10 sec and then by
60°C for 30 sec. RT-qPCR was performed using a Roche LightCycler
480 system (Roche Diagnostics, Indianapolis, IN, USA). The relative
levels of CTDP1 mRNA expression were normalized to the internal
control gene, GAPDH. The specific primers were as follows: CTDP1
forward, 5′-ATATGGATCCATGCAAAATCGAGCTCGAGA-3′ and reverse,
5′-GCGGCCGCTAATCTTCAATTTACCCTAATA-3′; GAPDH forward,
5′-GAAGGTGAAGGTCGGAGTC-3′ and reverse,
5′-GAAGATGGTGATGGGATTTC-3′.
Lentivirus-mediated siRNA gene
silencing
The CTDP1 targeting siRNA sequence was
5′-CCCAGTTGCAGAGTAAGAA-3′. The sequence of the scrambled siRNA
(SCR-siRNA), which served as a negative RNA interference control,
was 5′-TTCTCCGAACGTGTCACGT-3′. The siRNA sequences were inserted
into the green fluorescent protein (GFP) expression vector pGCL-GFP
(GeneChem Co., Ltd., Shanghai, China). The recombinant virus was
packaged using the Lentivector Expression system (GeneChem Co.,
Ltd), and SGC-7901 cells were infected. Three days subsequently,
GFP-positive cells were counted under a fluorescence microscope
(IX71 System; Olympus Corp., Tokyo, Japan). The effect of siRNA
infection on CTDP1 expression was determined by RT-qPCR analysis on
the fourth day. PCR cycling conditions were initially performed for
4 min at 95°C, followed by 40 cycles at 95°C for 10 sec and then by
60°C for 30 sec.
Western blotting
Cell lysates were subjected to 8% SDS-PAGE (Sangon
Biotech Co., Ltd., Shanghai, China). The blots were subsequently
incubated with rabbit anti-human polyclonal CTDP1 antibody
(dilution, 1:2,000; catalogue no. ab137683; Abcam, Cambridge, UK)
overnight at 4°C and then with secondary antibody (donkey
anti-rabbit immunoglobulin G H&L horseradish
peroxidase-conjugated polyclonal antibody; dilution, 1:10,000;
catalogue no. ab16284; Abcam) at room temperature for 2 h and
chemiluminescent substrates (catalogue no. ab5801; Abcam) at room
temperature for 30 sec. Hybridization with rabbit anti-rat
polyclonal anti-GAPDH antibody (dilution, 1:2,000; catalogue no.
ab9485; Abcam) was used to confirm equal protein loading.
Cell proliferation assessment
GFP-positive cells with siRNA infection were
separated by fluorescence-activated cell sorting (FACS) using a
FACSCalibur (BD Biosciences, Franklin Lakes, NJ, USA) according to
the manufacturer's instructions. FACS-sorted cells
(1.0×103 per well) were cultured in 96-well plates, and
then observed and counted using a fluorescence microscope (IX71
System; Olympus Corp.) each day for 5 days to assess proliferative
ability.
Cell cycle assay
Cells were cultured in 6-well plates for 48 h and
harvested by centrifugation at 200 × g for 5 min at 4°C. The cells
were washed twice with pre-cooled phosphate-buffered saline (PBS;
pH 7.4; Sangon Biotech Co., Ltd.,) and then fixed in 70% alcohol.
Propidium iodide (PI; 50 µg/ml; catalogue no. P4864; Sigma-Aldrich,
St. Louis, MO, USA) staining was used to determine the percentage
of cells at each stage of the cell cycle. The distribution of cells
within the cell cycle was analysed by FACS (FACSCalibur).
Cell apoptosis
Those cells exhibiting exponential growth were
harvested and stained with allophycocyanin (APC)-labeled Annexin V
(catalogue no. 88-8007; eBioscience, San Diego, CA, USA) to detect
apoptotic (Annexin V positive) cells. A total of 1.0×106
cells were washed twice with pre-cooled PBS (pH 7.4), and incubated
for 15 min in 100 µl staining buffer including 5 µl APC-labeled
Annexin V. FACS analysis for Annexin V staining was subsequently
performed by flow cytometry (FACSCalibur).
Colony formation assay
Cells at the exponential growth phase were harvested
and re-seeded into 6-well plates at a density of 200 cells/well.
The plates were maintained at 37°C under humidified air containing
5% CO2 for two weeks. Following methyl-alcohol fixation
with methyl-alcohol (Sinopharm Chemical Regent Co., Ltd., Shanghai,
China), the colonies were stained with crystal violet (Beyotime
Biotechnology, Haimen, China) for 15 min, followed by using a Sony
DSC-H7 Digital camera (Sony Corporation, Tokyo, Japan) and counting
the number of colonies per well (visible to naked eye).
Statistical analysis
Data are presented as the mean ± standard deviation.
Statistical significance was determined using Student's t-test with
GraphPad Prism 6.01 software (GraphPad Software Inc., La Jolla, CA,
USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
Lentivirus-delivered siRNA stably
inhibits CTDP1 expression in GC cells
Although CTDP1 has a crucial role in gene
transcription, studies regarding CTDP1 expression in cancer, and
particularly in GC, remain rare. Therefore, the expression of CTDP1
was detected in the AGS, BGC-823, SGC-7901, HGC-27 and MGC80-3 GC
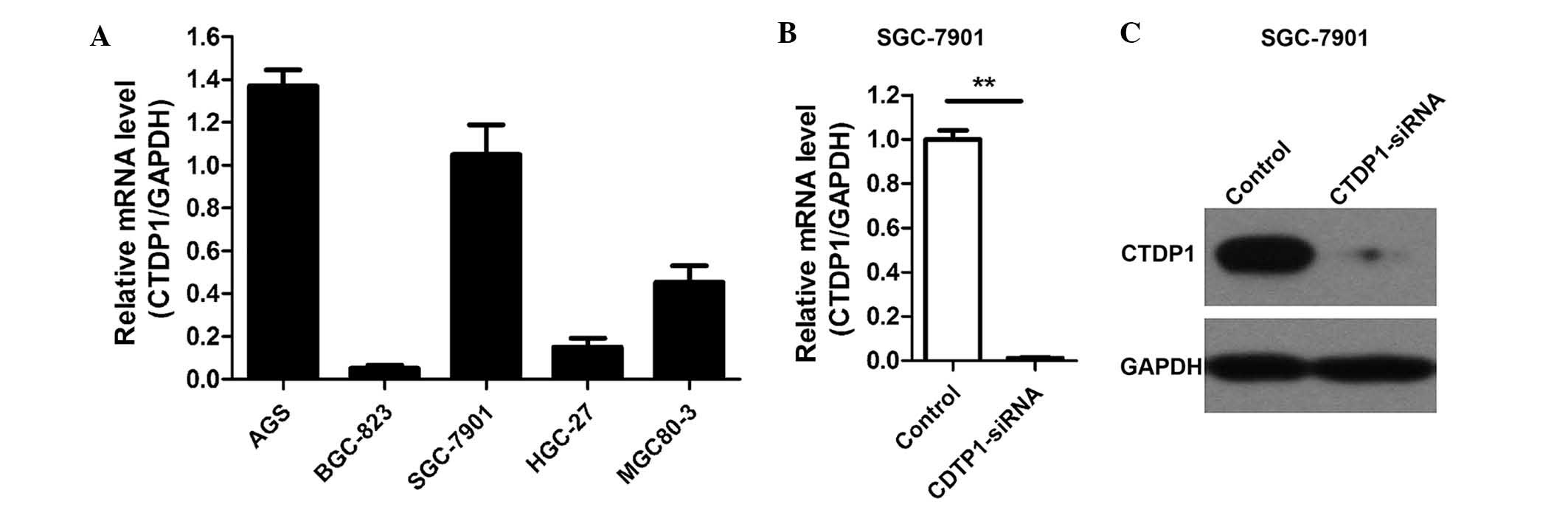
cell lines using RT-qPCR. The results revealed that CTDP1 was
expressed in all cell lines evaluated, and was particularly highly
expressed in the AGS and SGC-7901 cell lines (Fig. 1A). SGC-7901 cells were therefore
selected for further analysis of the role of CTDP1 in GC.
Lentivirus-delivered siRNA was used to attenuate CTDP1 expression
in SGC-7901 cells, and the efficiency of siRNA lentivirus infection
was >90%. Three days following infection, >90% of the
infected SGC-7901 cells expressed GFP fluorescein. The silencing
effectiveness of CTDP1-siRNA on CTDP1 expression was further
evaluated by RT-qPCR and western blotting. The results indicated
that CTDP1 expression was efficiently inhibited by CTDP1-siRNA
infection in SGC-7901 cells (Fig. 1B and
C). These data demonstrated that CTDP1 was highly expressed in
certain GC cell lines and was able to be effectively and
sustainably silenced by lentivirus-delivered CTDP1-specific
siRNA.
CTDP1 inhibition decreases cell
proliferation ability of SGC-7901 cells
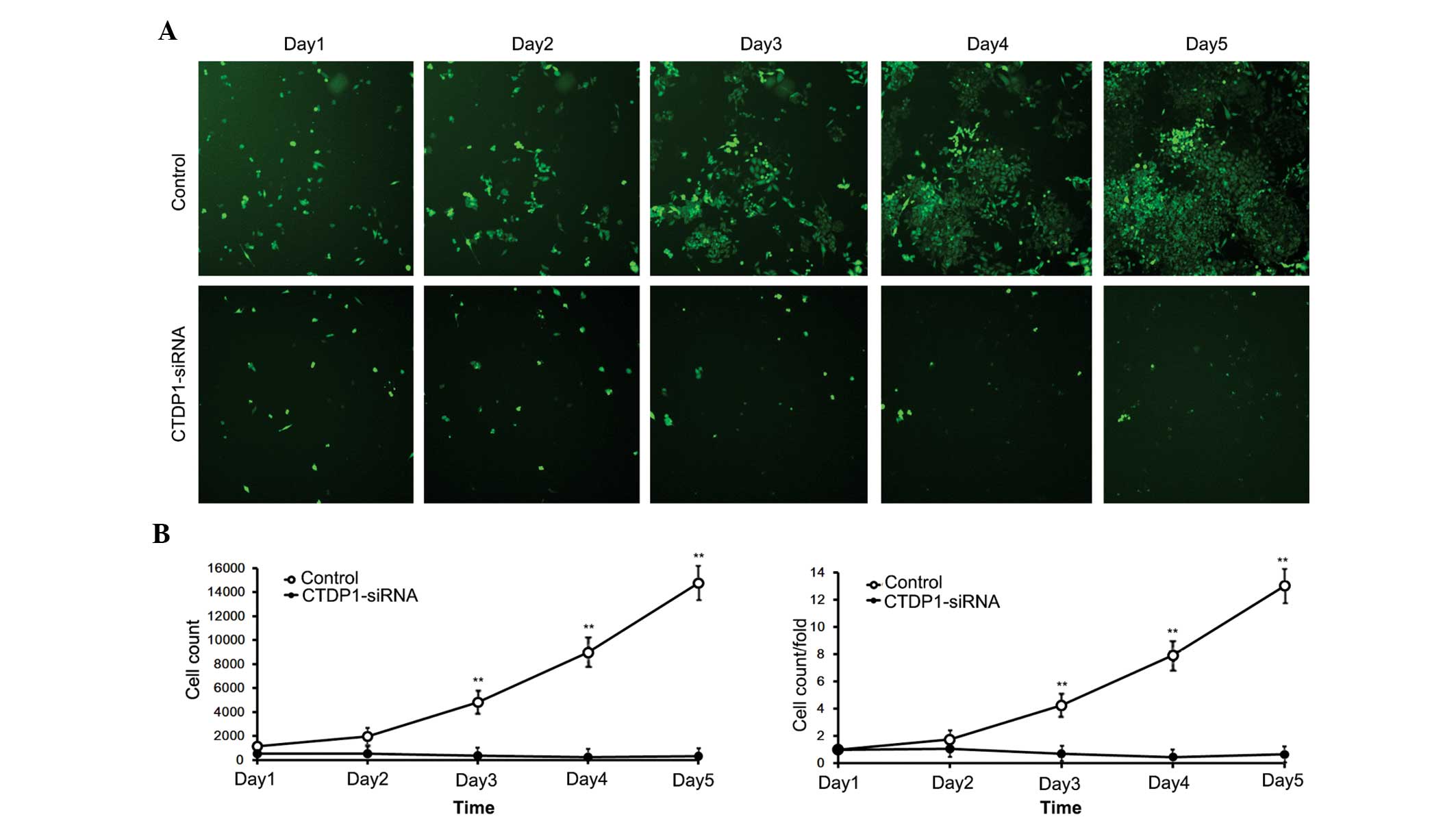
The effects of CTDP1 inhibition on GC cell
proliferation. SGC-7901 cells infected with siRNA expressed GFP
fluorescein. Therefore, cells that were effectively infected with
siRNA were able to be separated by flow cytometry. The separated
cells were harvested and monitored for 5 days. The numbers of
control cells (infected with SCR-siRNA) increased ~15-fold in 5
days, while cells infected with CTDP1-siRNA exhibited no
significant proliferation, and demonstrated a decrease in cell
number (Fig. 2). This result
indicated that inhibition of CTDP1 by siRNA infection significantly
reduced the cell proliferation ability of SGC-7901 cells
(P<0.05).
CTDP1 inhibition arrests the cell
cycle in SGC-7901 cells
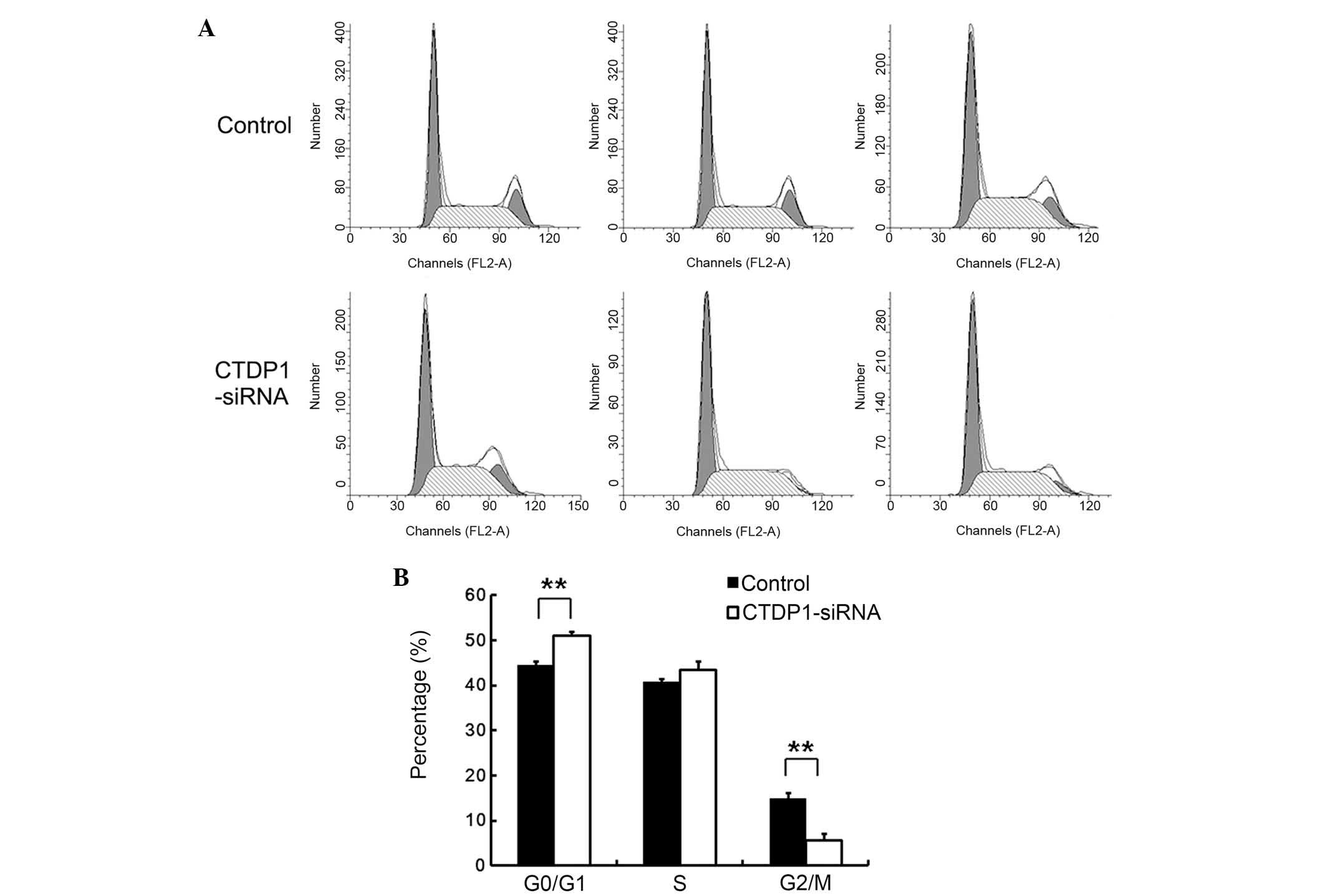
In order to investigate the underlying cause of the
decrease in cell proliferation in SGC-7901 cells infected with
CTDP1-siRNA, the effect of CTDP1 inhibition on the cell cycle was
evaluated by PI staining and flow cytometric analysis. The results
indicated that cells treated with SCR-siRNA had a lower percentage
at G0/G1 phase than that of those treated with CTDP1-siRNA
(43.7±1.6 vs. 51.2±1.3%; P<0.01). In addition,
SCR-siRNA-infected cells had 16.3±1.4% at G2/M phase, while the
CTDP1-siRNA cells only had 7.5±2.4% at G2/M phase (Fig. 3). These data suggested that CTDP1
inhibition resulted in the arrest of SGC-7901 cells at the
quiescent phase.
CTDP1 inhibition promotes cell
apoptosis in SGC-7901 cells
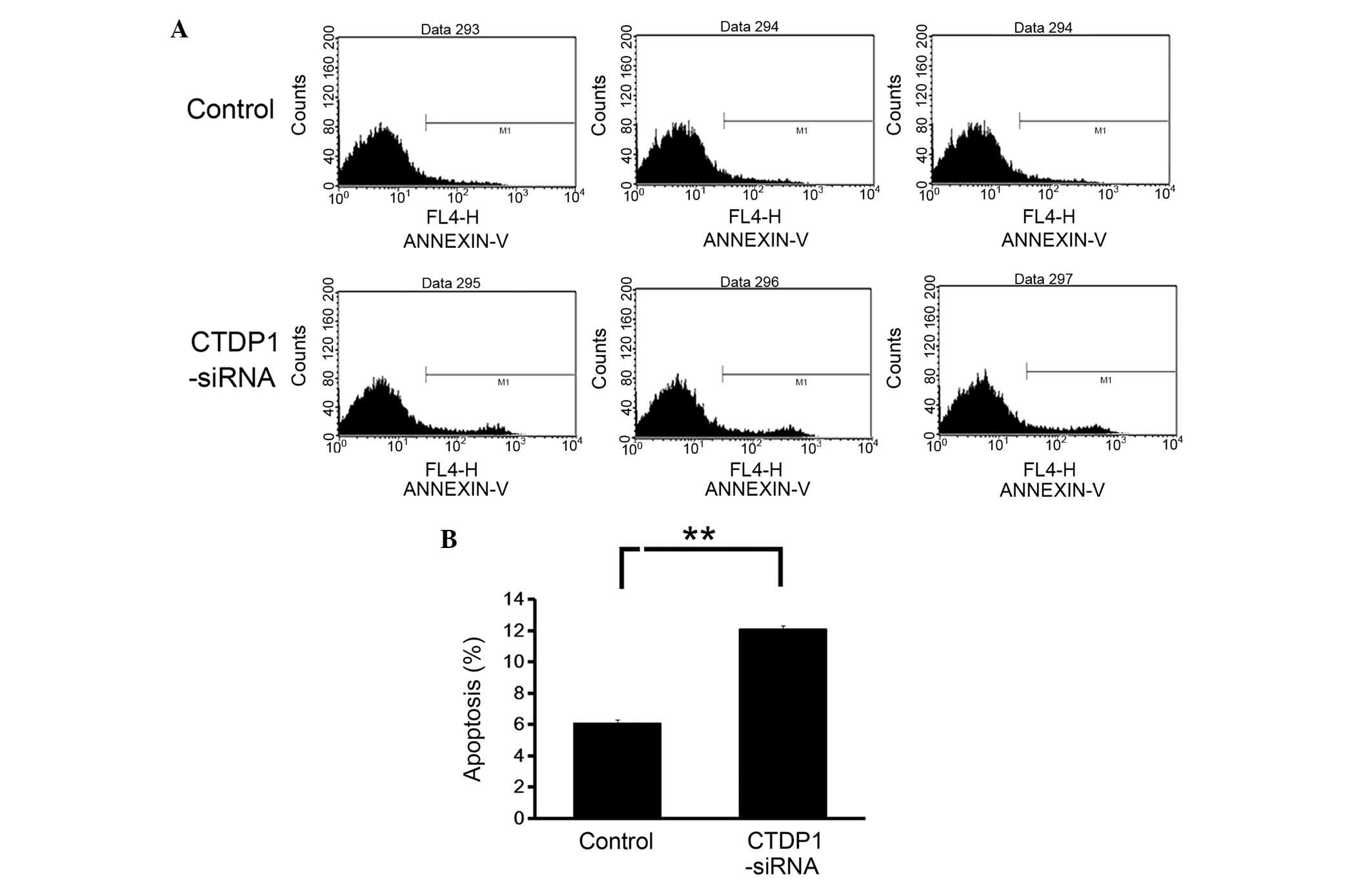
The effects of CTDP1 inhibition on cell apoptosis in
GC cells was also evaluated using Annexin V-APC staining and flow
cytometric analysis. The data revealed that the downregulation of
CTDP1 expression resulted in a marked increase in the percentage of
apoptotic cells (Annexin V positive) in SGC-7901 cells (from
6.1±0.4 to 11.9±0.7%) (Fig. 4). This
result demonstrated that CTDP1 inhibition enhanced apoptosis in
SGC-7901 cells.
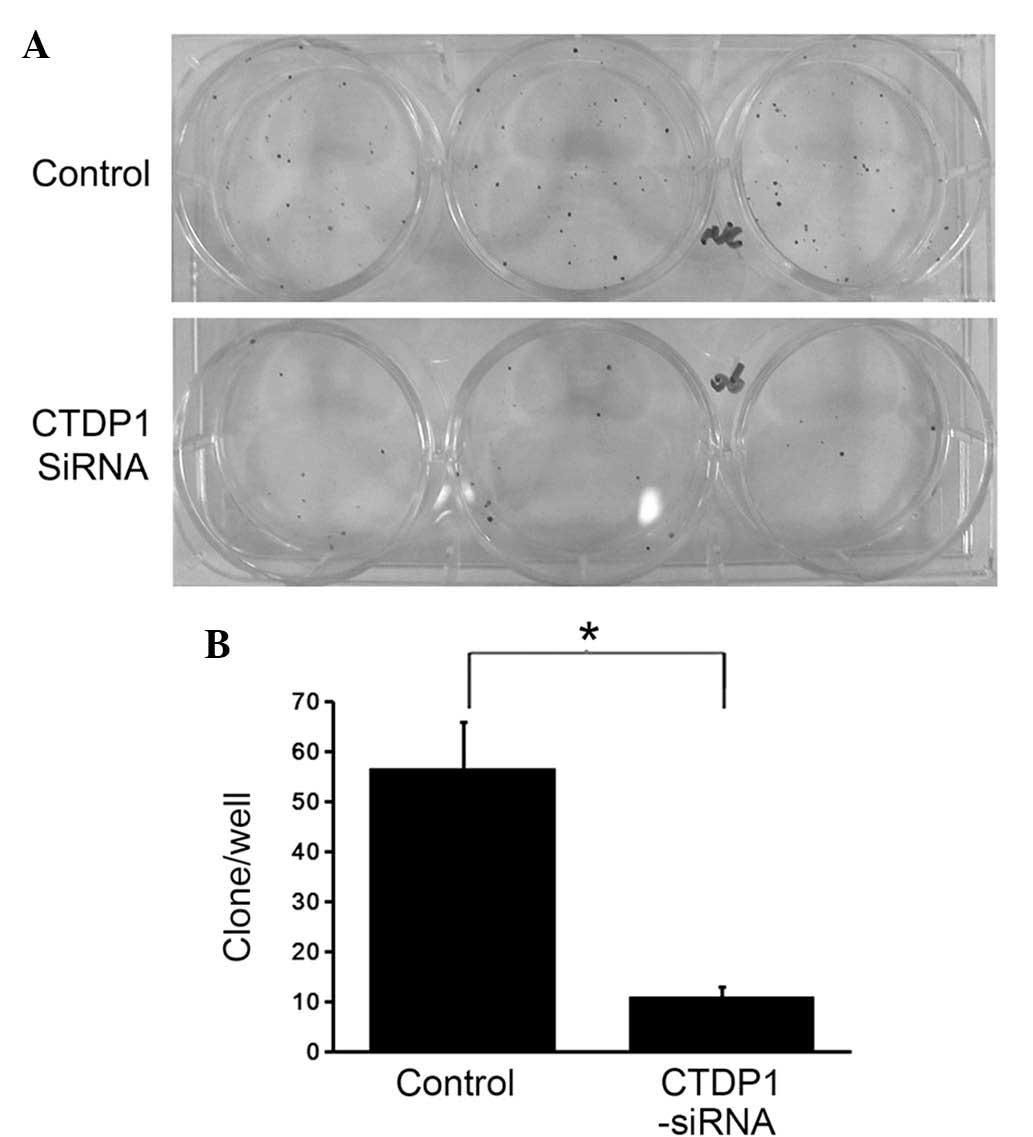
CTDP1 inhibition decreases colony
formation ability in GC cells
The aforementioned experiments indicated that
inhibition of CTDP1 induced a decrease in cell proliferation
ability, cell cycle arrest and an increase in cell apoptosis in
SGC-7901 cells. To further investigate the impact of CTDP1
inhibition on GC cells, differences in colony formation ability
between CTDP1-siRNA-infected and SCR-siRNA-infected SGC-7901 cells
were evaluated. Cells were seeded in 6-well plates at a density of
200 cells/well. Following two weeks of incubation, the SCR-siRNA
infected cells exhibited 5.5-fold higher colony numbers compared
with those of the CTDP1-siRNA infected cells (Fig. 5). This suggested that CTDP1 inhibition
decreased the colony formation ability of SGC-7901 cells.
Discussion
In the present study, CTDP1 expression was detected
in various GC cell lines, CTDP1 expression was stably and
efficiently silenced by a specific siRNA-mediated system in
SGC-7901 cells, and the effects of CTDP1 inhibition on SGC-7901
cells were evaluated. It was revealed that inhibition of CTDP1
decreased cell proliferation, arrested the cell cycle at G0/G1
phase and increased cell apoptosis in SGC-7901 cells. Furthermore,
the colony formation ability of SGC-7901 cells was suppressed by
silencing CTDP1. These results suggested that CTDP1 silencing
reduced the tumor formation ability of GC cells.
The primary function of FCP1, the protein encoded by
CTDP1, is dephosphorylation of the CTD of RNA polymerase II, which
has a critical role in the initial synthesis of mRNA and the
post-transcriptional modification of mRNA (8,9). The
phosphorylation of CTD in the largest subunit of RNA polymerase II
mediates the assemblage of regulatory factors during the initial
stages of mRNA synthesis (10,11). FCP1
dephosphorylates the CTD of RNA polymerase II in order to recycle
it and further initiate a novel round of transcription. Various
phosphorylation sites and potential conformational states make CTD
a transcriptional controller that is able regulate mRNA production
and processing (12).
In addition to regulating RNA polymerase II, FCP1 is
also involved in the regulation of RNA polymerase I. Bierhoff et
al (13) found that CK2
facilitated a novel round of transcription initiation of RNA
polymerase I via the phosphorylation of FCP1. Furthermore,
FCP-mediated phosphorylation is not only associated with the
assembly of transcription factors, but also temporally controls the
cell cycle through activation of cyclin-dependent kinases and
Greatwall kinase (14,15).
The results of the present study revealed that CTDP1
silencing resulted in proliferation inhibition and cell cycle
arrest in GC cells. This indicated that normal expression of CTDP1
may be a decisive factor in cell regulation. Overexpression or
silencing of CTDP1 may inhibit its associated regulation
mechanisms. Schauer et al (16) found that FCP1 misregulation, whether
FCP1 overexpression or silencing, induced p53-dependent and
enhanced levels of caspase-mediated apoptosis in Drosophila
melanogaster. The present study also found that CTDP1 silencing
induced an increase in the apoptotic rate of GC cells.
In conclusion, the results of the present study
suggest that CTDP1 silencing is able to suppress cell
proliferation, induce cell cycle arrest, increase cell apoptosis
and inhibit colony formation in SGC-7901 cells. This finding
reveals that CTDP1 has a significant role in GC development, and
that CTDP1 may be a promising therapeutic target in the clinical
treatment of GC.
Acknowledgements
The present study was supported by Scientific
Research Foundation for Youth Scholars of The Second Military
University affiliated Shanghai Changzheng Hospital (no.
2012CZQN12).
References
|
1
|
Torpy JM, Lynm C and Glass RM: JAMA
patient page. Stomach cancer. JAMA. 303:17712010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jang BG and Kim WH: Molecular pathology of
gastric carcinoma. Pathobiology. 78:302–310. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chambers RS and Dahmus ME: Purification
and characterization of a phosphatase from HeLa cells which
dephosphorylates the C-terminal domain of RNA polymerase II. J Biol
Chem. 269:26243–26248. 1994.PubMed/NCBI
|
|
5
|
Maniatis T and Reed R: An extensive
network of coupling among gene expression machines. Nature.
416:499–506. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kalaydjieva L: Congenital cataracts-facial
dysmorphism-neuropathy. Orphanet J Rare Dis. 1:322006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Varon R, Gooding R, Steglich C, Marns L,
Tang H, Angelicheva D, Yong KK, Ambrugger P, Reinhold A, Morar B,
et al: Partial deficiency of the C-terminal-domain phosphatase of
RNA polymerase II is associated with congenital cataracts facial
dysmorphism neuropathy syndrome. Nat Genet. 35:185–189. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Proudfoot NJ, Furger A and Dye MJ:
Integrating mRNA processing with transcription. Cell. 108:501–512.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Svetlov V and Nudler E: Basic mechanism of
transcription by RNA polymerase II. Biochim Biophys Acta.
1829:20–28. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dahmus ME: Reversible phosphorylation of
the C-terminal domain of RNA polymerase II. J Biol Chem.
271:19009–19012. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jasnovidova O and Stefl R: The CTD code of
RNA polymerase II: A structural view. Wiley Interdiscip Rev RNA.
4:1–16. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Buratowski S: The CTD code. Nat Struct
Biol. 10:679–680. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bierhoff H, Dundr M, Michels AA and Grummt
I: Phosphorylation by casein kinase 2 facilitates rRNA gene
transcription by promoting dissociation of TIF-IA from elongating
RNA polymerase I. Mol Cell Biol. 28:4988–4998. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Visconti R, Palazzo L, Monica R Della and
Grieco D: Fcp1-dependent dephosphorylation is required for
M-phase-promoting factor inactivation at mitosis exit. Nat Commun.
3:8942012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hégarat N, Vesely C, Vinod PK, Ocasio C,
Peter N, Gannon J, Oliver AW, Novák B and Hochegger H: PP2A/B55 and
Fcp1 regulate Greatwall and Ensa dephosphorylation during mitotic
exit. PLoS Genet. 10:e10040042014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Schauer T, Tombácz I, Ciurciu A, Komonyi O
and Boros IM: Misregulated RNA Pol II C-terminal domain
phosphorylation results in apoptosis. Cell Mol Life Sci.
66:909–918. 2009. View Article : Google Scholar : PubMed/NCBI
|