Introduction
Alveolar soft part sarcoma (ASPS) is a rare,
malignant, soft-tissue tumor that accounts for ~1.2% of all
soft-tissue sarcomas (1). ASPS
belongs to the group of malignant tumors with uncertain
differentiation. With regard to molecular genetics, it is
characterized by an unbalanced translocation,
der(17)t(X;17)(p11;q25) (2). The most
common age of onset is between 15 and 35 years old, and onset prior
to the age of 5 years old or after 50 years old is rare. It has
been reported (3) that the incidence
of the tumor in women is higher than that in men, particularly
among women under the age of 25 years old. The most common site of
tumor occurrence is in the deep soft tissues of the limb,
particularly in the thigh, while it may also occur in rarer sites,
such as the head and neck, mediastinum, retroperitoneum, breasts
and orbits (4). ASPS grows slowly,
with clinical symptoms that are not pronounced, so the tumor is not
easily identified (4). ASPS is also
prone to early metastases and has a high recurrence rate following
conservative surgical excision. Therefore, the prognosis of the
tumor is poor. Careful analysis of the imaging and
clinicopathological features, as performed in the present study,
will be useful for determining an accurate diagnosis.
Materials and methods
Patients
A total of 2 males and 4 females with ASPS confirmed
by surgery and pathology were treated the Affiliated hospital of
Inner Mongolia Medical University (Hohhot, China) between October
2013 and June 2014. The patient ages ranged from 16 to 45 years
old, with a mean of 31.4 years old. The locations of the tumor
included 1 case in the thigh, 1 case in the leg, 1 case in the
upper arm, and 3 cases in the gluteus muscles and iliopsoases. The
duration of the disease ranged between 3 months to 3 years, and the
main clinical manifestation was an increasingly soft-tissue mass
with pain.
Treatment and analysis
The patients underwent computed tomography (CT)
scans, and plain and enhanced magnetic resonance imaging prior to
surgery. The location, size, morphology and enhanced pattern of the
tumors were analyzed and evaluated by two experts separately.
Following radical surgical resection, the pathological sections
were analyzed using different methods in the paraffin-embedded
tissue samples: (i) Hematoxylin-eosin (HE) staining, (ii) periodic
acid-schiff (PAS) staining and (iii) immunohistochemical staining
(Envision methods). MyoD1 (cytoplasmic staining), desmin, NSA,
vimentin, AE1/AE3, cytokeratin, epithelial membrane antigen, smooth
muscle actin, muscle-specific actin and synaptophysin were
assessed.
Results
All tumors were located at the deep muscles. The
mean size of the tumors was 4.1×4.8×3.3 cm. In total, 5 tumors were
lobulated in shape and 3 tumors exhibited peritumoral soft-tissue
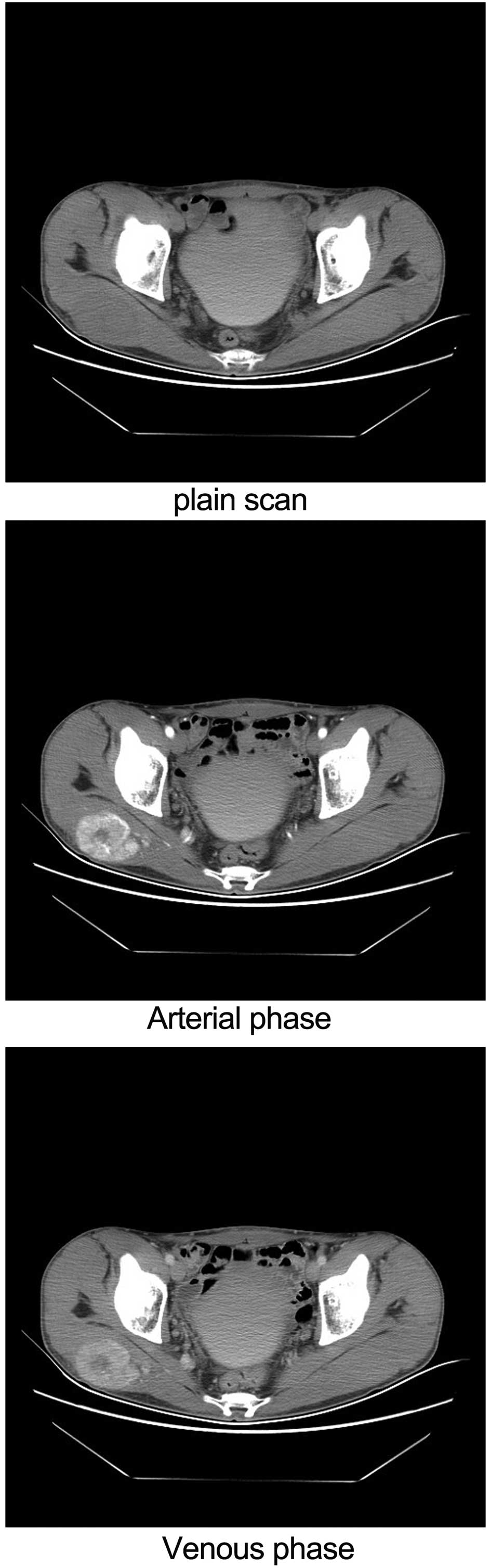
nodules. On CT, the tumor density was even, with marked enhancement
on contrast-enhanced imaging (Fig.
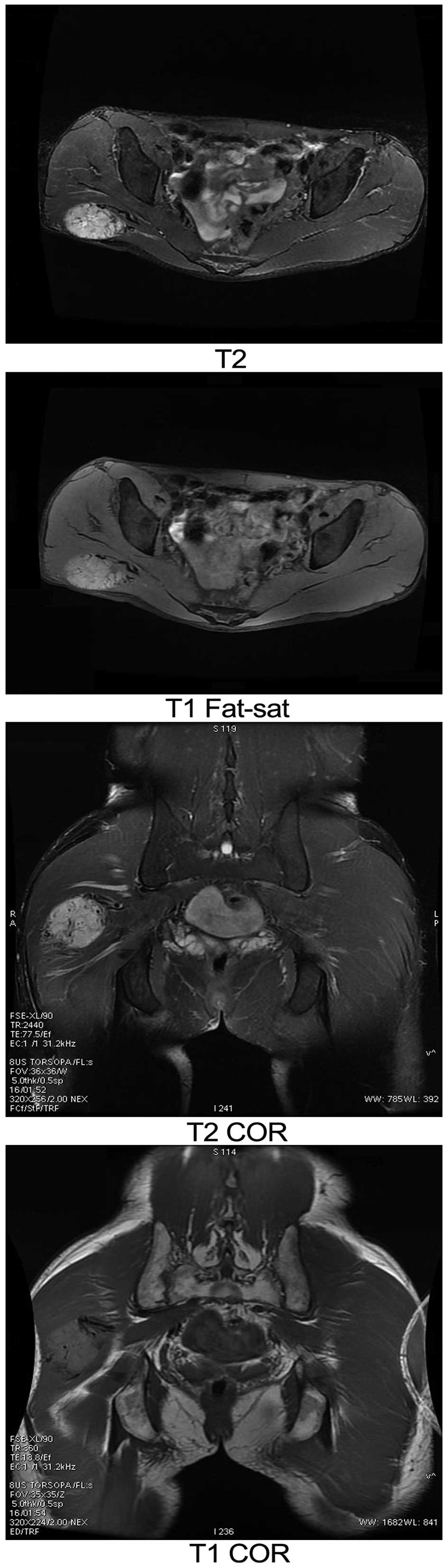
1). On MRI, the signal intensity was homogeneous, with
isointensity on T1-weighted imaging (WI) and hyperintensity on
T2WI. Cystic degeneration and necrosis were found in 2 cases. The
solid component of the tumors showed marked enhancement on
contrast-enhanced MRI (Fig. 2).
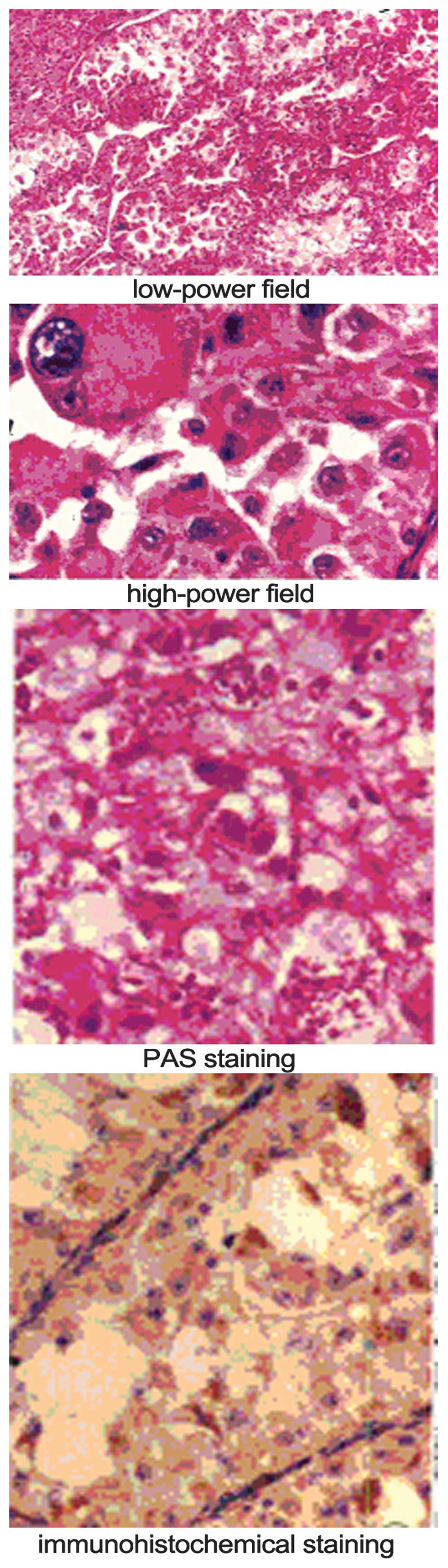
Microscopic analysis revealed tumor cells with granular cytoplasm
arranged in alveolar or solid structures separated by sinusoidal
vessels. Crystals were present in the cytoplasm of the tumor cells
following periodic acid-Schiff (PAS) staining.
Immunohistochemically, 4 cases were positive for MyoD1, 1 was
positive for desmin, 2 were positive for S-100, 5 were positive for
neuron-specific enolase and 5 were positive for vimentin. All ASPS
cases were negative for AE1/AE3, cytokeratin, epithelial membrane
antigen, smooth muscle actin, muscle-specific actin and
synaptophysin (Fig. 3).
Discussion
Upon pathological examination, ASPS always has an
incomplete capsule and the edge is nodular. When the tumor becomes
large, it can cause hemorrhagic necrosis, with tumor cells arranged
loosely in substantial aciniform or nested structures. Central cell
nests can appear with degeneration or necrosis, the cytoplasm is
positive for PAS staining, and the cells contain rod- or bar-shaped
crystals, all of which contribute to the tumor diagnosis (5). The blood supply of the tumor tissue is
rich; the presence of bulky, twisted blood vessels around the tumor
and a large amount of bleeding can be observed during surgery
(6).
The imaging results, particularly on MRI, can
reflect the clinical and pathological characteristics of ASPS to a
certain extent. As patients are often experience a long course of
disease and the clinical symptoms are not pronounced, the majority
of patients present to hospital with a larger tumor or serious
symptoms prior to treatment. In the present study, the mean tumor
volume was ~4.1×4.8×3.3 cm, which is consistent with the tumor
sizes of ASPS. Upon pathological examination, the tumors had a
compact nature and were elliptical in shape. MRI exhibited moderate
T1 and long T2 signals for the soft-tissue mass. Upon growth of the
tumor, hemorrhage and necrosis can be caused, and in certain cases
in the present study, the central region exhibited necrosis or/and
cystic change. The shape of the cystic region was irregular,
showing markedly abnormal long T1 and T2 signals. The blood supply
of the tumor was rich, so it exhibited strong enhancement.
The MRI of the tumor boundary reflects its invasive
ability (6,7), and a previous study (8) has reported that the tumor peripheral
nodule formation is due to the breakthrough of sarcoma cells
through the cell membrane and into the ‘tumor response area’. In
the present study, 2 tumors exhibited no clear boundary between the
local and surrounding tissue, showing continuity. There were
soft-tissue nodules around the tumors in 4 cases, which meant that
the tumor cells grew to break through the thin film, indicating a
malignant tumor. Another study (9)
reported that the transfer of ASPS metastases mainly occurred via
the blood, and that the lung was the most common metastatic site,
being affected in 42–65% of all patients, while lymph node
metastasis occurred in only 10% of the patients (10).
For the differential diagnosis, ASPS must be
distinguished from rhabdomyosarcoma, fibrosarcoma, malignant
fibrous histiocytoma, synovial sarcoma and atypical lipoma.
Rhabdomyosarcoma is large and lobulated, with moderate T1 and long
T2 or mixed signals. Tumor necrosis in common, with enhanced scans
showed marked enhancement. Pathological diagnosis may also aid the
differentiation. With regard to fibrosarcoma, T1WI demonstrates
equal or slightly low signals, and T2WI demonstrates uneven
slightly high signal within the tumor. Hemorrhage and necrosis can
occur, and the tumor easily invades neighboring bone. Malignant
fibrous histiocytoma consists of lobulated soft-tissue masses, with
moderate T1 signals and heterogeneous high signals on T2WI. The
tumor can present as a bleeding cyst, without enhancement on T1W1.
When the fiber content in the tumor is large, an irregular low
signal is visible on T2WI, which can be used for identification
purposes. Synovial sarcoma is a nodular or lobulated soft-tissue
mass. The tumor commonly exhibits focal calcification, with
moderate T1 and long T2 signals due to hemorrhage, necrosis and
calcification. The tumor signal is often uneven (11). With regard to atypical lipoma, the fat
content of well-differentiated liposarcoma is rich and easy to
identify. However, less-differentiated liposarcoma, particularly
pleomorphic liposarcoma, contains a large quantity of immature
adipose tissue, with a soft-tissue signal, which requires careful
identification (12).
Overall, ASPS is a rare, malignant, soft-tissue
tumor, and although the incidence rate is low, the clinical and
imaging diagnosis should not be overlooked. For young and
middle-aged patients, the possibility of ASPS should be considered
for slow-growing tumors that occur in the deep muscles. The
characteristics of ASPS upon imaging are mainly the presence of a
large, lobulated mass with an incomplete capsule and nodules of
soft tissue around the tumor. The tumor can exhibit necrosis or
metastasize when it becomes larger. On MRI, the tumor parenchyma
show moderate T1 and long T2 signals, with marked enhancement on
the contrast scans. Although these signs are non-specific and the
diagnosis still relies on the pathology, the MRI manifestations can
reflect the characteristics of malignant tumors to a certain
extent, enabling a qualitative diagnosis and guiding clinical
pre-operative treatment.
References
|
1
|
Enzinger FM and Weiss SW: Alveolar soft
part sarcoma. Soft tissue tumors (4th). (St. Louis). Mosby Press.
1509–1521. 2002.
|
|
2
|
Ladanyi M, Lui MY, Antonescu CR,
Krause-Boehm A, Meindl A, Argani P, Healey JH, Ueda T, Yoshikawa H,
Meloni-Ehrig A, et al: The der(17)t(x;17)(p11;q25) of human
alveolar soft part sarcoma fuses the TFE3 transcription factor gene
to ASPL, a novel gene at 17q25. Oncogene. 20:48–57. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Marchac A, Picard A, Landman-Parker J,
Larroquet M, Vazquez MP and Franchi G: A pediatric case of alveolar
soft part sarcoma. Rev Stomatol Chir Maxillofac. 108:547–550.
2007.(In French). View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Folpe AL and Deyrup AT: Alveolar Soft-part
Sarcoma: A review and update. J Clin Pathol. 59:1127–1132. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Akiyama Y, Baba T, Ibayashi Y, Asai Y and
Houkin K: Alveolar soft part sarcoma in brain with cardiac
metastasis: A case report. Int J Cardiol. 114:e93–e95. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nakano H, Tateishi A, Imamura T, Miki H,
Ohno T, Moue T, Sekiguchi M, Unno K, Abe S, Matsushita T, et al:
RT2PCR suggests human skeletal muscle origin of alveolar soft part
sarcoma. Oncology. 58:319–323. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Moulton JS, Blebea JS, Dunco DM, Braley
SE, Bisset GS III and Emery KH: MR imaging of soft tissue masses:
diagnostic efficacy and value of distinguishing between benign and
malignant lesions. AJR Am J Roentgenol. 164:1191–1199. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Saito T, Oda Y, Kawaguchi K, Takahira T,
Yamamoto H, Sakamoto A, Tamiya S, Iwamoto Y and Tsuneyoshi M:
Possible association between tumor-suppressor gene mutations and
hMSH2 /hMLH1 inactivation in alveolar soft part sarcoma. Hum
Pathol. 34:841–849. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang CK, Li CW, Hsieh TJ, Chien SH, Liu GC
and Tsai KB: Characterization of bone and soft-tissue tumors with
in vivo 1h mr spectroscopy: Initial results. Radiology.
232:599–605. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Negendank WG, Sauter R, Brown TR, Evelhoch
JL, Falini A, Gotsis ED, Heerschap A, Kamada K, Lee BC and Mengeot
MM: Proton magnetic resonance spectroscopy in patients with glial
tumors: A multicenter study. J Neurosurg. 84:449–458. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lorigan JG, O'Keeffe FN, Evans HL and
Wallace S: The radiologic manifestations of alveolar soft-part
sarcoma. AJR Am J Roentgenol. 153:335–339. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Aiken AH and Stone JA: Alveolar soft-part
sarcoma of the tongue. AJNR Am J Neuroradiol. 24:1156–1158.
2003.PubMed/NCBI
|