Introduction
Breast cancer is the most frequently diagnosed
cancer amongst females and the second most common cause of
cancer-related mortality among women (1). One third of novel cancer diagnoses in
females are breast cancer and a total of one in eight women will be
diagnosed with breast cancer, with a lifetime risk of mortality due
to breast cancer of 3.4% (1).
Chemotherapy is one of the major systemic therapies available for
the treatment of breast cancer. A recent meta-analysis of the
outcome of 100,000 patients with breast cancer, confirmed the
benefit of cyclophosphamide-, methotrexate- and fluorouracil-
therapies and anthracycline-based therapies with absolute reduction
of mortalities of 6.2 and 6.5%, respectively, at 10 years (2). Despite progress in the improvement of
chemotherapeutic strategies, the ability to treat advanced breast
cancer remains poor, mainly due to the chemoresistance of cancer
cells to standard chemotherapy. Numerous anti-apoptotic and
cytoprotective pathways have been associated with the
chemoresistance of cancer cells (3,4).
Heme oxygenase (HMOX) is a microsomal enzyme, which
catalyzes the initial, rate-limiting step in the degradation of
heme, and has a crucial role in the recycling of iron (5). The enzymatic activity of HMOX also
produces CO, ferrous iron and biliverdin. Thus, HMOX is able to
reduce oxidative stress, attenuate inflammatory responses and lower
the rate of apoptosis (6). HMOX-1, an
isoform of HMOX, may be induced in response to cellular stress and
diverse oxidative stimuli (7). HMOX-1
is frequently overexpressed in a range of cancers, including
hepatoma, prostate cancer, melanoma and brain tumors (8–11). HMOX-1
promotes proliferation in human melanoma and pancreatic cancer cell
lines (10,12). As HMOX-1 is a potent inducer of
vascular endothelial growth factor (VEGF), a crucial factor
involved in tumor angiogenesis, it has also been recognized to
stimulate angiogenesis and thus support tumor progression (13). Additionally, overexpression of HMOX-1
promoted metastasis in a murine model of melanoma (14).
Expression of HMOX-1 may be induced by radiation and
chemotherapy in cancer cells (15),
and the expression of HMOX-1 was predictive of poorer survival in
patients with breast carcinoma (16).
HMOX-1 has also been found to protect cancer cells against
apoptosis by exerting anti-apoptotic activities (17,18).
Studies have indicated that upregulation of HMOX-1 is correlated
with the inhibition of autophagy (19,20).
Autophagy, which describes the process of cellular self-digestion,
is considered to be a cytoprotective response, which may occur
following the withdrawal of growth factors or under stressful
conditions (21). However, under
certain conditions, autophagy may function as a mechanism of cell
death (22). HMOX-1 and autophagy are
induced in response to cell stress (15,21).
Therefore, HMOX-1 may function as a specific regulator of autophagy
in breast cancer and may have a role in the survival of breast
cancer cells during chemotherapy.
In the present study, HMOX-1 expression and the
effects of doxorubicin in breast cancer cell lines were evaluated.
In addition, the effects of HMOX-1 knockdown in breast cancer cells
were investigated.
Materials and methods
Cell culture
The human breast cancer cell lines MDA-MB-231,
BT549, HS578T, MDA-MB-468, MCF-7 and ZR751, as well as the
non-malignant MCF-10A breast cell line were purchased from the
American Type Culture Collection (Manassas, VA, USA) and cultured
in Dulbecco's modified Eagles medium (DMEM) supplemented with 10%
fetal bovine serum and 1% penicillin and streptomycin (Gibco,
Karlsruhe, Germany) in a 5% CO2 atmosphere at 37°C.
Doxorubicin was obtained from Keygen Biotech (Nanjing, China),
dissolved in water and stored at 4°C.
Transfection of short interfering
(si)RNAs
HMOX-1 siRNA and control siRNA was
synthesized by Guangzhou RiboBio Co., Ltd (Guangzhou, China). The
sequence of HMOX-1 siRNA was as follows: Sense, 5′-CCA GCA ACA AAG
UGC AAG AdTdT-3′ and antisense, 3′-dTdTGGUCGUUGUUUCACGUUCU-5′.
Breast cancer cell lines were transfected with 50 nM siRNA using
RNAiMAX transfection reagent (Invitrogen Life Technologies,
Carlsbad, CA, USA) according to the manufacturer's
instructions.
Reverse transcription
quantitative-polymerase chain reaction (RT-qPCR) for mRNA
quantification
Total RNA was extracted from the cells using TRIzol
(Invitrogen Life Technologies) and complementary (c)DNA was
synthesized from 1,000 ng total RNA using the PrimeScript RT
reagent kit (Takara Biotechnology, Co., Ltd, Dalian, China),
according to the manufacturer's instructions. PCR was performed
using the PrimeScript RT Master Mix (Takara Biotechnology, Co.,
Ltd) on a Bio-Rad CFX96 real-time PCR machine (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). The primer sequences were
as follows: HMOX-1 forward, 5′-TACCACATC TATGTGGCCCTG-3′ and
reverse, 5′-TGGCTGGTG TGTAGGGGAT-3′; GAPDH forward,
5′-GCACCGTCA AGGCTGAGAAC-3′ and reverse, 5′-TGGTGAAGACGC
CAGTGGA-3′. Data analysis was performed using the comparative
CT method (23) with
Bio-Rad Manager 2.1 software (Bio-Rad Laboratories, Inc.).
Western blotting
Cells were lysed in radioimmunoprecipitation assay
lysis buffer (Cell Signaling Technology, Inc. Danvers, MA, USA)
supplemented with protease inhibitor (Roche Diagnostics, Basel,
Switzerland). The concentration of total protein was determined
using a bicinchoninic acid assay kit (Keygen Biotech). Equal
amounts of protein (35 µg) were separated by 10% SDS-PAGE,
transferred onto polyvinylidene difluoride membranes (EMD
Millipore, Bedford, MA, USA) and blotted with the following
monoclonal IgG rabbit anti-human antibodies at a dilution of
1:1,000: Anti-HMOX-1 (cat. no. ab52947; Abcam, Cambridge, UK),
anti-B cell lymphoma-2 (Bcl-2; cat. no. 2870), anti-Bcl-extra large
(xL; cat. no. 2764), anti-Bcl-2-like protein 4 (Bax; cat. no.
5023), anti-Beclin-1 (cat. no. 3495), anti-LC3B (cat. no. 3868) or
anti-β-tubulin (cat. no. 2128) (Cell Signaling Technology, Inc.),
followed by incubation overnight at 4°C. The bands were visualized
using Luminol reagent (Pierce Biotechnology, Inc.; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) and imaged using GE ImageQuant
Las 4000 mini (GE Healthcare Life Sciences, Chalfont, UK).
Drug sensitivity assay
MDA-MB-231 and BT549 cells were seeded in 96-well
culture plates at a density of 5,000 and 10,000 cells/well,
respectively, and transfected with siRNA, 12 h prior to treatment
with 0.02, 0.05, 0.10, 0.15 or 0.20 µg/ml doxorubicin for 48 h.
Cell viability was assessed using the Cell Counting kit-8 (CCK-8;
Dojindo, Kumamoto, Japan) according to the manufacturer's
instructions. The absorbance values were measured at 450 nm using a
Sunrise Absorbance Reader (Tecan US, Inc., Morrisville, NC,
USA).
Cell apoptosis assay
Apoptosis was assessed using an Annexin
V-fluorescein isothiocyanate (FITC) apoptosis kit (Dojindo)
according to the manufacturer's instructions. Briefly, the cells
were transfected with siRNA for 12 h prior to treatment with 0.2
µg/ml doxorubicin for 48 h. The cells were harvested, resuspended
in 500 µl binding solution, and incubated with 5 µl Annexin V and 5
µl propidium iodide (PI) for 10 min in the dark. Apoptosis was
analyzed by flow cytometry using a Beckman Coulter EPICS XL-MCL and
the results were analyzed using Kaluza software version 1.2 (both
from Beckman Coulter Inc., Brea, CA, USA).
Cell autophagy assay
MDA-MB-231 and BT549 cells were seeded in 24-well
plates at a density of 20,000 and 40,000 cells/well, respectively,
and transfected with siRNA for 12 h prior to treatment with 0.1
µg/ml doxorubicin for 48 h. The cells were then washed in
phosphate-buffered saline (PBS; Gibco Life Technologies, Shanghai,
China), fixed in 4% formaldehyde (Xilong Chemical Co., Ltd.,
Shantou, China) for 15 min, permeabilized with 0.1% Triton X-100
(Beyotime Institute of Biotechnology, Shanghai, China) for 10 min,
blocked with 0.1% bovine serum albumin (Beyotime Institute of
Biotechnology) for 1 h and incubated with anti-light chain 3B
(LC3B) antibody (Cell Signaling Technology, Inc.) overnight at 4°C.
Subsequently, cells were incubated with a green fluorescent protein
(GFP)-tagged secondary antibody (Life Technologies, Grand Island,
NY, USA) for 1 h at room temperature, then incubated with DAPI
(Roche Diagnostics) for 10 min and images were acquired using a
fluorescence microscope (Carl Zeiss Axio Observer Z1; Carl Zeiss,
Jena, Germany).
Statistical analysis
Data are expressed as the mean ± standard deviation.
Differences between the treatment groups and control group were
assessed with Student's t-test using GraphPad Prism version 5.0
(GraphPad Software Inc., San Diego, CA, USA). P<0.05, P<0.01
and P<0.001 were considered to indicate statistically
significant differences.
Results
HMOX-1 is overexpressed and able to be
induced by doxorubicin in breast cancer cell lines
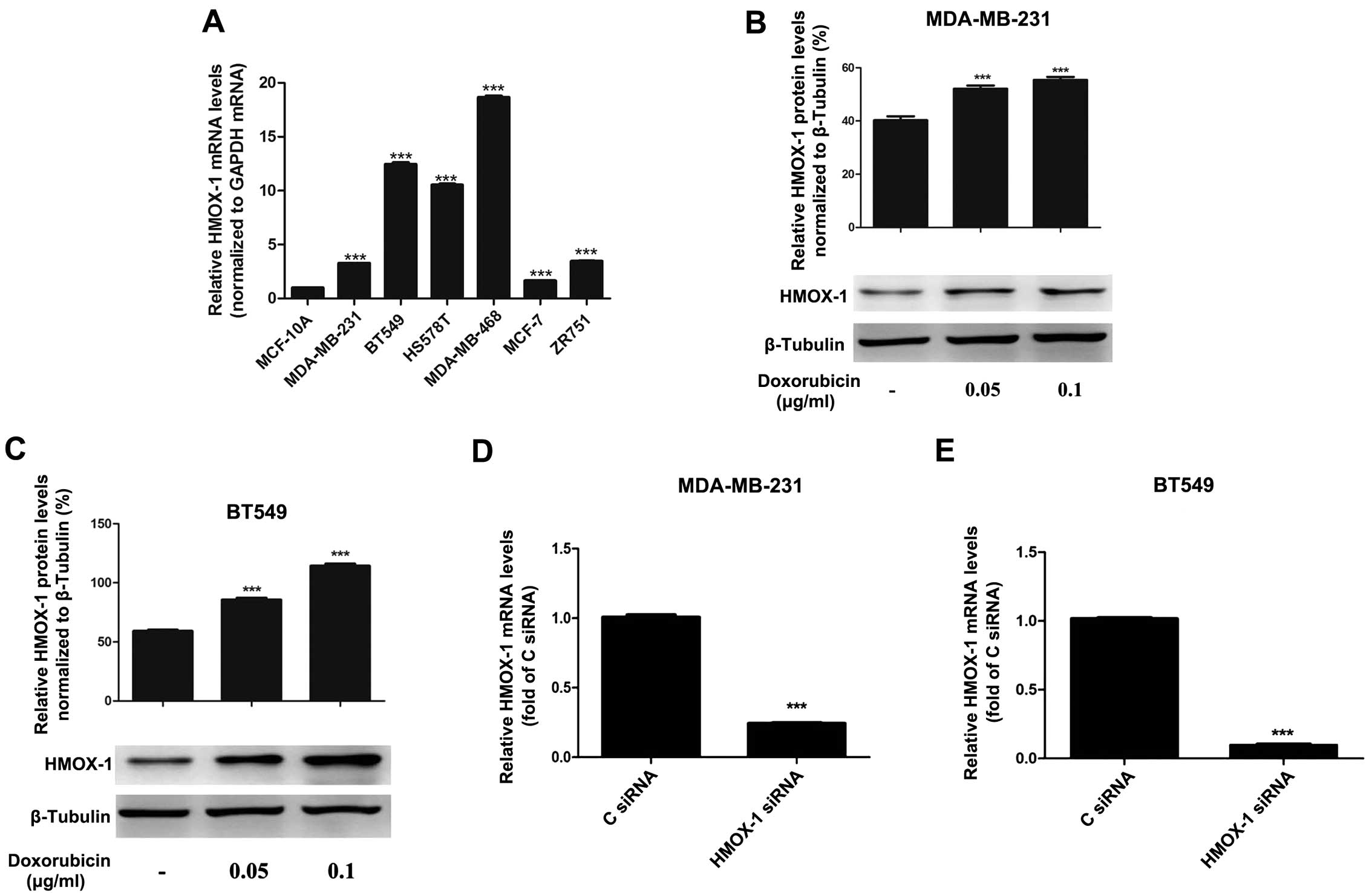
RT-qPCR analysis indicated that HMOX-1 was
overexpressed in the breast cancer cell lines evaluated. Compared
with that of the non-malignant breast cell line MCF-10A, the
expression of HMOX-1 was 3.3-fold higher in MDA-MB-231
cells, 12.4-fold higher in BT549 cells, 10.5-fold higher in HS578T
cells, 18.6-fold higher in MDA-MB-468 cells, 1.6-fold higher in
MCF-7 cells and 3.4-fold higher in ZR751 cells (Fig. 1A). In addition, doxorubicin treatment
significantly upregulated HMOX-1 protein expression in MDA-MB-231
and BT549 cells (Fig. 1B and C).
Silencing HMOX-1 increases the
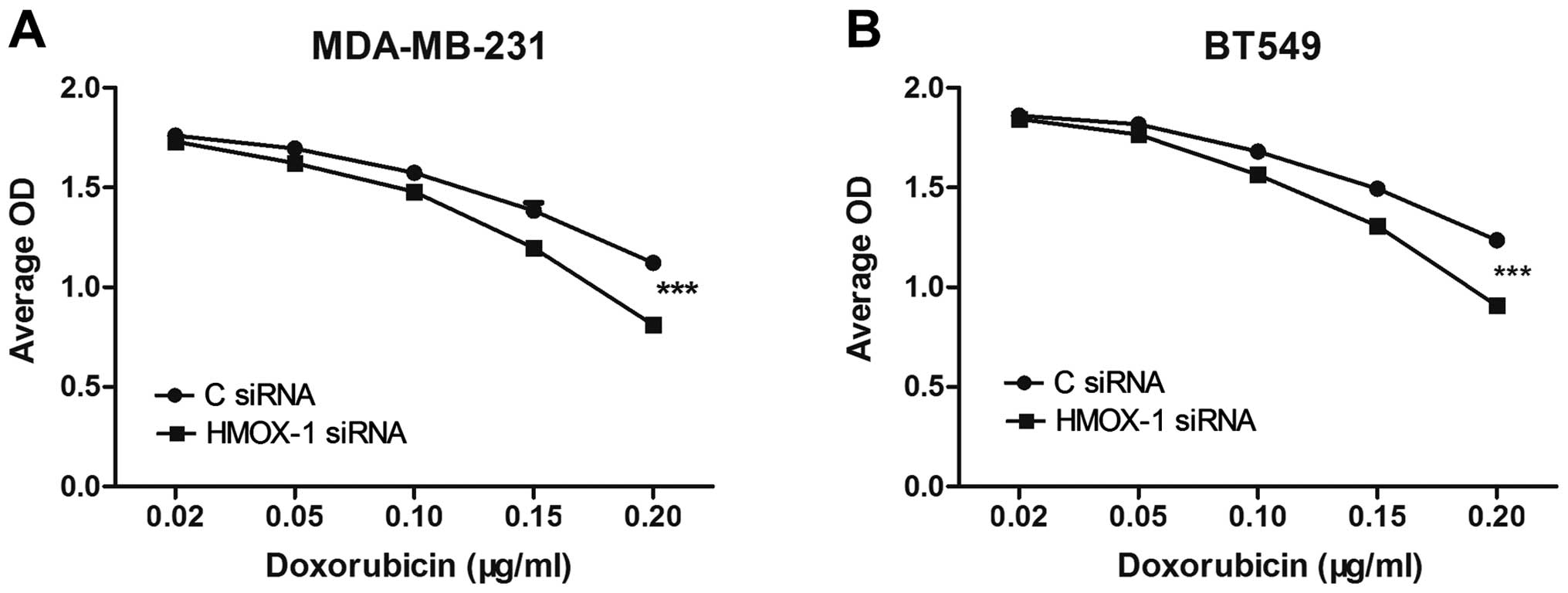
sensitivity of breast cancer cell lines to doxorubicin
A cytotoxicity assay was performed to investigate
the effects of HMOX-1 on the sensitivity of breast cancer cell
lines to doxorubicin. Breast cancer cells were transfected with 50
nM HMOX-1 siRNA or the negative control siRNA. At 48 h
post-transfection, HMOX-1 messenger (m)RNA expression was
significantly knocked down in the MDA-MB-231 and BT549 cell lines
(Fig. 1D and E). The transfected
cells were subsequently exposed to 0.02, 0.05, 0.1, 0.15 or 0.2
µg/ml doxorubicin for 48 h. The CCK-8 assay demonstrated that
knockdown of HMOX-1 significantly enhanced the cytotoxicity
of doxorubicin in MDA-MB-231 and BT549 cells (Fig. 2).
HMOX-1 knockdown enhances
doxorubicin-induced apoptosis in breast cancer cell lines
MDA-MB-231 and BT549 cells were transfected with
HMOX-1 siRNA or control siRNA, treated with 0.2 µg/ml
doxorubicin for 48 h, then stained using Annexin V and PI and
subjected to flow cytometric analysis to quantify apoptotic cells.
Silencing of HMOX-1 significantly increased the apoptotic
rate in doxorubicin-treated MDA-MB-231 and BT549 cells (Fig. 3A and B). Furthermore, western blot
analysis indicated that knockdown of HMOX-1 reduced the
expression of Bcl-xL and Bcl-2, but did not alter the expression of
Bax (Fig. 3C).
 | Figure 3.Knockdown of HMOX-1 enhances
doxorubicin-induced apoptosis in breast cancer cell lines. (A and
B) Rate of apoptosis in (A) MDA-MB-231 cells and (B) BT549 cells
transfected with C siRNA or HMOX-1 siRNA and then treated
with 0.20 µg/ml doxorubicin for 48 h. The rates of apoptosis were
determined using Annexin V and PI staining and flow cytometry. (C)
Western blotting revealed that siRNA knockdown of HMOX-1
downregulated HMOX-1, Bcl-2 and Bcl-xl protein expression, but not
Bax protein expression, in MDA-MB-231 and BT549 cells at 72 h
post-transfection. Results are representative of three independent
experiments. HMOX-1, heme oxygenase-1; C, control; PI, propidium
iodide; Bcl-2, B cell lymphoma-2; Bcl-xl, B cell lymphoma-extra
large; Bax, Bcl-2-like protein 4; FITC, fluorescein
isothiocyanate. |
HMOX-1 silencing enhances
doxorubicin-induced autophagy in breast cancer cell lines
Doxorubicin is able to promote cell death in cancer
cells via autophagy (21). Silencing
HMOX-1 markedly increased the accumulation of autophagic
vacuoles in doxorubicin-treated MDA-MB-231 and BT549 cells
(Fig. 4A and B). Furthermore, western
blotting confirmed that the expression of LC3B was upregulated in
HMOX-1-silenced MDA-MB-231 and BT549 cells compared with
that of the respective control cells, while Beclin-1 expression was
unaffected (Fig. 4C).
Discussion
HMOX-1 is involved in the maintenance of cellular
homeostasis and has a significant cytoprotective role (5). HMOX-1 is frequently upregulated in tumor
tissues and promotes tumor growth and metastasis (8–14).
Induction of HMOX-1 is often associated with chemoresistance in
cancer cells, and the inhibition of HMOX-1 in combination with
chemotherapy may enhance the efficacy of cancer treatment (24–26).
In the present study, it was demonstrated that
HMOX-1 was overexpressed in breast cancer cell lines, and was also
induced by doxorubicin. Therefore, the effect of HMOX-1 on the
chemoresistance of breast cancer cells was investigated. Knockdown
of HMOX-1 significantly enhanced the cytotoxicity of doxorubicin in
MDA-MB-231 and BT549 cells. Therefore, the mechanism underlying the
effects of knockdown of HMOX-1 on the cytotoxicity of
doxorubicin was investigated. The Bcl-2 family includes
pro-apoptotic (Bax, Bcl-2-antagonist/killer 1 and Bcl-2-associated
death promoter) and anti-apoptotic (Bcl-2 and Bcl-xL) members and
is a key regulator of apoptosis in cancer cells. Additionally,
Bcl-2 and Bcl-xL have been implicated in the resistance of cancer
cells to chemotherapeutic drugs (27,28). The
results of the present study indicated that knockdown of
HMOX-1 significantly enhanced doxorubicin-induced apoptosis
and downregulated the expression of Bcl-2 and Bcl-xL in breast
cancer cells. These findings indicated that HMOX-1 may modulate the
survival of breast cancer cells via regulation of the expression of
Bcl-2 and Bcl-xL.
Furthermore, knockdown of HMOX-1 enhanced
doxorubicin-induced autophagy, without altering the expression of
autophagic regulator Beclin-1 in MDA-MB-231 and BT549 cells.
Autophagy is the process of lysosomal degradation, by which cells
self-destruct, and has been associated with cell survival in
various types of cancer (21).
Autophagy is also able to protect cells from apoptosis and induce
drug-resistance. Blocking autophagy restored anti-estrogen
sensitivity to an MCF-7-derived anti-estrogen resistant cell line
(29). Autophagy has also been
implicated in the development of trastuzumab resistance in human
epidermal growth factor receptor 2-positive breast cancer (30). A number of chemotherapeutic agents
(anthracyclines and taxanes) are able to induce autophagy, and
inhibition of autophagy increases drug toxicity via the induction
of apoptosis in breast cancer cells (31,32).
However, studies have indicated that excessive
autophagy may result in autophagic cell death (also known as type
II programmed cell death), which occurs in association with
increased autophagosome formation in a caspase-independent manner
(33). Scarlatti et al
(34) reported that resveratrol,
which is found in grapes and peanuts, induced Beclin-1-independent
cytotoxic autophagy in MCF-7 cells. Di et al (35) demonstrated that breast cancer cells
treated with doxorubicin and z-VAD-FMK, a general inhibitor of
caspases, underwent cell death via a non-apoptotic pathway,
characterized by dramatic accumulation of autophagic vacuoles.
Doxorubicin is one of the most commonly used
chemotherapeutic drugs, and primarily functions by inhibiting
topoisomerases and intercalating into the DNA double helix to
interfere with DNA uncoiling, which induces cell death (36). Cell lines resistant to doxorubicin
often exhibit defective apoptosis due to overexpression of Bcl-2
family proteins; therefore, chemotherapeutic agents that evoke
autophagic cell death may be more cytotoxic in apoptosis-defective
and drug-resistant tumor cells (37).
In the present study, knockdown of HMOX-1 induced
accumulation of autophagic vacuoles and markedly increased the
expression of LC3B in breast cancer cells, without altering the
expression of Beclin-1. Therefore, knockdown of HMOX-1
induced Beclin-1-independent autophagy in breast cancer cells.
Under certain conditions, autophagy and apoptosis contribute to the
rate of cell death as parallel pathways, and silencing Bcl-2 may
induce autophagic cell death (31).
Knockdown of HMOX-1 also reduced the expression of Bcl-2 and
promoted autophagy in breast cancer cells, suggesting that
depletion of HMOX-1 may induce autophagic cell death by
regulating the levels of Bcl-2. These results demonstrate that
knockdown of HMOX-1 enhanced the cytotoxic effects of
doxorubicin and induced apoptosis and autophagy in breast cancer
cells. These results indicate that HMOX-1 may have a role in tumor
cell survival and chemoresistance in breast cancer. Additionally,
HMOX-1 represents a potential therapeutic target, as targeting
HMOX-1 may enhance the therapeutic efficacy of chemotherapy in
breast cancer.
In conclusion, the present study demonstrated that
HMOX-1 is overexpressed and able to be induced by doxorubicin in
breast cancer cell lines. Knockdown of HMOX-1 enhanced the
cytotoxicity of doxorubicin by downregulating anti-apoptotic genes
and promoting apoptosis and autophagy in breast cancer cells.
Therefore, HMOX-1 may represent a potential therapeutic target for
breast cancer.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (no. 81172337) and the
Guangdong Department of Science and Medicine Center (no.
2011A080300002).
References
|
1
|
Libson S and Lippman M: A review of
clinical aspects of breast cancer. Int Rev Psychiatry. 26:4–15.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Peto R, Davies C, Godwin J, Gray R, Pan
HC, Clarke M, Cutter D, Darby S, McGale P, Taylor C, et al: Early
Breast Cancer Trialists' Collaborative Group (EBCTCG): Comparisons
between different polychemotherapy regimens for early breast
cancer: Meta-analyses of long-term outcome among 100,000 women in
123 randomised trials. Lancet. 379:432–444. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Videira M, Reis RL and Brito MA:
Deconstructing breast cancer cell biology and the mechanisms of
multidrug resistance. Biochim Biophys Acta. 1846:312–325.
2014.PubMed/NCBI
|
|
4
|
Kontos CK, Christodoulou MI and Scorilas
A: Apoptosis-related BCL2-family members: Key players in
chemotherapy. Anticancer Agents Med Chem. 14:353–374. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Maines MD: The heme oxygenase system: A
regulator of second messenger gases. Annu Rev Pharmacol Toxicol.
37:517–554. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tenhunen R, Marver HS and Schmid R: The
enzymatic conversion of heme to bilirubin by microsomal heme
oxygenase. Proc Natl Acad Sci USA. 61:748–755. 1968. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Alam J, Shibahara S and Smith A:
Transcriptional activation of the heme oxygenase gene by heme and
cadmium in mouse hepatoma cells. J Biol Chem. 264:6371–6375.
1989.PubMed/NCBI
|
|
8
|
Doi K, Akaike T, Fujii S, Tanaka S, Ikebe
N, Beppu T, Shibahara S, Ogawa M and Maeda H: Induction of haem
oxygenase-1 nitric oxide and ischaemia in experimental solid
tumours and implications for tumour growth. Br J Cancer.
80:1945–1954. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Maines MD and Abrahamsson PA: Expression
of heme oxygenase-1 (HSP32) in human prostate: Normal,
hyperplastic, and tumor tissue distribution. Urology. 47:727–733.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Torisu-Itakura H, Furue M, Kuwano M and
Ono M: Co-expression of thymidine phosphorylase and heme
oxygenase-1 in macrophages in human malignant vertical growth
melanomas. Jpn J Cancer Res. 91:906–910. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hara E, Takahashi K, Tominaga T, Kumabe T,
Kayama T, Suzuki H, Fujita H, Yoshimoto T, Shirato K and Shibahara
S: Expression of heme oxygenase and inducible nitric oxide synthase
mRNA in human brain tumors. Biochem Biophys Res Commun.
224:153–158. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sunamura M, Duda DG, Ghattas MH, Lozonschi
L, Motoi F, Yamauchi J, Matsuno S, Shibahara S and Abraham NG: Heme
oxygenase-1 accelerates tumor angiogenesis of human pancreatic
cancer. Angiogenesis. 6:15–24. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cherrington JM, Strawn LM and Shawver LK:
New paradigms for the treatment of cancer: The role of
anti-angiogenesis agents. Adv Cancer Res. 79:1–38. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Was H, Cichon T, Smolarczyk R, Rudnicka D,
Stopa M, Chevalier C, Leger JJ, Lackowska B, Grochot A, Bojkowska
K, et al: Overexpression of heme oxygenase-1 in murine melanoma:
Increased proliferation and viability of tumor cells, decreased
survival of mice. Am J Pathol. 169:2181–2198. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Berberat PO, Dambrauskas Z, Gulbinas A,
Giese T, Giese N, Künzli B, Autschbach F, Meuer S, Büchler MW and
Friess H: Inhibition of heme oxygenase-1 increases responsiveness
of pancreatic cancer cells to anticancer treatment. Clin Cancer
Res. 11:3790–3798. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Noh SJ, Bae JS, Jamiyandorj U, Park HS,
Kwon KS, Jung SH, Youn HJ, Lee H, Park BH, Chung MJ, et al:
Expression of nerve growth factor and heme oxygenase-1 predict poor
survival of breast carcinoma patients. BMC Cancer. 13:5162013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Busserolles J, Megías J, Terencio MC and
Alcaraz MJ: Heme oxygenase-1 inhibits apoptosis in Caco-2 cells via
activation of Akt pathway. Int J Biochem Cell Biol. 38:1510–1517.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen GG, Liu ZM, Vlantis AC, Tse GM, Leung
BC and van Hasselt CA: Heme oxygenase-1 protects against apoptosis
induced by tumor necrosis factor-alpha and cycloheximide in
papillary thyroid carcinoma cells. J Cell Biochem. 92:1246–1256.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bolisetty S, Traylor AM, Kim J, Joseph R,
Ricart K, Landar A and Agarwal A: Heme oxygenase-1 inhibits renal
tubular macroautophagy in acute kidney injury. J Am Soc Nephrol.
21:1702–1712. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Banerjee P, Basu A, Wegiel B, Otterbein
LE, Mizumura K, Gasser M, Waaga-Gasser AM, Choi AM and Pal S: Heme
oxygenase-1 promotes survival of renal cancer cells through
modulation of apoptosis- and autophagy-regulating molecules. J Biol
Chem. 287:32113–32123. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Eisenberg-Lerner A, Bialik S, Simon HU and
Kimchi A: Life and death partners: Apoptosis, autophagy and the
cross-talk between them. Cell Death Differ. 16:966–975. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shen HM and Codogno P: Autophagic cell
death: Loch Ness monster or endangered species? Autophagy.
7:457–465. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mayerhofer M, Florian S, Krauth MT,
Aichberger KJ, Bilban M, Marculescu R, Printz D, Fritsch G, Wagner
O, Selzer E, et al: Identification of heme oxygenase-1 as a novel
BCR/ABL-dependent survival factor in chronic myeloid leukemia.
Cancer Res. 64:3148–3154. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nuhn P, Künzli BM, Hennig R, Mitkus T,
Ramanauskas T, Nobiling R, Meuer SC, Friess H and Berberat PO: Heme
oxygenase-1 and its metabolites affect pancreatic tumor growth in
vivo. Mol Cancer. 8:372009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Al-Owais MM, Scragg JL, Dallas ML, Boycott
HE, Warburton P, Chakrabarty A, Boyle JP and Peers C: Carbon
monoxide mediates the anti-apoptotic effects of heme oxygenase-1 in
medulloblastoma DAOY cells via K+ channel inhibition. J
Biol Chem. 287:24754–24764. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jäger R, Herzer U, Schenkel J and Weiher
H: Overexpression of Bcl-2 inhibits alveolar cell apoptosis during
involution and accelerates c-myc-induced tumorigenesis of the
mammary gland in transgenic mice. Oncogene. 15:1787–1795. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Boise LH, González-García M, Postema CE,
Ding L, Lindsten T, Turka LA, Mao X, Nuñez G and Thompson CB:
Bcl-x, a bcl-2-related gene that functions as a dominant regulator
of apoptotic cell death. Cell. 74:597–608. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Samaddar JS, Gaddy VT, Duplantier J,
Thandavan SP, Shah M, Smith MJ, Browning D, Rawson J, Smith SB,
Barrett JT, et al: A role for macroautophagy in protection against
4-hydroxytamoxifen-induced cell death and the development of
antiestrogen resistance. Mol Cancer Ther. 7:2977–2987. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cufí S, Vazquez-Martin A,
Oliveras-Ferraros C, Corominas-Faja B, Cuyàs E, López-Bonet E,
Martin-Castillo B, Joven J and Menendez JA: The anti-malarial
chloroquine overcomes primary resistance and restores sensitivity
to trastuzumab in HER2-positive breast cancer. Sci Rep. 3:24692013.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sun WL, Chen J, Wang YP and Zheng H:
Autophagy protects breast cancer cells from epirubicin-induced
apoptosis and facilitates epirubicin-resistance development.
Autophagy. 7:1035–1044. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Veldhoen RA, Banman SL, Hemmerling DR,
Odsen R, Simmen T, Simmonds AJ, Underhill DA and Goping IS: The
chemotherapeutic agent paclitaxel inhibits autophagy through two
distinct mechanisms that regulate apoptosis. Oncogene. 32:736–746.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Denton D, Nicolson S and Kumar S: Cell
death by autophagy: Facts and apparent artefacts. Cell Death
Differ. 19:87–95. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Scarlatti F, Maffei R, Beau I, Codogno P
and Ghidoni R: Role of non-canonical Beclin 1-independent autophagy
in cell death induced by resveratrol in human breast cancer cells.
Cell Death Differ. 15:1318–1329. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Di X, Shiu RP, Newsham IF and Gewirtz DA:
Apoptosis, autophagy, accelerated senescence and reactive oxygen in
the response of human breast tumor cells to adriamycin. Biochem
Pharmacol. 77:1139–1150. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Box VG: The intercalation of DNA double
helices with doxorubicin and nogalamycin. J Mol Graph Model.
26:14–19. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wirawan E, Van de Walle L, Kersse K,
Cornelis S, Claerhout S, Vanoverberghe I, Roelandt R, De Rycke R,
Verspurten J, Declercq W, et al: Caspase-mediated cleavage of
Beclin-1 inactivates Beclin-1-induced autophagy and enhances
apoptosis by promoting the release of proapoptotic factors from
mitochondria. Cell Death Dis. 1:e182010. View Article : Google Scholar : PubMed/NCBI
|