Introduction
Gastric cancer (GC) is one of the most common types
of cancer and the second highest cause of cancer mortality
worldwide (1). A large number of
gastric cancer patients are diagnosed once the tumor has
metastasized and has reached an advanced stage (2). Clinicians treat patients using
conventional and targeted therapies, but these methods have little
therapeutic effect.
A large number of molecular markers are associated
with the metastasis of tumors, and one of the most important
factors leading to this malignancy is epithelial mesenchymal
transition (EMT), which is characterized by the gain of stem cell
properties and the promotion of tumor invasion and metastasis
(3,4).
The features of EMT include the loss of the adhesion molecule
epithelial-cadherin (E-cadherin) and gain of mesenchymal tissue
markers.
The cancer stem cell (CSC) hypothesis has been
proposed to explain the pathogenesis of a number of types of cancer
(5). The notable properties of CSCs
are their ability for self-renewal and cell proliferation, they can
initiate tumor formation, self-renewal, differentiation and cause
cancer recurrence and metastasis. Furthermore, CSCs are more
chemoresistant and radioresistant than their differentiated,
daughter cancer cells, which may be the reason for treatment
failure for malignant tumors.
Notch signaling, which serves a pivotal role in
cellular differentiation, proliferation and apoptosis, is disrupted
in several malignancies and offers a potential target for
therapeutic intervention. Abnormal activation of Notch1 signaling
has been observed in gastric cancer cells (6) and correlates with colony-forming ability
and xenografted tumor growth (7).
Inhibition of Notch1 signaling with a γ-secretase inhibitor
resulted in a significant reduction in GBM cell growth in
vitro and in vivo (3). In
addition, the Notch1 signaling pathway is also critical in
maintaining the characteristics of CSCs and is associated with the
self-renewal of various types of CSCs, such as breast and
pancreatic cancer (8). However, the
role of Notch1 signaling in gastric CSCs (GCSCs) is not clear.
The present study aimed to examine the role of
Notch1 in GCSCs by treating those cells with the γ-secretase
inhibitor DAPT. In addition, the role of Notch1 signaling in EMT
within GCSCs was investigated.
Materials and methods
Cells and animals
The human gastric cancer cell line MKN-45 was
purchased from the Type Culture Collection of the Chinese Academy
of Sciences (TCCCA; Shanghai, China). The cell line was cultured in
RPMI-1640 (GE Healthcare Life Sciences, Logan, UT, USA)
supplemented with 10% fetal bovine serum (FBS, GE Healthcare Life
Sciences) and were maintained at 37°C in a 5% CO2
atmosphere.
A total of 50 4-week-old female nude mice were
obtained from the Shanghai Experimental Animal Center of the
Chinese Academy of Science (Shanghai, China). The mice were
maintained in cages (5 mice/cage) in a room with a constant
temperature (22±1°C) and a dark-light cycle. The present study was
conducted in strict accordance with the recommendations of the
Guide for the Care and Use of Laboratory Animals of Chongqing
Medical University. The protocol was approved by the Committee on
the Ethics of Animal Experiments of Chongqing Cancer Institute
(Chongqing, China).
Preparation of CD44+ and CD44−
MKN45 cells for in vitro and in vivo analysis of tumorigenicity.
CD44+ and CD44− populations were sorted from
the human gastric cancer cell line, MKN45. For
fluorescence-activated cell sorting (FACS), 5–10×106
cells were harvested and incubated for 30 min at room temperature
with a 10-fold dilution of the following antibodies:
Anti-CD44-fluorescein isothiocyanate rat monoclonal antibody and
anti-CD44-PE (eBioscience, San Diego, CA, USA). Then, the cells
were detected using a FACS-LSRII flow cytometer (Becton Dickinson,
Franklin Lakes, NJ, USA). The cells were routinely sorted twice and
reanalyzed for purity (XDP, Beckman-Coulter).
For in vivo experiments, CD44+ and
CD44− cells were resuspended in PBS and were injected
subcutaneously into the limbs of mice. Groups of mice were
inoculated with CD44+ or CD44− cells at
1×103, 3×103, 1×104 and
5×104 (5 mice/group), and tumor growth was monitored
every 2 days after the second week of inoculation. Another 2 groups
of mice were injected with 5×104 CD44+ MKN45
cells for intraperitoneal treatment with γ-secretase inhibitor
N-[N-(3,5-difluorophenacetyl)-l-ananyl]-S-phenyglycine t-butyl
ester (DAPT, Sigma-Aldrich, St. Louis, MO, USA). For in
vitro experiments, the sorted cells were cultured in RPMI-1640
and assessed by western blotting, proliferation, self-renewal,
tumor-initiation, migration and invasion assays.
Drug and treatment
For in vitro experiments, DAPT was prepared as a 10
µM stock in DMSO (Sigma-Aldrich). CD44+ and
CD44− cells were treated with DMSO or DAPT (10 µM) and
were analyzed after 72 h. Animals were treated intraperitoneally
with a drug concentration of 10 mg/kg/body weight or with the
vehicle (control) once daily for 5 weeks, using a 3-days-on and
4-days-off intermittent-dose schedule, as described previously
(9).
Spheroid colony formation assay
Cells were seeded into each well (20 cells per well)
of ultra-low-attachment 48-well plates (Beyotime Institute of
Biotechnology, Shanghai, China) and supplemented with 300 µl of
RPMI-1640 plus 40 ng/ml bFGF and 20 ng/ml EGF (Invitrogen Life
Technologies, Carlsbad, CA, USA). After 4 weeks, the total number
of spheroid colonies/well were counted.
Cell chemosensitivity examination
Cells cultured in medium were incubated and treated
with 5-fluorouracil (6 mM) (Sigma-Aldrich). After 48 h of exposure
to the chemotherapeutic agents, 20 ml of
3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium bromide
(MTT, Sigma-Aldrich) solution (0.5 mg/ml) was added for an
additional 4 h before 100 ml dimethyl sulfoxide (DMSO,
Sigma-Aldrich) was added for 15 min. The plates were then shaken
gently for 5 min and measured at 570 nm using a spectrophotometer.
A total of 5 wells were assayed for each condition.
Migration and invasion assays
The cells were added to the upper chambers, and the
lower chambers were filled with 750 ml of RPMI-1640 media with 10%
FBS. The cells were incubated for 24 h at 37°C in 5%
CO2. After 24 h, the non-migrated/non-invading cells
were removed from the upper sides, and the migrated/invaded cells
that were on the lower sides of the inserts were stained. The
absorbance of the wells were read at 560 nm using a RF-5301PC
fluorescence spectrometer (Shimadzu Corporation, Kyoto, Japan)
according to the manufacturer's protocol.
Immunoblotting
Total protein for immunoblots was extracted from
cells using RIPA lysis buffer (Beyotime Institute of Biotechnology)
according to the manufacturer's instructions. After the protein
extracts were quantified using a BCA protein assay, equivalent
amounts of lysates were resolved by 10% SDS polyacrylamide gel
electrophoresis (Beyotime Institute of Biotechnology) and
transferred onto a polyvinylidene fluoride (PVDF) membrane
(Beyotime Institute of Biotechnology), which was then blocked in 5%
non-fat milk in TBST (Beyotime Institute of Biotechnology) for 1 h
at 4°C. Then the blots were incubated with primary antibodies
overnight at 4°C and washed with PBST 3 times (each time for 5
min), subsequently incubated with HRP-conjugated secondary
antibodies for 1 h at room temperature and washed with PBST 3 times
(each time for 5 min). The signal was detected using an enhanced
chemiluminescence reagent (Millipore, Billerica, MA, USA).
The mouse monoclonal antibodies against GAPDH and
Snail were purchased from BD Biosciences (Franklin Lakes, NJ, USA).
The rabbit monoclonal antibodies against ZO1 N-cadherin, E-cadherin
and Vimentin were purchased from Abcam (Abcam; Cambridge, UK). The
monoclonal goat anti-rabbit IgG and goat anti-mouse Horseradish
peroxidase (HRP)-conjugated antibodies were purchased from Santa
Cruz Biotechnology.
The antibodies were diluted in 5% non-fat milk as
follows: anti-Notch1, 1:1,200; anti-Hes1, 1:10,000;
anti-E-cadherin, 1:1,200; anti-N-cadherin, 1:1,200; anti-vimentin,
1:1,200; anti-Snail, 1:500; anti-ZO1, 1:500; anti-GAPDH, 1:500; and
HRP-conjugated IgG, 1:7,000.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
RNA was purified from cell lines using RNAiso
(Takara Bio, Inc., Otsu, Japan), and cDNA was synthesized using the
Synthesis Kit (Takara Bio, Inc.). RT-qPCR was performed using a
CFX96 Real-Time PCR Detection System (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA) with SYBR® Premix Ex Taq II (Takara Bio, Inc.).
The PCR conditions were as follows: 95°C for 30 sec, followed by 40
cycles of 95°C for 5 sec, then 60°C for 30 sec, and the data were
normalized against the β-actin RNA. The sequences of the PCR
primers for each of the gene transcripts were as follows: Notch1,
sense 5′-TGC CGA ACC AAT ACA ACC CTC-3′ and anti-sense
5′-TGG TAG CTC ATC ATC TGG GACA-3′; Hes1, sense 5′-GTG CAT GAA CGA
GGT GAC CC-3′ and anti-sense 5′-GTA TTA ACG CCC TCG CAC GT-3′;
β-actin, sense 5′-CCA CGA AAC TAC CTT CAA CTCC-3′ and
anti-sense 5′-GTG ATC TCC TTC TGC ATC CTGT-3′.
Histological examination
Tumor tissues were fixed in 10% neutral-buffered
formalin and embedded in paraffin and then sectioned and stained
with hematoxylin and eosin (HE, Sigma-Aldrich). Histological
differences were examinedusing an Optical Microscope (Olympus
Corporation, Tokyo, Japan).
Statistical analysis
All the experiments were repeated 3 times, and the
results were analyzed using the SPSS software, version 16.0 (SPSS,
Inc., Chicago, IL, USA). Data are presented as the mean ± standard
deviation (SD). Group comparisons were performed using the t-test,
the nonparametric test and one-way analysis of variance.
Differences were considered statistically significant when
P<0.05.
Results
CD44+ cells isolated from
the MKN45 cell line display the characteristics of CSCs
Tumors contain a small number of CSCs that have
self-renewal and tumor-initiating abilities (10). In the spheroid colony formation assay,
the CD44+ MKN45 cells formed a greater number of
spheroids compared with CD44− MKN45 cells (Fig. 1A; P<0.05). In the tumorigenicity
assay, nude mice were injected with 1×103 to
5×104 CD44+ or CD44− MKN45 cells.
Transplantation of 1×103, 5×103 or
1×104 CD44− cells consistently failed to form
tumors in all mice, while 5×104 CD44− cells
resulted in tumor formation in 1/5 mice. In contrast,
transplantation of 1×103 CD44+ cells failed
to form tumors in all mice, however, the transplantation of
5×103, 1×104 or 5×104
CD44+ cells into nude mice resulted in tumor formation
in 2/5, 4/5 or 5/5 mice, respectively (Table I).
 | Table I.Tumorigenicity of CD44+
and CD44− cells in nude mice. |
Table I.
Tumorigenicity of CD44+
and CD44− cells in nude mice.
|
| Cell numbers of
injection |
|---|
|
|
|
|---|
|
CD44+/− |
1×103 |
5×103 |
1×104 |
5×104 |
|---|
| CD44+
cells | 0/5 | 2/5 | 4/5 | 5/5 |
| CD44−
cells | 0/5 | 0/5 | 0/5 | 1/5 |
Since chemotherapy resistance is a common
characteristic of CSCs, the susceptibility of CD44+ and
CD44− MKN45 cells susceptibility to 5-fluorouracil
(5-FU) treatments was assessed, which is generally used for the
treatment of GC (Fig. 1B).
CD44+ cells were more chemoresistant compared with
CD44− cells and exhibited a cell survival rate of
71.5±2.0% after 48-h incubation, compared with 32.6±1.9% for
CD44− cells (P<0.05).
These data indicate that CD44+ gastric
cancer cells were tumorigenic and possessed CSC
characteristics.
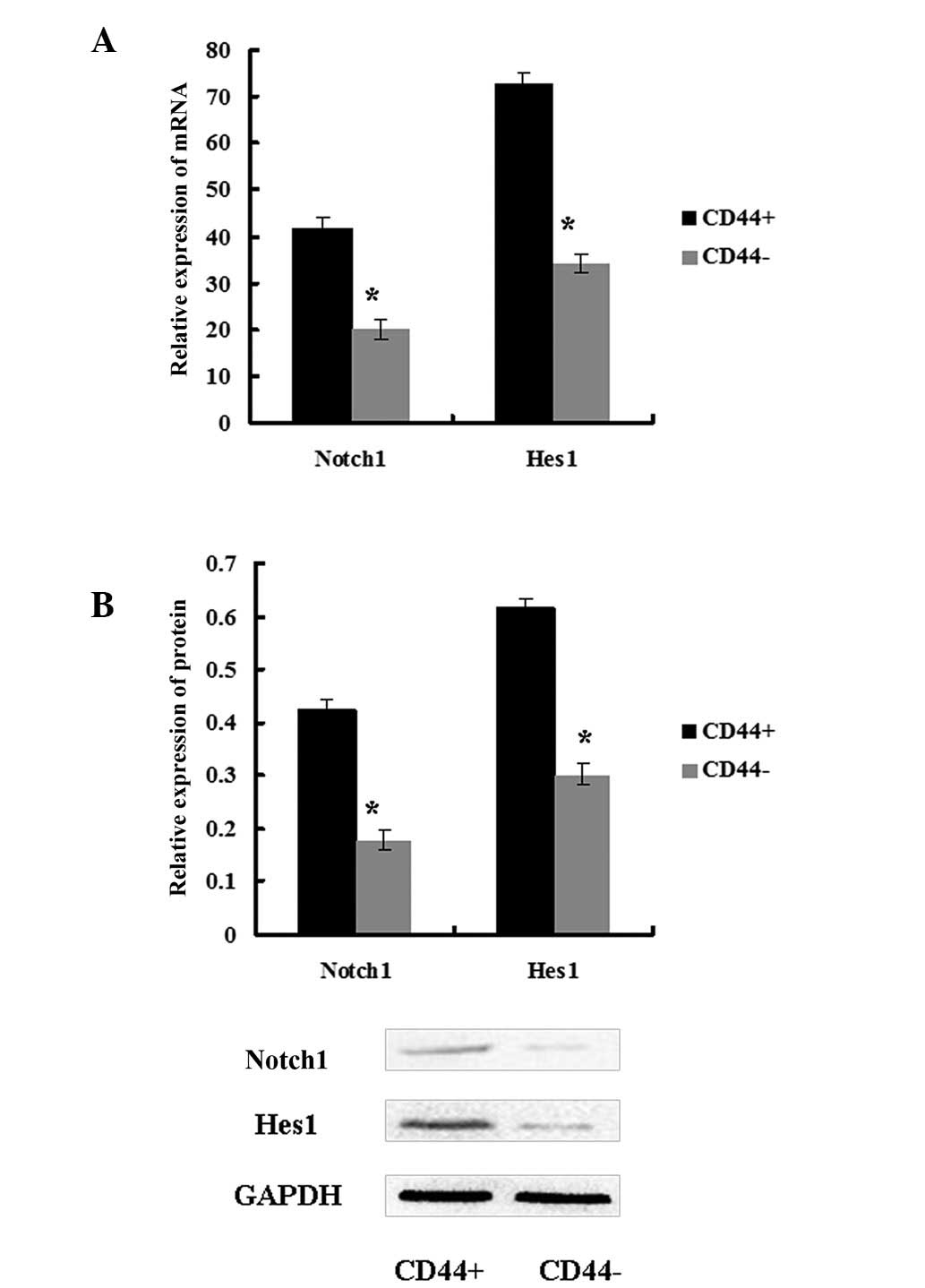
The Notch1 signaling pathway was
activated in CD44+ MKN45 cells
To explore the role of the Notch1 pathway in CSCs,
the expression of Notch1 and its downstream target Hes1 was
assessed in CD44+ and CD44− MKN45 cells.
Notch1 and Hes1 expression levels were higher in CD44+
cells compared with in CD44− cells (Fig. 2). These data demonstrated that the
Notch1 signaling pathway was activated in GCSCs.
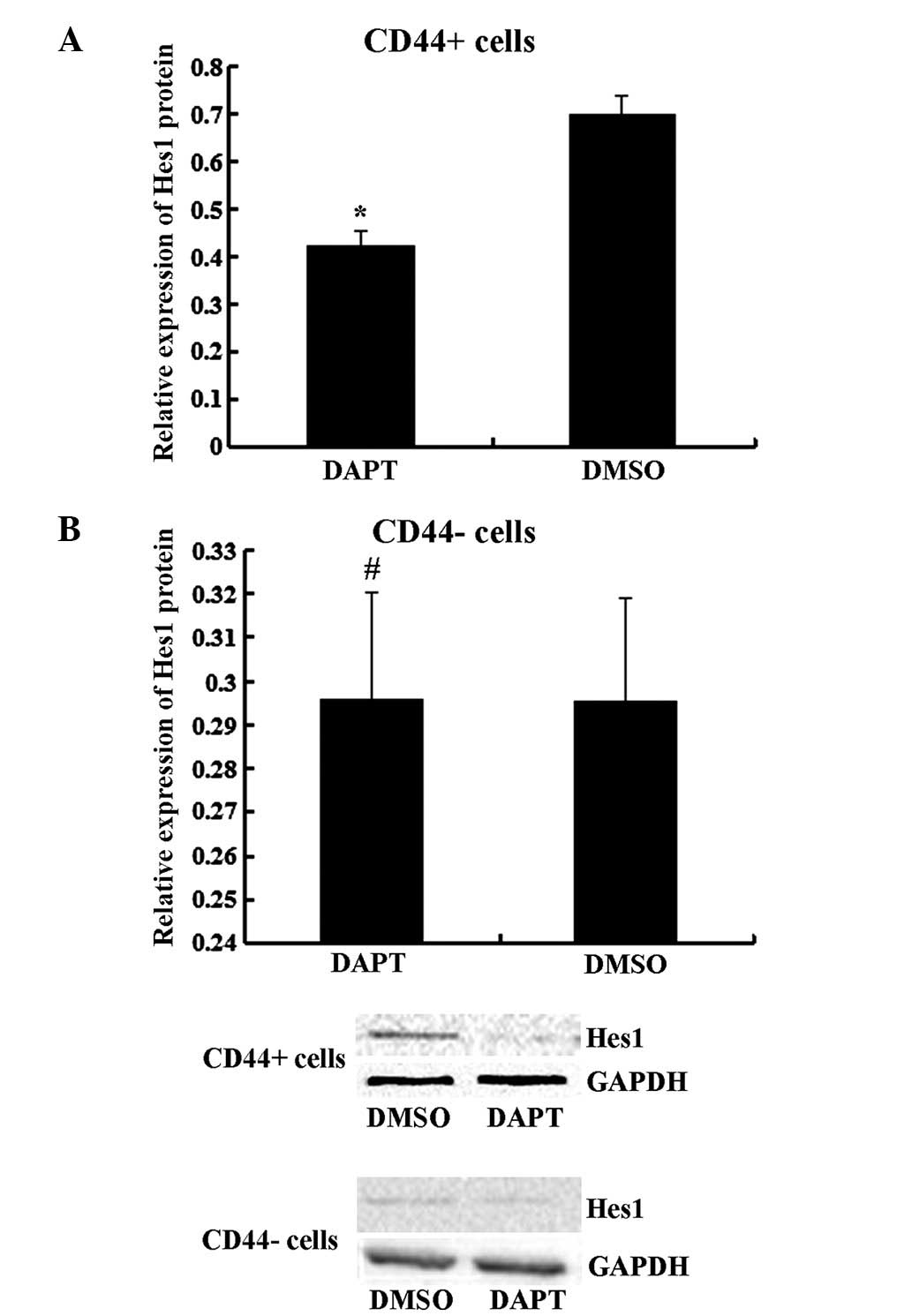
The γ-secretase inhibitor DAPT
attenuated the self-renewal, tumor-initiating, migration and
invasion abilities of CD44+ MKN45 cells
It has previously been demonstrated that Notch1
signaling serves a role in stem cell renewal and cell fate
determination in neural, hematopoietic and embryonic stem cells
(4). To further determine the effect
of the Notch1 pathway, CD44+ and CD44− cells
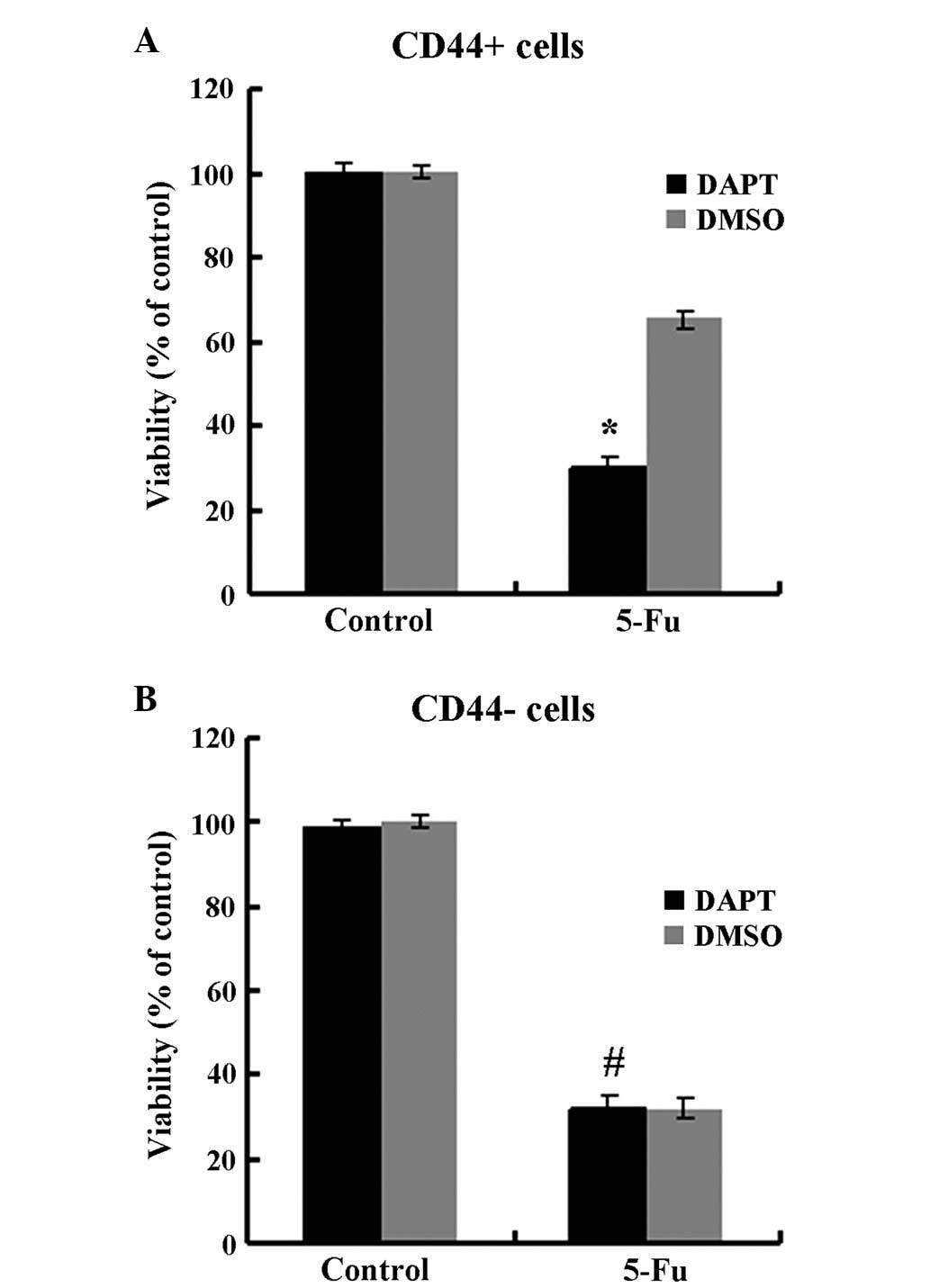
were treated with DAPT. As presented in Fig. 3, DAPT treatment suppressed the
expression of the Notch1 downstream target Hes1 in CD44+
cells (P<0.05) but not in CD44− cells (P>0.05).
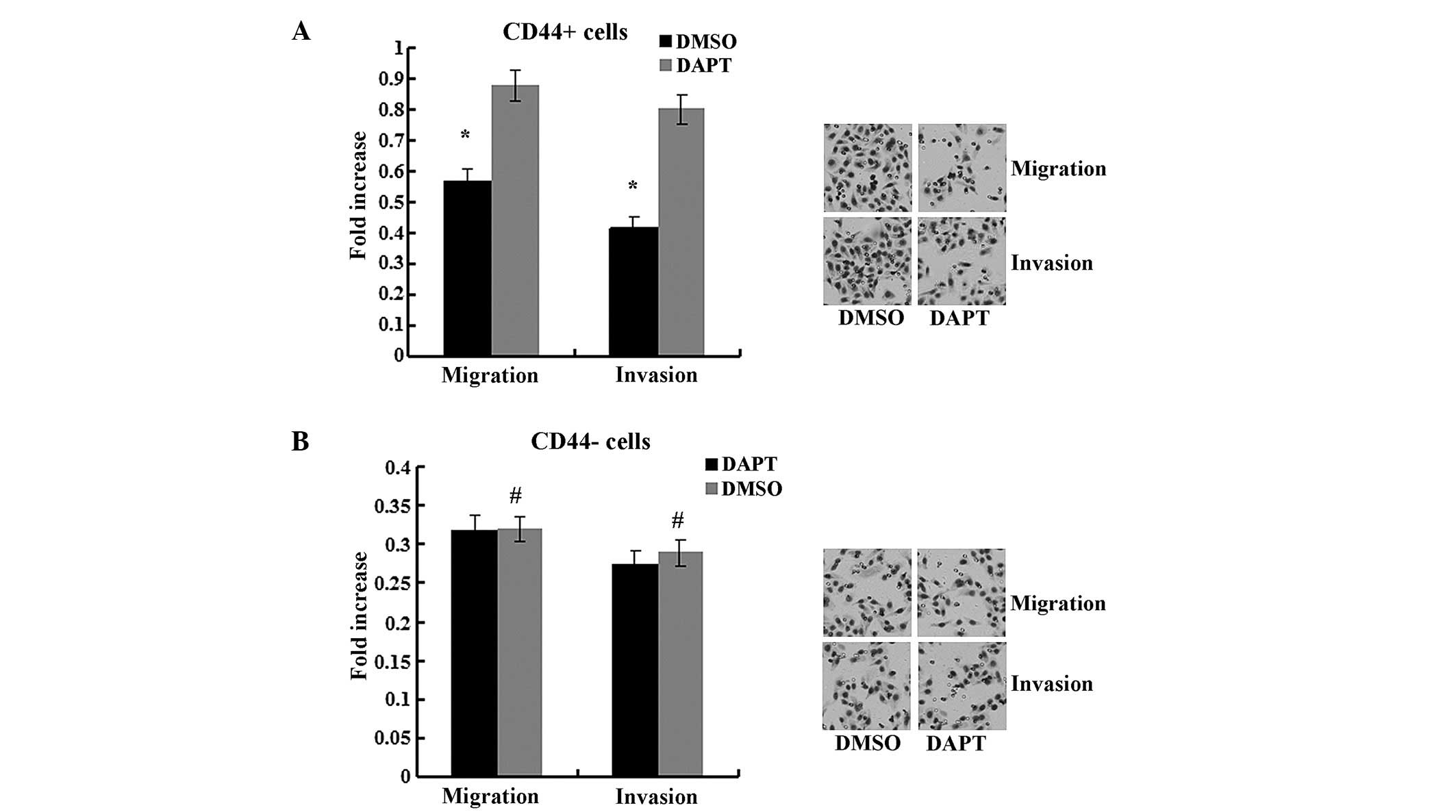
The migration and invasion abilities were impaired by DAPT in
CD44+ cells compared to cells treated with DMSO
(Fig. 4, P<0.05) but not in
CD44− cells (P>0.05). In the spheroid colony
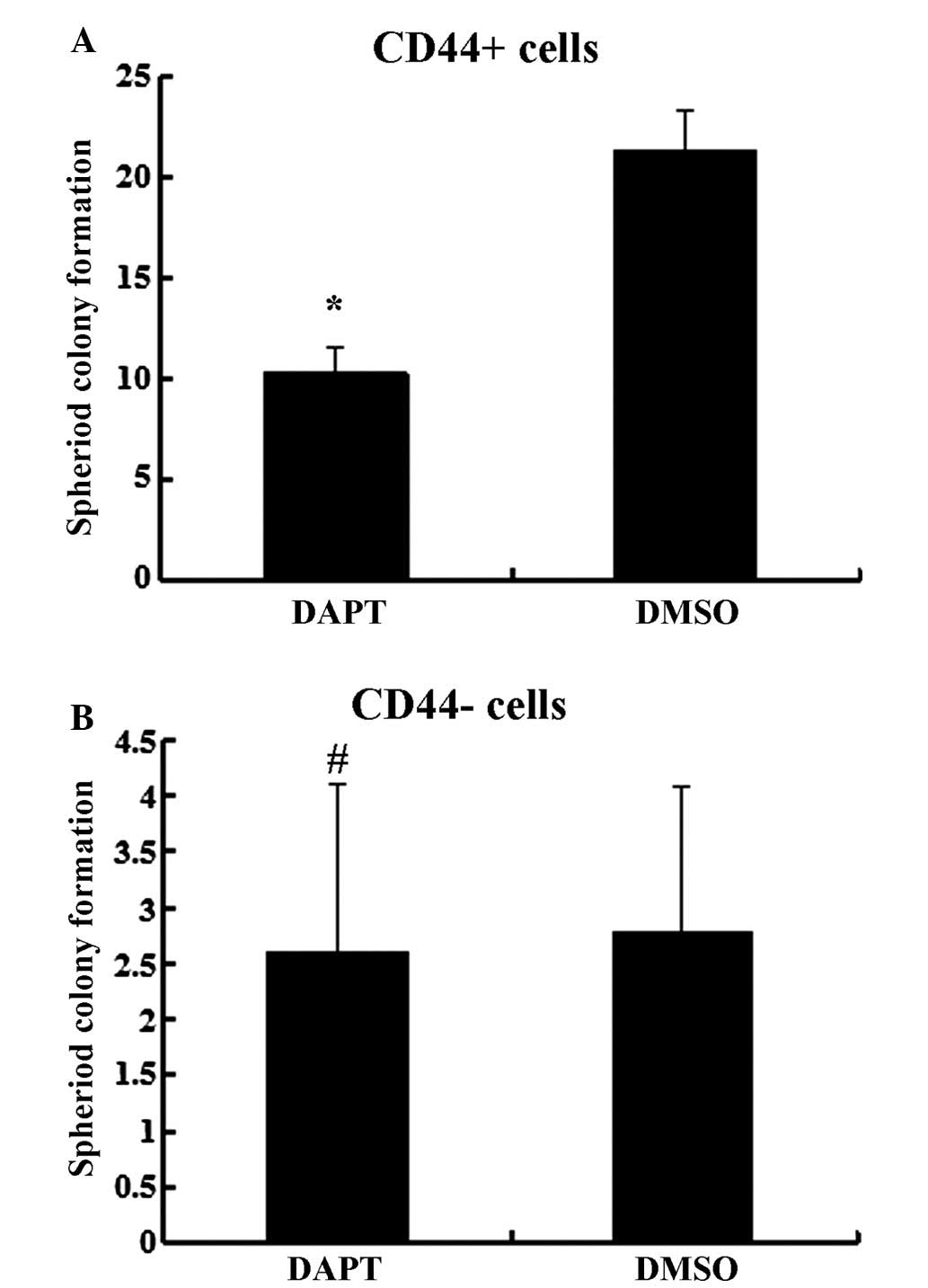
formation assay, CD44+ cells that were treated with DAPT
formed fewer spheroids compared with cells treated with DMSO
(Fig. 5; P<0.05), and results from
the MTT assay demonstrated that the chemotherapy susceptibility of
CD44+ cells that were treated with DAPT was upregulated
compared with cells treated with DMSO (Fig. 6; P<0.05). However, those changes in
migration and chemotherapeutic susceptibility were not observed in
CD44− MKN45 cells (Figs. 5
and 6; P>0.05). These data
demonstrated that the γ-secretase inhibitor DAPT suppressed the
Notch1 signaling pathway and inhibited the self-renewal,
tumor-initiating, migration and invasion abilities and improved
chemotherapy susceptibility of CD44+ MKN45 cells.
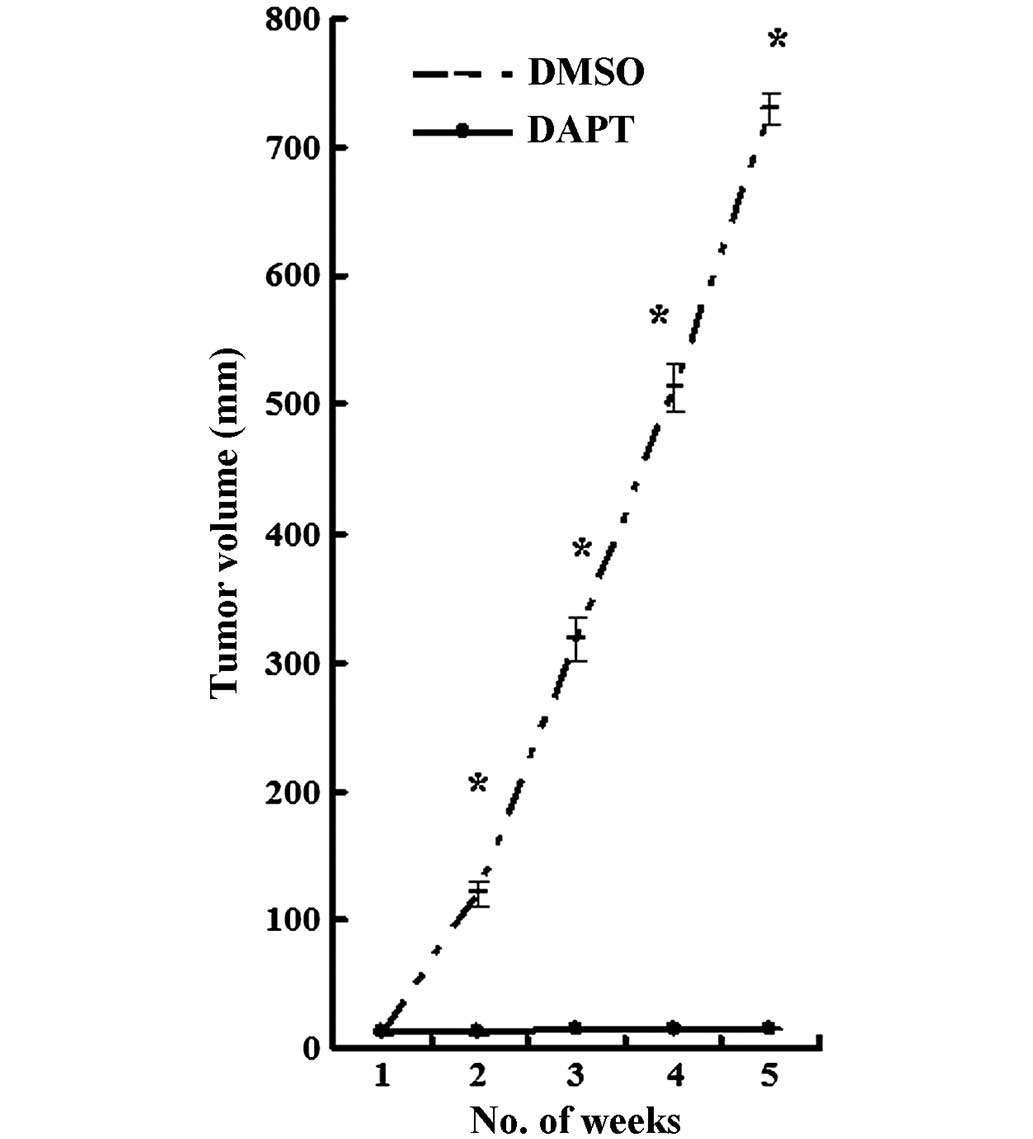
Intraperitoneal treatment with DAPT
effectively inhibited the growth of CD44+ MKN45 cell
xenograft tumors
Given the ability of DAPT to inhibit
CD44+ MKN45 cells in vitro, the role of DAPT was tested
in a nude mouse model. CD44+ MKN45 cells were
subcutaneously injected into the flanks of mice, and
intraperitoneal treatment with either DAPT or DMSO was initiated
when the tumor volume reached a size of 10 mm3. All mice
were treated for 5 weeks, using an established, 3-days-on and
4-days-off, intermittent dose schedule (9). Xenograft tumors grew continuously in
vehicle-treated animals, whereas DAPT treatment significantly
inhibited tumor growth (Fig. 7;
P<0.05).
The γ-secretase inhibitor DAPT
prevented epithelial-mesenchymal transition in CD44+
MKN45 cells
To further explore the molecular mechanism of the
inhibition of DAPT on EMT in CSCs, the expression of EMT markers
was examined. Western blot analysis demonstrated that the protein
expression levels of the epithelial markers E-cadherin and ZO1 were
upregulated, and expression of the mesenchymal markers N-cadherin,
Vimentin and Snail were downregulated in CD44+ cells
that were treated with DAPT compared with cells treated with DMSO.
The expression levels of these EMT markers were not changed by DAPT
treatin CD44− MKN45 cells (Fig. 8; P>0.05). Altogether, these results
indicated that the γ-secretase inhibitor DAPT could impair EMT in
CD44+ MKN45 cells (Fig.
8).
Discussion
CSCs have been identified in a number of
malignancies and are functionally defined by their ability to
undergo self-renewal and produce differentiated progeny (11,12). CSCs
may display certain properties and they may be isolated based on
cell surface-marker profiles (13).
CSCs exhibit increased resistance against conventional chemotherapy
(14,15), and they may initiate tumors at
limiting dilutions in animals (5). A
number of previous studies have validated the CSC hypothesis by
isolating CSCs from gastric cancer patients, and CD44 has been
identified as a surface marker of GCSCs (16–20). In
the present study, CD44+ MKN45 cells were sorted by
FACS. CD44+ cells exhibited increased resistance against
chemotherapy and formed a greater number of spheroids in
vitro and tumors in vivo, which indicated that
CD44+ MKN45 cells possessed properties of CSCs.
Numerous studies have focused specifically on the
signaling pathways that may mediate the resistance of CSCs
(21,22). Notch signaling is involved in the
development and progression of several solid tumors (23). In addition, Notch1 signaling is
implicated in the self-renewal of various types of CSCs, including
breast cancer, medulloblastoma and pancreatic cancer (8). Notch1 activation induces up-regulation
of Hes1 expression, which dictated cell fate decisions in
hematopoietic stem and progenitor cells (24). In the present study, the role of the
Notch signaling pathway was investigated in GCSCs. Gastric cancer
cell line MKN45 was derived from gastric cancer metastatic tissue
(25). In addition, Notch1 expression
was upregulated in MKN45 cells compared with other cells derived
from non-metastatic gastric cancer in our previous studies
(26). Therefore, MKN45 cells were
selected for the present study. In the present study, the
expression levels of Notch1 and its downstream target Hes1 were
higher in CD44+ MKN45 cells compared with
CD44− cells, which is in accordance with previous
studies (27).
The interaction of Notch ligands with their
receptors promotes a γ-secretase-dependent cleavage of the Notch
receptor and releases the Notch intracellular domain (NICD), which
results in activation of the pathway and induces target genes such
as Hes1. Therefore, suppressing γ-secretase function with the
γ-secretase inhibitor DAPT blocks the Notch signaling pathway
(9). To explore the function of
Notch1 signaling, CD44+ and CD44− cells were
treated with DAPT. Hes1 expression was downregulated in
CD44+ cells. Meanwhile, the self-renewal,
tumor-initiation, migration and invasion abilities of
CD44+ cells were inhibited, and chemotherapy
susceptibility was improved. Treatment with DAPT resulted in a
significant inhibition of gastric CD44+ cells in
vivo. However, those phenomena did not appear in
CD44− cells. These data indicate that the Notch1 pathway
may contribute to maintaining properties of GCSCs and their ability
for migration and invasion.
To explore the molecular mechanisms of DAPT's effect
on GCSCs, the expression of EMT markers were determined. During
EMT, epithelial cells lose many of their epithelial
characteristics, such as the expression of E-cadherin and ZO1, and
instead, acquire properties that are typical for mesenchymal cells
such as the expression of vimentin. DAPT treatment inhibited the
expression of Notch1 signaling pathway members and its downstream
target Hes1, which reduced Snail expression and impaired EMT in
CD44+ cells: These results were in accordance with other
previous studies (28,29). It has been proposed that transformed
epithelial cells may activate embryonic programs for epithelial
plasticity and switch from sessile and epithelial phenotypes to
motile and mesenchymal phenotypes (30). Therefore, the induction of EMT may
lead to the invasion of surrounding stroma, intravasation,
dissemination and colonization of distant sites (31). Under the CSC hypothesis, sustained
metastatic growth requires the dissemination of a CSC from the
primary tumor followed by its reestablishment at a secondary site
(32). In EMT, cancer cells acquire
the ability to invade the surrounding microenvironment and, thus,
may lead to relapse and metastases (33).
The findings of the present study support the idea
that the CD44+ population in human gastric cancer
possess properties of CSCs and corroborate the CSC hypothesis. In
addition, the role of the Notch1 signaling pathway in GCSCs was
demonstrated and inhibition by DAPT was shown to impair properties
of GCSCs and the process of EMT. In the future, clinical
investigations are needed to confirm these results and to establish
DAPT as part of the treatment for gastric cancer patients.
Acknowledgements
The authors would like to acknowledge grant support
from the National Natural Science Foundation of China (no.
81172387) and the National Natural Science Foundation of Chongqing
(CSTC 2011BB5119).
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wagner AD, Unverzagt S, Grothe W, et al:
Chemotherapy for advanced gastric cancer. Cochrane Database Syst
Rev. 3:CD0040642010.PubMed/NCBI
|
|
3
|
Chen J, Kesari S, Rooney C, et al:
Inhibition of notch signaling blocks growth of glioblastoma cell
lines and tumor neurospheres. Genes Cancer. 1:822–835. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Takebe N, Harris PJ, Warren RQ and Ivy SP:
Targeting cancer stem cells byinhibiting Wnt, Notch and Hedgehog
pathways. Nat Rev Clin Oncol. 8:97–106. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Visvader JE and Lindeman GJ: Cancer stem
cells in solid tumours: Accumulating evidence and unresolved
questions. Nat Rev Cancer. 8:755–768. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Brzozowa M, Mielańczyk L, Michalski M, et
al: Role of Notch signaling pathway in gastric cancer pathogenesis.
Contemp Oncol (Pozn). 17:1–5. 2013.PubMed/NCBI
|
|
7
|
Piazzi G, Fini L, Selgrad M, et al:
Epigenetic regulation of Delta-Like1 controls Notch1 activation in
gastric cancer. Oncotarget. 2:1291–1301. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pannuti A, Foreman K, Rizzo P, et al:
Targeting Notch to target cancer stem cells. Clin Cancer Res.
16:3141–3152. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Palagani V, El Khatib M, Kossatz U, et al:
Epithelial mesenchymal transition and pancreatic tumor initiating
CD44+/EpCAM+ cells are inhibited by γ-secretase inhibitor IX. PLoS
One. 7:e465142012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yin B, Zeng Y, Liu G, Wang X, Wang P and
Song Y: MAGE-A3 is highly expressed in a cancer stem cell-like side
population of bladder cancer cells. Int J Clin Exp Pathol.
7:2934–2941. 2014.PubMed/NCBI
|
|
11
|
Ailles LE and Weissman IL: Cancer stem
cells in solid tumors. Curr Opin Biotechnol. 18:460–466. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Alison MR, Murphy G and Leedham S: Stem
cells and cancer: A deadly mix. Cell Tissue Res. 331:109–124. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ricci-Vitiani L, Lombardi DG, Pilozzi E,
Biffoni M, Todaro M, Peschle C and De Maria R: Identification and
expansion of human colon-cancer-initiating cells. Nature.
445:111–115. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tan S, Chen JS, Sun LJ and Yao HR:
Selective enrichment of hepatocellular cancer stem cells by
chemotherapy. J Int Med Res. 37:1046–1056. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bao S, Wu Q, McLendon RE, Hao Y, Shi Q,
Hjelmeland AB, Dewhirst MW, Bigner DD and Rich JN: Glioma stem
cells promote radioresistance by preferential activation of the DNA
damage response. Nature. 444:756–760. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Han ME, Jeon TY, Hwang SH, Lee YS, Kim HJ,
Shim HE, Yoon S, Baek SY, Kim BS, Kang CD and Oh SO: Cancer spheres
from gastric cancer patients provide an ideal model system for
cancer stem cell research. Cell Mol Life Sci. 68:3589–3605. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen T, Yang K, Yu J, Meng W, Yuan D, Bi
F, Liu F, Liu J, Dai B, Chen X, et al: Identification and expansion
of cancer stem cells in tumor tissues and peripheral blood derived
from gastric adenocarcinoma patients. Cell Res. 22:248–258. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jiang J, Zhang Y, Chuai S, Wang Z, Zheng
D, Xu F, Zhang Y, Li C, Liang Y and Chen Z: Trastuzumab (herceptin)
targets gastric cancer stem cells characterized by CD90 phenotype.
Oncogene. 31:671–682. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang C, Li C, He F, Cai Y and Yang H:
Identification of CD44+CD24+ gastric cancer stem cells. J Cancer
Res Clin Oncol. 137:1679–1686. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xu G, Shen J, Ou Yang X, Sasahara M and Su
X: Cancer stem cells: The 'heartbeat' of gastric cancer. J
Gastroenterol. 48:781–797. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Teng Y, Wang X, Wang Y and Ma D:
Wnt/beta-catenin signaling regulates cancer stem cells in lung
cancer A549 cells. Biochem Biophys Res Commun. 392:373–379. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Song Z, Yue W, Wei B, Wang N, Li T, Guan
L, Shi S, Zeng Q, Pei X and Chen L: Sonic hedgehog pathway is
essential for maintenance of cancer stem-like cells in human
gastric cancer. PLoS One. 6:e176872011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Koch U and Radtke F: Notch signaling in
solid tumors. Curr Top Dev Biol. 92:411–455. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Oh P, Lobry C, Gao J, et al: In vivo
mapping of notch pathway activity in normal and stress
hematopoiesis. Cell Stem Cell. 13:190–204. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zheng HC, Takahashi H, Li XH, et al:
Downregulated parafibromin expression is a promising marker for
pathogenesis, invasion, metastasis and prognosis of gastric
carcinomas. Virchows Arch. 452:147–155. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li LC, Peng Y, Liu YM, Wang LL and Wu XL:
Gastric cancer cell growth and epithelial-mesenchymal transition
are inhibited by γ-secretase inhibitor DAPT. Oncol Lett.
7:2160–2164. 2014.PubMed/NCBI
|
|
27
|
Yan B, Liu L, Zhao Y, et al: Xiaotan
Sanjie decoction attenuates tumor angiogenesis by manipulating
Notch-1-regulated proliferation of gastric cancer stem-like cells.
World J Gastroenterol. 20:13105–13118. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ischenko I, Seeliger H, Kleespies A,
Angele MK, Eichhorn ME, Jauch KW and Bruns CJ: Pancreatic cancer
stem cells: New understanding of tumorigenesis, clinical
implications. Langenbecks Arch Surg. 395:1–10. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tang SN, Singh C, Nall D, Meeker D,
Shankar S and Srivastava RK: The dietary bioflavonoid quercetin
synergizes with epigallocathechin gallate (EGCG) to inhibit
prostate cancer stem cell characteristics, invasion, migration and
epithelial-mesenchymal transition. J Mol Signal. 5:142010.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Abell AN and Johnson GL: Implications of
mesenchymal cells in cancer stem cell populations: relevance to
EMT. Curr Pathobiol Rep. 2:21–26. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Shankar S, Nall D, Tang SN, Meeker D,
Passarini J, Sharma J and Srivastava RK: Resveratrol inhibits
pancreatic cancer stem cell characteristics in human and KrasG12D
transgenic mice by inhibiting pluripotency maintaining factors and
epithelial-mesenchymal transition. PLoS One. 6:e165302011.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Guo W: Concise review: breast cancer stem
cells: regulatory networks, stem cell niches, and disease
relevance. Stem Cells Transl Med. 3:942–948. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Dave B, Mittal V, Tan NM and Chang JC:
Epithelial-mesenchymal transition, cancer stem cells and treatment
resistance. Breast Cancer Res. 14:2022012. View Article : Google Scholar : PubMed/NCBI
|