Introduction
Chondrosarcomas are the second most common type of
bone malignancy, worldwide (1). Spine
chondrosarcoma account for <10% of all chondrosarcomas (2,3) and the
majority are located in the thoracic spine (1,3,4). Diagnosis is based on computed tomography
and magnetic resonance imaging, however, pathological diagnosis
based on needle biopsies or lesion resection is the gold standard
criteria for diagnosis (5). Surgical
resection is the most effective treatment strategy (2,3,6), as these tumors are relatively resistant
to chemotherapy and radiotherapy (7–9). However,
chondrosarcoma of the spine is associated with a poor prognosis;
previous studies determined a 5-year survival rate of 64%. In
addition, patients have a median survival time of 6 years following
surgery and surgical margins are an important prognostic factor
(6,10).
Malignancy is one of the primary risk factors for
tumor embolism. Embolism refers to the lodging of an embolus, which
may be a blood clot, fat globule, gas bubble or foreign material in
the bloodstream. Thromboembolisms are the most common type of tumor
embolism, commonly occurring in the pulmonary artery. Tumor
embolization is a rare but unique complication of malignancy.
Patients with cancer have an increased risk of developing embolism
due to tumor- and treatment-mediated hypercoagulability (11–13). In an
analysis oncology outpatient cohort at Dana-Farber Cancer Institute
(Boston, MA, USA), CNS, pancreatic, upper gastrointestinal and
lung/pleural malignancies were associated with a significantly
higher risk of pulmonary embolism than other malignancies, whereas
hematological and breast malignancies had a significantly lower
risk of pulmonary embolism (14).
To the best of our knowledge, a right atrial
embolism from thoracic chondrosarcoma has not been reported
previously. In the present study, the case of an adult male with
thoracic chondrosarcoma who succumbed as a result of a right atrial
embolism is reported. Written informed consent was obtained from
the patient's family.
Case report
A 70-year-old male, who was previously diagnosed
with thoracic chondrosarcoma in 2008, presented to Guang'anmen
Hospital (Beijing, China) on May 7, 2012 with a fever and
exertional dyspnea that had persisted for two weeks. The patient
had previously received multimodality therapy for the treatment of
local recurrence, osseous metastasis and pulmonary metastasis,
which included surgical treatment of recurrent lesions, the
placement of an artificial vertebral body, radiotherapy, biological
therapy with interleukin-2, administration of bisphosphonates for
the treatment of bone metastasis and traditional Chinese medicine
(TCM) therapy, as follows. The patient underwent T5 vertebral
resection, replacement and posterior fixation for treatment of a
thoracic neoplasm at Peking University People's Hospital (Beijing,
China) on December 9, 2008. A postoperative pathological diagnosis
was chondrosarcoma was determined. The patient subsequently
underwent thoracic mass fixation and artificial vertebral body
implantation due to local recurrence on June 29, 2011. Chest
computed tomography on August 30, 2011 suggested T5-7 vertebral
bodies, bone metastases in the adjacent ribs and a mass surrounding
the vertebral bodies. Therefore, the patient underwent T5
extrapyramidal radiotherapy (6 MV X-rays at 95% planning target
volume administered in 20 fractions of 40 Gy (2 Gy/fraction)] at
Peking University Third Hospital (Beijing, China) between September
5, 2011 and October 7, 2011. Later, the patient received
interleukin-II therapy (1×106 IU three times a week).
Chest computed tomography performed at Peking University Third
Hospital on January 13, 2012, suggested bilateral pulmonary
nodules. Thus, the patient received additional interleukin-II
therapy (1×106 IU five times a week). In April 2012,
chest computed tomography suggested progress of the bilateral
pulmonary nodules, a mass surrounding vertebral bodies and bone
metastases. Consequently, the patient received TCM therapy [20 ml
cinobufotalin injection plus 250 ml 0.9% normal saline (NS)
intravenously (i.v.) drip daily for 13 days, followed by 20 ml
compound Kushen injection plus 250 ml 0.9% NS i.v. drip daily for
13 days] in Guang'anmen Hospital between May 23 and June 5, 2012.
On presentation to Guang'anmen Hospital, the patient reported mild
shortness of breath and a fever. Initial blood tests, performed on
June 7, 2012, revealed leukocytosis with neutrophilia [white blood
cell, 9.15×109/l (normal range, 4–10×109/l);
neutrophil count, 77.1% (normal range, 51–75%); lymphocyte count,
15.3% (normal range, 20–40%)]. In addition, Klebsiella
pneumoniae was identified in bacterial culture of the sputum.
The patient was diagnosed with a lung infection and subsequently
received anti-infection treatment (cefoperazone sodium and
sulbactam sodium, 3 g every 12 h for 12 days) between June 7 and
31, 2012. Following three days of treatment, the fever had gone and
the blood tests results were within the normal ranges. However,
three weeks later, the patient's breathing suddenly deteriorated
and shock developed. The blood pressure decreased to 75/35 mmHg
(normal range, 120/80–140/90 mmHg), and a cardiovascular
examination revealed tachycardia and systolic blowing murmurs. In
addition, diastolic rumbling noises were heard in the auscultatory
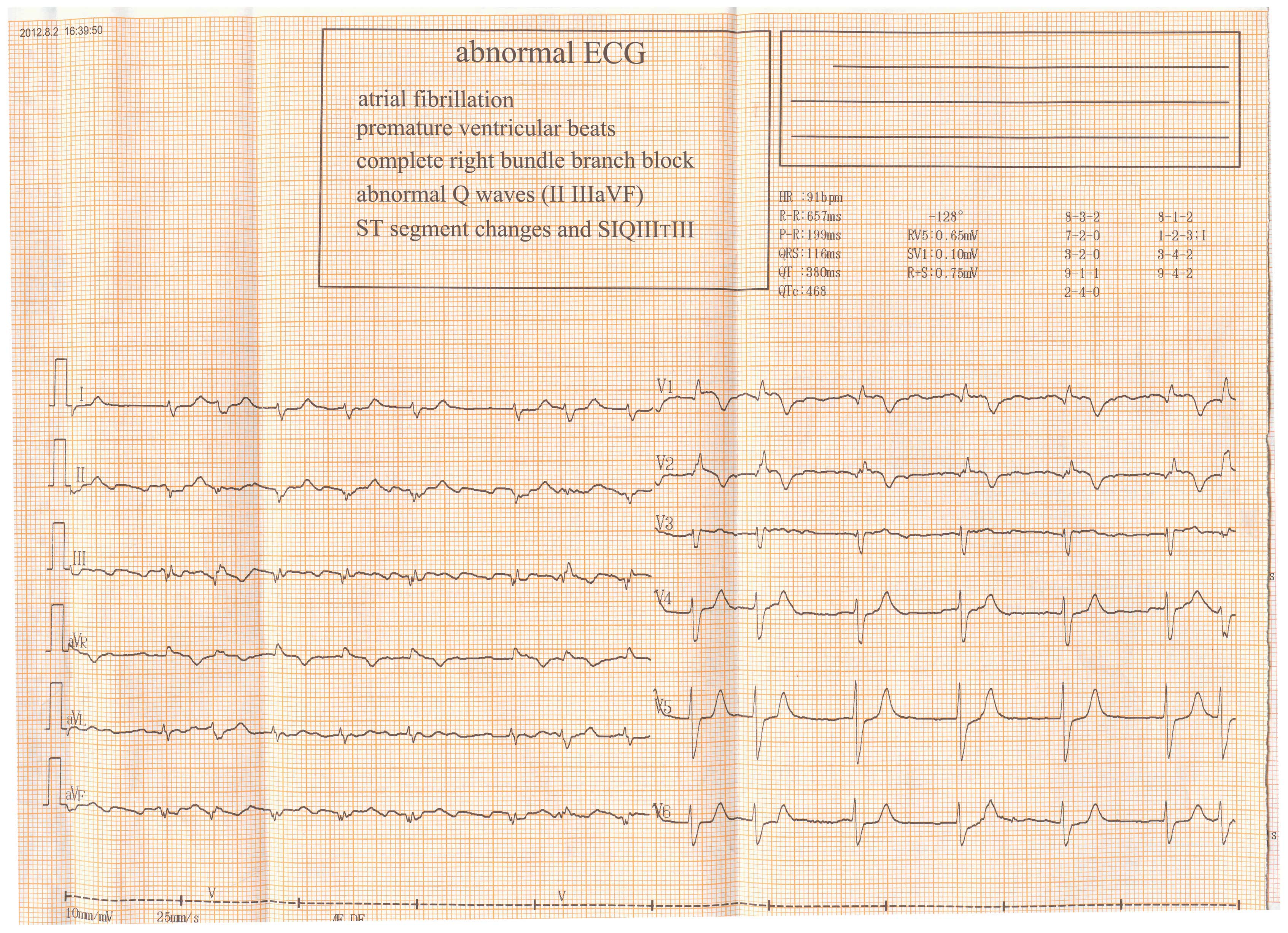
mitral area. Electrocardiogram (ECG) revealed atrial fibrillation,
ST segment changes and an
SIQIIITIII right bundle branch
block (lead I, apparent S waves; lead III, obvious Q and T wave
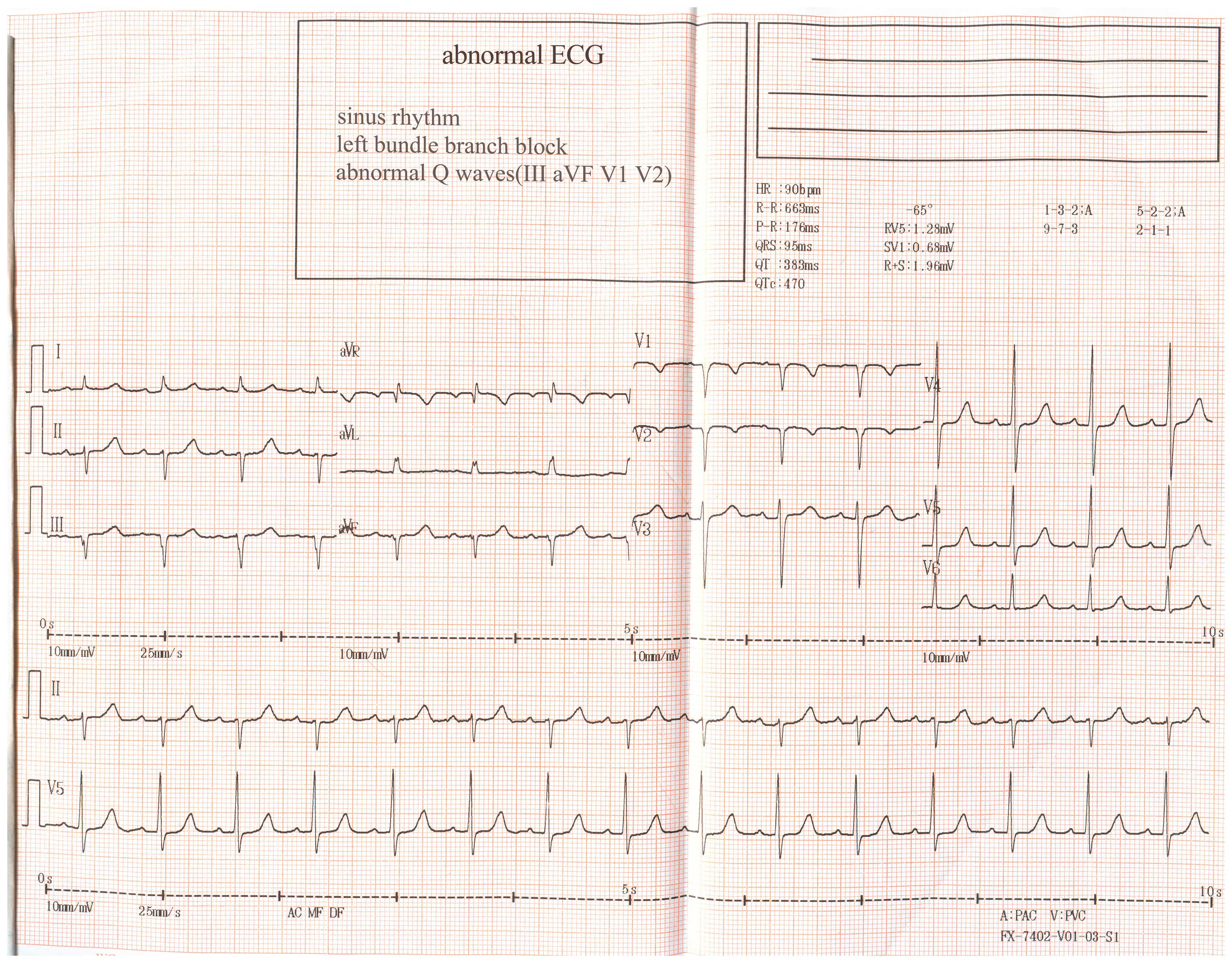
inversion; Fig. 1), which had not
been identified on the ECG performed previously following admission
(Fig. 2).
Analysis of the arterial blood gas revealed that the
partial pressure of O2 was 53 mmHg at room air
temperature (normal range, 80–100 mmHg) and that the partial
pressure of CO2 was 35.3 mmHg (normal range, 35–45
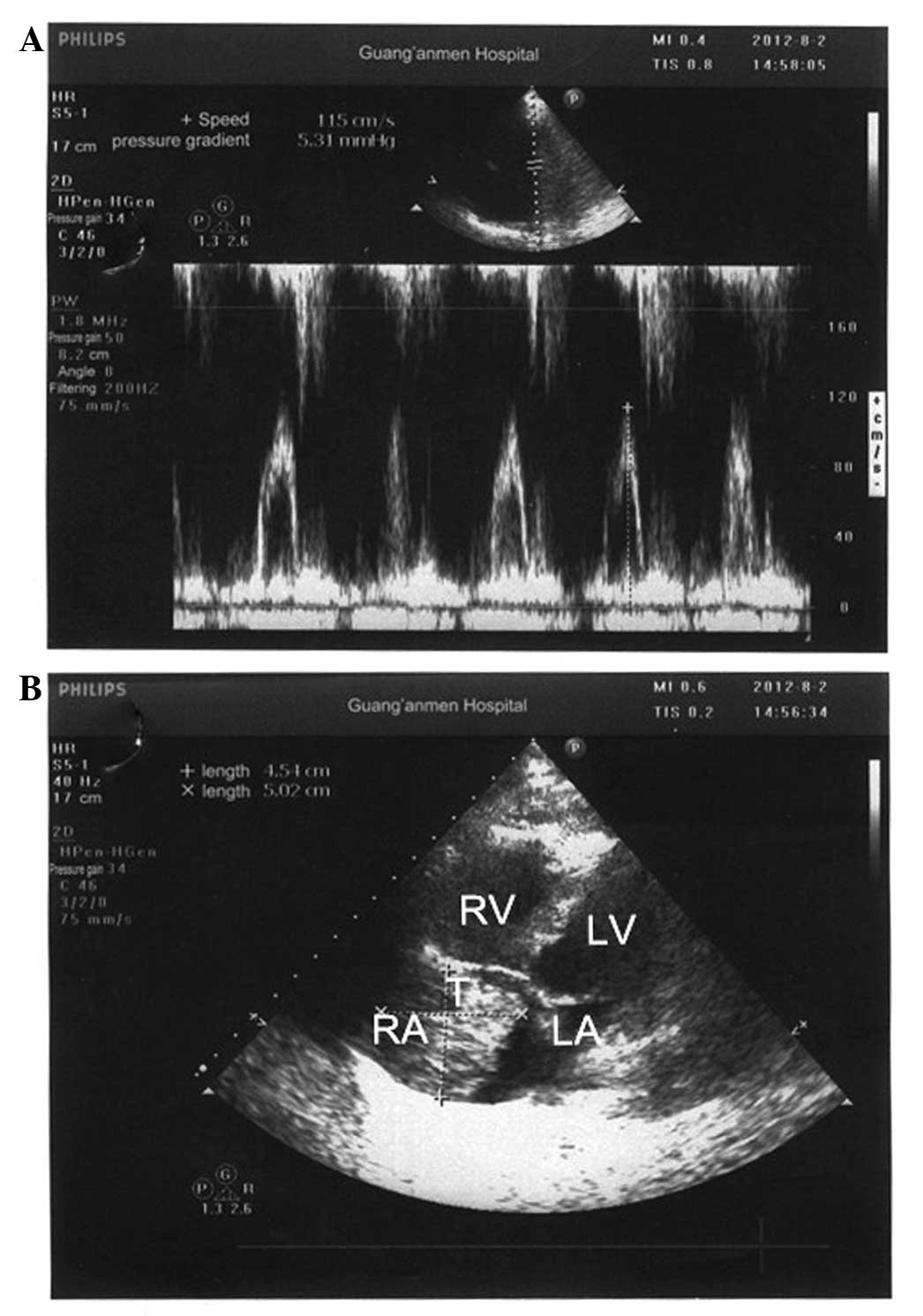
mmHg). Echocardiography revealed a 5.0×4.5-cm mass in the right
atrium (Fig. 3), severe tricuspid
regurgitation and severe pulmonary arterial hypertension.
Subsequently, anti-shock therapy, including high-flow (8 l/min)
oxygen therapy, intravenous fluids and dopamine, were administered.
However, the patient's condition continued to deteriorate and the
patient succumbed as a result of the atrial embolism.
Discussion
Chondrosarcoma accounts for ~15% of all primary
malignant bone tumors (15).
Chondrosarcoma of the spine is rare, and the majority of such cases
occur in the vertebral body or attachments (6,10,16). Chondrosarcoma of the spine is most
frequently identified in the cervical, thoracic and lumbar
vertebrae, with occurrence in the thoracic vertebrae being the most
common (5); this preponderance may be
due to the greater number of thoracic vertebrae (6). Chondrosarcoma is classified into five
types, namely, central, peripheral, mesenchymal, differentiated and
clear cell chondrosarcoma. The two most common types of
chondrosarcoma are central (arising within a bone) and peripheral
(arising from the surface of a bone) (17). In contrast to osteosarcoma,
chondrosarcoma usually occurs in adulthood (18).
Malignancy is one of the main risk factors for a
tumor embolism. A number of previous studies have reported the
occurrence of tumor embolisms in patients with chondrosarcoma
(19–21). A literature search of PubMed for
studies published between 1977 and 2013 was conducted using the
following keywords: (‘tumour embolism’ [All Fields] OR ‘neoplastic
cells, circulating’ [MeSH Terms] OR (‘neoplastic’ [All Fields] AND
‘cells’ [All Fields] AND ‘circulating’ [All Fields]) OR
‘circulating neoplastic cells’ [All Fields] OR (‘tumor’ [All
Fields] AND ‘embolism’ [All Fields]) OR (‘tumor embolism’ [All
Fields]) AND (‘chondrosarcoma’ [MeSH Terms]) OR ‘chondrosarcoma’
[All Fields]). The search identified 32 studies. Of these, 20
studies were associated with the subject of ‘tumor embolism with
chondrosarcoma’ (19,21–39), 14 of
which were reported with pulmonary tumor embolism and 3 of which
exhibited left atrial embolism. However, no previous studies
reporting a large tumor embolism to the right atrium were
found.
Intra-atrial tumor embolism is an extremely rare
manifestation of chondrosarcoma. Three pathways of cardiac
involvement exist: Hematogenous spread, direct invasion from
neighboring chest tumors or via the pericardial space, and
retrograde lymphatic spread (40–43). In
the present case, we hypothesize that circulating tumor cells
migrated to the heart hematogenously, via the superior vena cava,
and that subsequently, the tumor emboli reduced the blood flow
volume of the heart. The tumor emboli may have attached to the
endocardium, causing an intracardiac obstructive mass. A high
proportion of patients with tumor embolisms exhibit widespread
metastatic disease at the time of presentation. This is consistent
for the patient in this case, who exhibited pulmonary metastasis
and bone metastases at presentation.
The clinical presentation of embolisms may include
non-specific symptoms, such as chest pain, weight loss and dyspnea,
or more characteristic symptoms, such as congestive cardiac failure
secondary to intracardiac obstructions and embolic events, as
observed in the present case. Tumor site, size and tendency to
cause an embolism determine the clinical findings. Embolic events
must be considered in patients who develop unexplained heart
failure or dyspnea. In such patients, echocardiography presents a
useful diagnostic step for the detection of cardiac metastasis
(44), as it provides information
regarding the mobility of the tumor thrombus and the association
between the valve and cardiac muscle with respect to the thrombus
(45).
Surgery is the main method of treatment for spinal
chondrosarcoma (46,47), as the disease is not sensitive to
chemotherapy (48). Furthermore,
radiation therapy has been found to have no significant effect on
the post-operative outcome of patients with chondrosarcoma
(10). In the current study, the
patient developed shock following the identification of a mass in
the right atrium by echocardiography. Surgical embolectomy was not
suitable as the patient was in a state of shock with unstable vital
signs. In addition, chemotherapy and radiation therapy were not
suitable due to the insensitivity of chondrosarcoma to such
treatments. Thus, a high level of awareness is required to
establish the diagnosis of an intra-atrial tumor embolism.
Embolisms as a result of this disease may also lead to arrhythmias
and heart failure. As symptoms may mimic other cardiac conditions,
the possibility of an intra-atrial tumor embolism must be
considered in order to provide timely treatment.
In conclusion, chondrosarcoma is a tumor that rarely
extends to involve the right atrium. To the best of our knowledge,
based on the literature search conducted, this is the first case of
thoracic chondrosarcoma with a right atrial tumor embolism to be
reported in the literature. The patient exhibited poor prognosis
due to a number of complications, including pulmonary embolism. The
findings of this study may increase awareness with regard to this
rare tumor, leading to improved clinical treatment of
chondrosarcoma.
References
|
1
|
NCCN Clinical Practice Guidelines in
Oncology. Bone Cancer. Version 1. 2015.National Comprehensive
Cancer Network. 1–83. 2014.
|
|
2
|
Sundaresan N, Rosen G and Boriani S:
Primary maligant tumors of the spine. Orthop Clin North Am.
40:21–36. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Knoeller SM, Uhl M, Gahr N, Adler CP and
Herget GW: Differential diagnosis of primary malignant bone tumors
in the spine and sacrum. The radiological and clinical spectrum:
Minireview. Neoplasma. 55:16–22. 2008.PubMed/NCBI
|
|
4
|
Bergh P, Gunterberg B, Meis-Kindblom JM
and Kindblom LG: Prognostic factors and outcome of pelvic, sacral,
and spinal chondrosarcomas: A center-based study of 69 cases.
Cancer. 91:1201–1212. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lloret I, Server A and Bjerkehagen B:
Primary spinal chondrosarcoma: Radiologic findings with pathologic
correlation. Acta Radiol. 47:77–84. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Boriani S, De Iure F, Bandiera S,
Campanacci L, Biagini R, Di Fiore M, Bandello L, Picci P and
Bacchini P: Chondrosarcoma of the mobile spine: Report on 22 cases.
Spine. 25:804–812. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Harwood AR, Krajbich JI and Fornasier VL:
Radiotherapy of chondrosarcoma of bone. Cancer. 45:2769–2777. 1980.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Krochak R, Harwood AR, Cummings BJ and
Quirt IC: Results of radical radiation for chondrosarcoma of bone.
Radiother Oncol. 1:109–115. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lee FY, Mankin HJ, Fondren G, Gebhardt MC,
Springfield DS, Rosenberg AE and Jennings LC: Chondrosarcoma of
bone: An assessment of outcome. J Bone Joint Surg Am. 81:326–338.
1999.PubMed/NCBI
|
|
10
|
York JE, Berk RH, Fuller GN, Rao JS,
Abi-Said D, Wildrick DM and Gokaslan ZL: Chondrosarcoma of the
spine: 1954 to 1997. J Neurosurg. 90(Suppl): 73–78. 1999.PubMed/NCBI
|
|
11
|
Khorana AA: Venous thromboembolism and
prognosis in cancer. Thromb Res. 125:490–493. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Heit JA, Silverstein MD, Mohr DN,
Petterson TM, O'Fallon WM and Melton LJ III: Risk factors for deep
vein thrombosis and pulmonary embolism: A population-based
case-control study. Arch Intern Med. 160:809–815. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Horsted F, West J and Grainge MJ: Risk of
venous thromboembolism in patients with cancer: A systematic review
and meta-analysis. PLoS Med. 9:e10012752012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shinagare AB, Guo M, Hatabu H, Krajewski
KM, Andriole K, Van den Abbeele AD, DiPiro PJ and Nishino M:
Incidence of pulmonary embolism in oncologic outpatients at a
tertiary cancer center. Cancer. 117:3860–3866. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lee DH, Jung SH, Yoon TM, Lee JK, Joo YE
and Lim SC: Low grade chondrosarcoma of the nasal septum. World J
Clin Cases. 1:64–66. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Camins MB, Duncan AW, Smith J and Marcove
RC: Chondrosarcoma of the spine. Spine (Phila Pa 1976). 3:202–209.
1978. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Matanić D, Kukuljan M, Grgurević E,
Miletić B, Flego V and Muhvić D: Central type of chondrosarcoma
with a fulminant course-a case report. Coll Antropol. 36:1037–1040.
2012.PubMed/NCBI
|
|
18
|
Mahajan AM, Ganvir S, Hazarey V and
Mahajan MC: Chondrosarcoma of the maxilla: A case report and review
of literature. J Oral Maxillofac Pathol. 17:269–273. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hayashida K, Nishimura T, Uehara T, Naito
H, Takamiya M, Kozuka T, Sakakibara H, Imakita M, Yutani C and
Hamada T: A case of pulmonary tumor-embolism from chondrosarcoma of
the lower extremity. Kaku Igaku. 22:101–106. 1985.(In Japanese).
PubMed/NCBI
|
|
20
|
Leung DY, Seah PW, Lee LC, Cranney GB and
Walsh WF: Embolic chondrosarcoma: An unusual cause of pulmonary
embolism. Am Heart J. 126:732–734. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yoshida K, Miyashita N, Nakajima M, Niki Y
and Matsushima T: A case of sternal chondrosarcoma with multiple
pulmonary embolisms. Nihon Kokyuki Gakkai Zasshi. 40:166–170.
2002.(In Japanese). View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Morgan JA and Paone G: Chondrosarcoma
presenting as a saddle tumor pulmonary embolism. J Card Surg.
28:436–438. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Schleger S, Weingartner JS, Costi M and
Eichinger WB: A primary pulmonary artery chondrosarcoma manifesting
as acute pulmonary embolism. Ann Thorac Surg. 94:1731–1733. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kawaguchi T, Yamanouchi Y, Numa Y, Sakurai
Y, Yamahara T, Seno T, Shikata N, Asai A and Kawamoto K: A case of
metastatic brain tumor causing multifocal cerebral embolism. Brain
Tumor Pathol. 29:63–67. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chandrasekharan R, Bhagavaldas MC and
Mathew AJ: Chondrosarcoma presenting as dyspnea in a 19-year-old
man: A case report. J Med Case Rep. 15:1502011. View Article : Google Scholar
|
|
26
|
Oizumi H, Tanaka R, Shimura H, Sasaki K,
Koike H, Hattori N and Tanaka S: A case of cerebral embolism with
metastatic chondrosarcoma in the left atrium. J Stroke Cerebrovasc
Dis. 20:79–81. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Clark N, Goldenkranz RJ, Maeuser H, Brener
BJ, Brief DK, Huston J, Hertz S, Omeish E, Manicone J, Aueron F, et
al: Chondrosarcoma of the aorta: A rare source of bowel and lower
extremity emboli. J Vasc Surg. 28:939–943. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yutani C, Imakita M, Ishibashi-Ueda H,
Katsuragi M, Yoshioka T and Kunieda T: Pulmonary hypertension due
to tumor emboli: A report of three autopsy cases with morphological
correlations to radiological findings. Acta Pathol Jpn. 43:135–141.
1993.PubMed/NCBI
|
|
29
|
Giuliano CT, Kauffman WM, Haller JO,
Fletcher BD and Rao SP: Inferior vena cava-right atrial tumor
thrombus in malignant pelvic bone tumors in children. Pediatr
Radiol. 22:206–208. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ninomiya M, Nakanishi S, Mitsuda H, Egawa
H and Okuhara T: A case of chondrosarcoma of femur forming tumor
embolism in the left heart atrium. Nihon Naika Gakkai Zasshi.
80:270–271. 1991.(In Japanese). View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tiszlavicz L, Nyirádi L and Ormos J: Fatal
chondrosarcoma tumor-embolism. Morphol Igazsagugyi Orv Sz.
27:211–218. 1987.(In Hungarian). PubMed/NCBI
|
|
32
|
Cohen DE, Mora C and Keefe DL:
Echocardiographic findings of metastatic chondrosarcoma involving
the left atrium. Am Heart J. 111:993–996. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Boland TW, Winga ER and Kalfayan B:
Chondrosarcoma: A case report with left atrial involvement and
systemic embolization. J Thorac Cardiovasc Surg. 74:268–272.
1977.PubMed/NCBI
|
|
34
|
Woodring JH, Bognar B and van Wyk CS:
Metastatic chondrosarcoma to the lung with extension into the left
atrium via invasion of the pulmonary veins: Presentation as embolic
cerebral infarction. Clin Imaging. 26:338–341. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Mangiapan G, Parrot A, Antoine M and
Mayaud C: Pulmonary artery hypertension due to tumor
micro-embolism. Rev Mal Respir. 12:62–65. 1995.(In French).
PubMed/NCBI
|
|
36
|
Shepard JA, Moore EH, Templeton PA and
McLoud TC: Pulmonary intravascular tumor emboli: Dilated and beaded
peripheral pulmonary arteries at CT. Radiology. 187:797–801. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Miyawaki F, Matsumoto H, Takayama Y and
Asano K: Surgical treatment of transcardiac expansion of ingrowing
chondrosarcoma. Kyobu Geka. 36:632–635. 1983.(In Japanese).
PubMed/NCBI
|
|
38
|
Scott AD, Crane P and Staunton MD:
Chondrosarcoma - local recurrence and systemic embolization. JR Soc
Med. 83:48–49. 1990.
|
|
39
|
Hayashida K, Nishimura T, Uehara T, Naito
H, Takamiya M, Nakajima N and Kozuka T: Cor pulmonale due to
embolization of metastatic chondrosarcoma. Heart Vessels.
3:218–222. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Mahdi H, Swensen RE, Hanna R, Kumar S,
Ali-Fehmi R, Semaan A, Tamimi H, Morris RT and Munkarah AR:
Prognostic impact of lymphadenectomy in clinically early stage
malignant germ cell tumour of the ovary. Br J Cancer. 105:493–497.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Reynen K, Köckeritz U and Strasser RH:
Metastases to the heart. Ann Oncol. 2:375–381. 2004. View Article : Google Scholar
|
|
42
|
Butany J, Nair V, Naseemuddin A, Nair GM,
Catton C and Yau T: Cardiac tumours: Diagnosis and management.
Lancet Oncol. 6:219–228. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Neragi-Miandoab S, Kim J and Vlahakes GJ:
Malignant tumours of the heart: A review of tumour type, diagnosis
and therapy. Clin Oncol (R Coll Radiol). 19:748–756. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Tse HF, Lau CP, Lau YK and Lai CL:
Transesophageal echocardiography in the detection of inferior vena
cava and cardiac metastasis in hepatocellular carcinoma. Clin
Cardiol. 19:211–213. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Yoshitomi Y, Kojima S, Sugi T, Matsumoto
Y, Yano M, Ozeki Y and Kuramochi M: Echocardiography of a right
atrial mass in hepatocellular carcinoma. Heart Vessels. 13:45–48.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Weber KL: What's new in musculoskeletal
oncology. J Bone Joint Surg Am. 87:1400–1410. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Pring ME, Weber KL, Unni KK and Sim FH:
Chondrosarcoma of the pelvis. J Bone Joint Surg Am. 83:1630–1642.
2001.PubMed/NCBI
|
|
48
|
Dickey ID, Rose PS, Fuchs B, Wold LE,
Okuno SH, Sim FH and Scully SP: Dedifferentiated chondrosarcoma:
The role of chemotherapy with updated outcomes. J Bone Joint Surg
Am. 86:2412–2418. 2004.PubMed/NCBI
|