Introduction
Gastric cancer is the fourth leading cause of
cancer-related mortality worldwide. There are ~989,600 new cases
and 738,000 mortalities per year, accounting for ~8% of new cancer
cases (1). Complete surgical
resection is the only potentially curative treatment for localized
gastric cancer. However, clinical outcomes of patients with
advanced gastric cancer remain poor. The majority of patients with
advanced gastric cancer experience recurrence or metastasis despite
curative resection (2) and, even with
intensive chemotherapy, the median survival time of patients with
recurrent or metastatic disease is ≤13 months (3).
Receptor tyrosine kinases, including human epidermal
growth factor receptor (HER) and vascular endothelial growth
factor, are important in cancer progression and are associated with
survival in patients with gastric cancer (4–11). A
number of anticancer drugs designed to inhibit signaling pathways
of tyrosine kinases have been evaluated in patients with
unresectable or metastatic gastric cancer (12–14);
however, only trastuzumab (anti-HER2) has been demonstrated to be
effective (15). Furthermore, only
23–24% of cases of gastric cancer exhibit overexpression of HER2,
the target of trastuzumab (16,17). Thus,
an improved understanding of the molecular pathogenesis involved in
tumor progression and survival is necessary to establish more
effective therapeutic targets and to improve outcomes in patients
with gastric cancer.
Platelet-derived growth factors (PDGFs) are receptor
tyrosine kinases that regulate diverse cellular functions,
including cell proliferation, transformation, migration and
embryonic development (18). PDGFs
consist of four different polypeptide chains (PDGF-A, −B, −C and
−D) that are assembled into disulfide-bonded dimers via
homodimerization of heterodimers in order to play their functional
role. So far, four homodimers (PDGF-AA, −BB, −CC and −DD) and one
heterodimer (PDGF-AB) have been described (19). PDGF isoforms exert their biological
functions by activating two structurally related receptor tyrosine
kinases, PDGF receptors (PDGFRs) α and β. Upon binding of dimeric
PDGF to PDGFR-α and −β, dimerization and activation of these
receptors occurs. The receptors may combine to generate homo- or
heterodimers, resulting in three possible combinations: PDGFR-αα,
PDGFR-ββ and PDGFR-αβ, which have different affinities for the four
PDGFs. Activated PDGF-C is a high affinity ligand for PDGFR-α
homodimers, but fails to bind to and activate PDGFR-β homodimers.
By contrast, activated PDGF-D is a high affinity ligand for PDGFR-β
homodimers, but fails to bind to and activate PDGFR-α homodimers
(20). PDGF-C and PDGF-D are also
expressed in a number of types of tumor and various tumor cell
lines, and are associated with tumor progression and angiogenesis
(21,22). PDGF-C overexpression is observed in
glioblastoma, Ewing family sarcoma and lung carcinoma cell lines
(22–24), whilst PDGF-D is frequently upregulated
in prostate, lung, renal, ovarian, brain and pancreatic cancers
(21,22,25–29).
Although PDGF-D overexpression has been observed in gastric cancer
tissues when compared with normal tissues (30), the distribution, frequency and
prognostic value of PDGF-D and PDGF-C expression in gastric cancer
have not been clarified.
The purpose of the current study was to evaluate the
association between the expression of PDGF-C and PDGF-D,
clinicopathological factors and outcomes in patients with gastric
cancer.
Materials and methods
Patients
The study group comprised 204 patients with primary
gastric adenocarcinomas who underwent curative gastrectomy (R0)
between January 2003 and December 2007 at the Department of
Esophagogastric Surgery, Tokyo Medical and Dental University
Hospital (Tokyo, Japan). Patient characterisitcs are summarized in
Table I. No patient received
anticancer treatment prior to surgery. Each tumor was classified
according to the tumor-node-metastasis classification criteria
recommended by the Union for International Cancer Control (31). All patients were evaluated for
recurrent disease by diagnostic imaging, including computed
tomography, ultrasonography and endoscopy, every 3–6 months. The
median follow-up time was 60 months (range, 5–111 months).
Recurrent disease was diagnosed in 51 patients (25%). There were 48
mortalities (24%) due to metastatic gastric cancer, and 11 (5%) due
to other diseases in the absence of recurrence. This study was
approved by the Institutional Review Board of Tokyo Medical and
Dental University. Written informed consent was obtained from all
patients.
 | Table I.Characteristics of the studied
patients (n=204). |
Table I.
Characteristics of the studied
patients (n=204).
| Characteristic | Value |
|---|
| Age, years; median
(range) | 64 (21–92) |
| Gender, n (%) |
|
| Male | 156 (76) |
|
Female | 48
(24) |
| Main location, n
(%) |
|
| Upper
third of stomach | 43
(21) |
|
Middle/lower third of
stomach | 161 (79) |
| WHO pathological
type, n (%) |
|
|
Differentiated | 104 (51) |
|
Undifferentiated | 100 (49) |
| Depth of invasion, n
(%) |
|
| T1a | 12 (6) |
| T1b | 75
(37) |
| T2 | 30
(15) |
| T3 | 37
(18) |
| T4 | 50
(25) |
| Lymph node
metastasis, n (%) |
|
|
Positive | 91
(45) |
|
Negative | 113 (55) |
| TNM stage, n
(%) |
|
| IA | 73
(36) |
| IB | 33
(16) |
|
IIA | 19 (9) |
|
IIB | 17 (8) |
|
IIIA | 19 (9) |
|
IIIB | 21
(10) |
|
IIIC | 22
(11) |
Immunohistochemical staining of PDGF-C
and PDGF-D
Immunohistochemical staining was conducted using the
Simple Stain MAX PO method with a Histofine Simple Stain MAX PO
(MULTI) (Nichirei Biosciences, Inc., Tokyo, Japan). The goat
polyclonal IgG antibody against human PDGF-C (#sc-18228) was
purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA,
USA), and the rabbit polyclonal antibody against human PDGF-D
(#PAB4843) was purchased from Abnova (Taipei, Taiwan). All
available hematoxylin and eosin-stained slides of the surgical
specimens were reviewed. For each case, representative paraffin
blocks were selected for immunohistochemical studies. The 4
µm-thick sections were cut from formalin-fixed, paraffin-embedded
tissue blocks. Following deparaffinization and rehydration in
graded concentrations of ethanol, antigen retrieval treatment was
performed at 98°C (microwave) for 15 min in a pH 9.0 retrieval
solution (Nichirei Biosciences, Inc.) prior to treatment with 3%
hydrogen peroxide (Wako Pure Chemical Industries, Ltd., Osaka,
Japan) for 15 min to quench endogenous peroxidase activity. The
slides were incubated with the primary antibodies against PDGF-C
(dilution, 1:50) or PDGF-D (dilution, 1:50) overnight at 4°C.
Sections were incubated with peroxidase-labeled anti-goat or
anti-rabbit antibodies [Histofine Simple Stain MAX PO (G) or
(MULTI); #414161 and #424152; Nichirei Biosciences, Inc.] for 30
min at room temperature. Peroxidase activity was detected with
diaminobenzidine (Histofine Simple Stain DAB solution; Nichirei
Biosciences, Inc.). The slides were counterstained with 1% Mayer's
hematoxylin (Wako Pure Chemical Industries, Ltd.). Expression
levels of PDGF-C and PDGF-D were evaluated based on cytoplasmic
staining intensity and positive frequency, and were classified into
two groups (high expression or low expression). Staining intensity
was scored into four grades: 0 (none), 1 (weak positive), 2
(moderate positive) or 3 (strong positive). Staining extent
(positive frequency) was scored into four grades: 0, <25%; 1,
25% to <50%; 2, 50% to <75%; or 3, ≥75%. Composite scores
were derived by adding the intensity score to the extent score. For
statistical analysis, composite scores of ≥4 were defined as high
expression, and scores of <4 were considered low expression.
Normal tissues from the same patients were used as controls. In
negative controls, the antibodies were replaced by normal goat or
rabbit IgG (Santa Cruz Biotechnology, Inc.). Colorectal cancer and
hepatocellular carcinoma tissues, which exhibit high expression,
served as positive controls.
Statistical analysis
The χ2 test was used to test possible
associations between the expression of PDGF-C or PDGF-D and
clinicopathological factors. It was also used to assess
correlations between PDGF-C and PDGF-D expressions. Kaplan-Meier
curves were plotted to assess the association between PDGF-C and
PDGF-D expression and relapse-free survival (RFS). Survival curves
were compared using the log-rank-test. P<0.05 was considered to
indicate statistical significance. A multivariate Cox proportional
hazards regression model was used to assess the prognostic
significance of PDGF-C and PDGF-D expression and a number of
clinicopathological factors. Statistical analysis was conducted
using SPSS software, version 20 (IBM SPSS, Armonk, NY, USA).
Results
PDGF-C and PDGF-D immunostaining
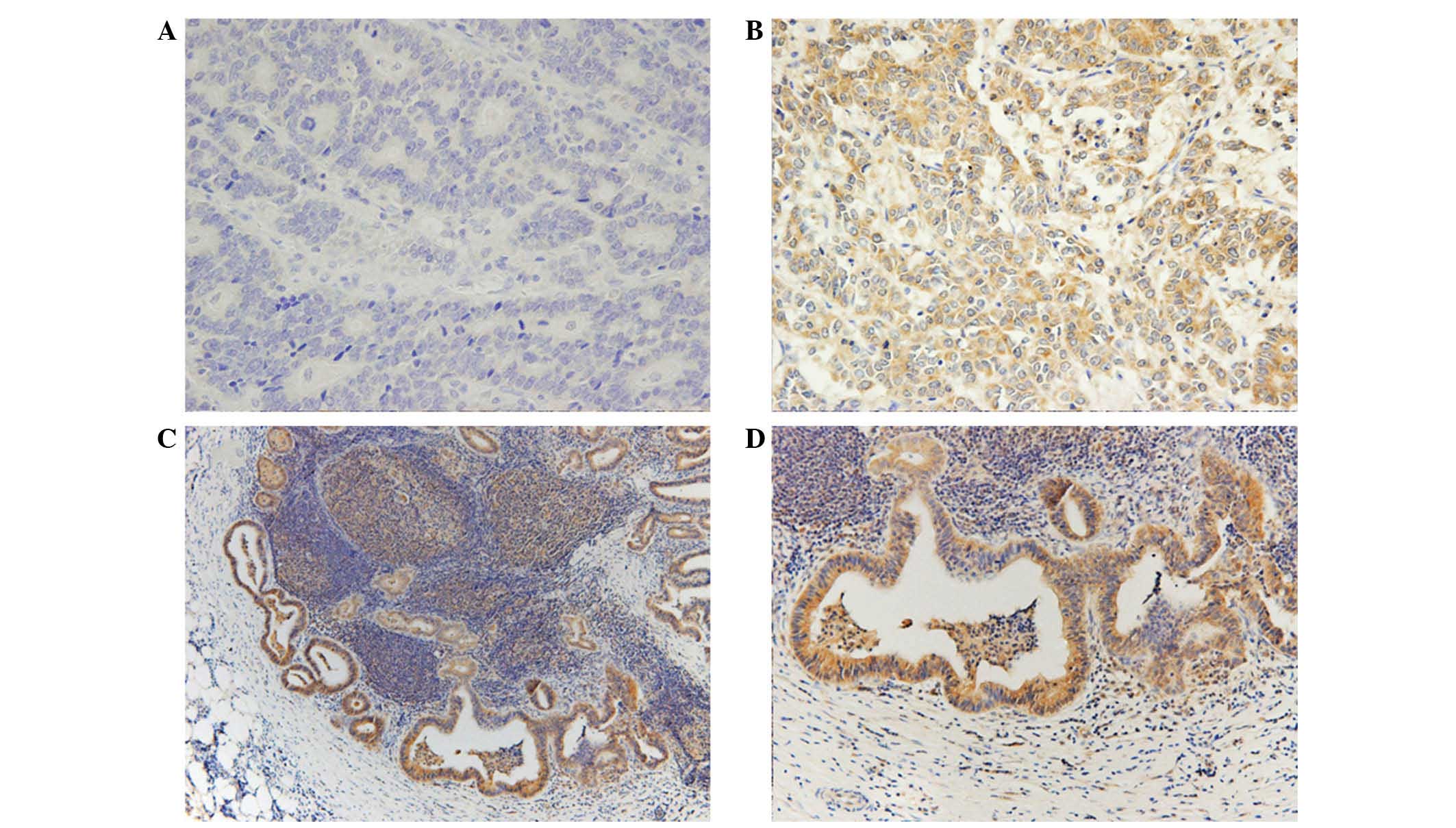
PDGF-C expression was predominantly located in the
cytoplasm, with some in the nucleus of the tumor cells, whilst
PDGF-D expression was observed only in the cytoplasm (Figs. 1 and 2).
Adjacent non-malignant tissue exhibited weak or no staining of
either protein. High expression of cytoplasmic PDGF-C and PDGF-D
was detected in 114 (56%) and 151 (74%) samples, respectively.
Expression of both PDGF-C and PDGF-D was observed in 98 (48%)
tumors, while 37 (18%) tumors exhibited low expression of PDGF-C
and PDGF-D. PDGF-C expression correlated with PDGF-D expression
(P<0.01). The expression of PDGF-C and PDGF-D was also evaluated
in 89 metastasis-positive lymph nodes; 81% of the samples were
found to exhibit high expression of PDGF-C, and 87% displayed high
expression of PDGF-D. These frequencies were higher than those
observed in the primary tumors. However, there was no significant
association between expression of either PDGF-C of PDGF-D in the
primary tumor and that in metastatic lymph nodes (Table II).
 | Table II.Association between PDGF-C and PDGF-D
expression, primary tumor and metastatic lymph nodes. |
Table II.
Association between PDGF-C and PDGF-D
expression, primary tumor and metastatic lymph nodes.
|
| Metastatic lymph
nodes |
|
|---|
|
|
|
|
|---|
| Primary tumor | Low | High | P-value |
|---|
| PDGF-C |
|
| 0.88 |
|
Low | 6 | 24 |
|
|
High | 11 | 48 |
|
| PDGF-D |
|
| 0.92 |
|
Low | 2 | 12 |
|
|
High | 10 | 65 |
|
Clinicopathological parameters and
expression of PDGF-C and PDGF-D
High expression of PDGF-C and PDGF-D was observed
more often in differentiated-type tumors than in
undifferentiated-type tumors (P=0.05). High PDGF-C expression
tended to be associated with distant metastasis and recurrence
(P=0.07). High PDGF-D expression significantly correlated with
gender, tumor depth, tumor stage and distant metastasis and
recurrence (P=0.02, P=0.04, P=0.01 and P<0.01, respectively) and
tended to be associated with lymph node metastasis (P=0.07)
(Table III).
 | Table III.Clinicopathological factors and
expression of PDGF-C and PDGF-D. |
Table III.
Clinicopathological factors and
expression of PDGF-C and PDGF-D.
|
| PDGF-C expression,
n |
| PDGF-D expression,
n |
|
|---|
|
|
|
|
|
|
|---|
| Variables | Low (n=90) | High (n=114) | P-value | Low (n=53) | High (n=151) | P-value |
|---|
| Age, years |
|
| 0.9 |
|
|
0.19 |
|
<70 | 60 | 75 |
| 39 | 96 |
|
|
≥70 | 30 | 39 |
| 14 | 55 |
|
| Gender |
|
| 0.59 |
|
|
0.02 |
|
Male | 70 | 85 |
| 34 | 121 |
|
|
Female | 20 | 29 |
| 19 | 30 |
|
| Histopathology |
|
| 0.05 |
|
| <0.01 |
|
Differentiated | 37 | 63 |
| 16 | 84 |
|
|
Undifferentiated | 53 | 51 |
| 37 | 67 |
|
| Depth of
invasion |
|
| 0.11 |
|
|
0.04 |
| T1 | 44 | 43 |
| 29 | 58 |
|
|
T2/T3/T4 | 46 | 71 |
| 24 | 93 |
|
| Lymph node
metastasis |
|
| 0.14 |
|
|
0.07 |
| N0 | 55 | 58 |
| 35 | 78 |
|
|
N1/N2/N3 | 33 | 53 |
| 18 | 73 |
|
| Recurrence |
|
| 0.07 |
|
| <0.01 |
|
Absent | 73 | 80 |
| 49 | 104 |
|
|
Present | 17 | 34 |
|
| 47 |
|
| TNM stage |
|
| 0.15 |
|
|
0.01 |
| I | 58 | 62 |
| 39 | 81 |
|
|
II/III | 32 | 52 |
| 14 | 70 |
|
Prognostic significance of PDGF-C and
PDGF-D expression
High PDGF-D expression was associated with
significantly shorter RFS time relative to the low expression group
(mean, 81 vs. 101 months; P<0.01), whilst high PDGF-C expression
was associated with marginally, but not significantly, shorter RFS
compared with the low expression group (mean, 82 vs. 90 months;
P=0.10). The prognostic relevance of high PDGF-C and PDGF-D
expression was assessed using a multivariate proportional hazards
regression model adjusted for the established clinical prognostic
factors (i.e., histopathology, tumor depth, lymph node metastasis).
High PDGF-D expression was determined to be an independent
prognostic factor [hazard ratio (HR), 3.6; 95% confidence interval
(CI), 1.3–10.4; P=0.02], whereas PDGF-C was not (P=0.48).
Histopathology (HR, 1.8; 95% CI, 1.0–3.3; P=0.05), tumor depth (HR,
9.5; 95% CI, 2.2–41.0; P<0.01) and lymph node metastasis (HR,
5.4; 95% CI, 2.2–13.1; P<0.01) were also independent prognostic
factors (Table IV).
 | Table IV.Prognostic factors according to a
multivariate Cox proportional hazards regression model for relapse
free survival. |
Table IV.
Prognostic factors according to a
multivariate Cox proportional hazards regression model for relapse
free survival.
| Variables | Patients, n | Univariate analysis
P-value | Hazard ratio (95%
confidence interval) | Multivariate
analysis P-value |
|---|
| Age |
|
0.70 |
|
|
| <70
years | 135 |
|
|
|
| ≥70
years | 69 |
|
|
|
| Gender |
|
0.87 |
|
|
|
Male | 155 |
|
|
|
|
Female | 49 |
|
|
|
| Histopathology |
| <0.01 | 1.8 (1.0–3.3) |
0.05 |
|
Differentiated | 100 |
|
|
|
|
Undifferentiated | 104 |
|
|
|
| Tumor depth |
| <0.01 | 9.5
(2.2–41.0) | <0.01 |
| T1 | 87 |
|
|
|
|
T2/T3/T4 | 117 |
|
|
|
| Lymph node
metastasis |
| <0.01 | 5.4
(2.2–13.1) | <0.01 |
| N0 | 113 |
|
|
|
|
N1/N2/N3 | 91 |
|
|
|
| PDGF-C
expression |
|
0.10 | 0.8 (0.4–1.5) |
0.48 |
|
Low | 90 |
|
|
|
|
High | 114 |
|
|
|
| PDGF-D
expression |
| <0.01 | 3.6
(1.3–10.4) |
0.02 |
|
Low | 53 |
|
|
|
|
High | 151 |
|
|
|
Discussion
The present study demonstrated that high PDGF-D
expression was significantly associated with tumor depth,
recurrence, distant metastasis and poor survival in patients with
gastric cancer, whereas high PDGF-C expression tended to be
associated (non-significantly) with distant metastasis, recurrence
and shorter RFS.
PDGF-D is frequently upregulated in various types of
cancer and plays an important role in tumor progression,
angiogenesis and metastasis through multiple oncogenic pathways,
including the phosphatidylinositol 3-kinase/Akt, nuclear factor-κB
(NF-κB), extracellular signal-regulated kinase, mammalian target of
rapamycin, mitogen-activated protein kinase and Notch pathways
(12,25,26,29). Wang
et al (26) demonstrated that
PDGF-D was associated with cancer invasion and angiogenesis in
pancreatic carcinomas via the regulation of Notch-1 and NF-κB
signaling. Ustach et al (27)
demonstrated that PDGF-D expression markedly accelerated tumor
growth in prostate carcinoma cells, suggesting the potential
oncogenic activity of PDGF-D. Xu et al (29) reported that overexpression of PDGF-D
in renal cell carcinoma cells promoted tumor growth, angiogenesis
and metastasis. These data suggest that PDGF-D overexpression may
be associated with human cancer progression. Accordingly, the
present results support the idea that high expression of PDGF-D in
cancer may be important in tumor progression.
Furthermore, PDGF-D may also be associated with the
epithelial-to-mesenchymal transition (EMT), an important process
for tumor metastasis, via a number of signaling pathways, including
Notch and NF-κB (32–34). Kong et al (32) reported that high expression of PDGF-D
was significantly associated with the induction of EMT in prostate
cancer cells.
As PDGF-D exerts oncogenic activity via the
regulation of tumor cell growth, invasion and metastasis, PDGF-D
signaling pathways are a potential therapeutic target for the
treatment of human cancers. Notably, Kong et al (25) reported that blocking the expression
and activation of PDGF-D in prostate cancer cells led to the
inhibition of cell proliferation, invasion and angiogenesis. In
addition, Zhao et al (35)
reported that silencing PDGF-D using RNA interference significantly
attenuated the proliferation and invasion of gastric cancer cells
that overexpressed PDGF-D. Furthermore, Lokker et al
(22) demonstrated that blocking
PDGF-D/PDGFR signaling inhibited survival and mitogenic pathways in
glioblastoma cell lines and prevented glioma formation in a nude
mouse xenograft model. However, antagonizing PDGF-D via
small-molecule inhibitors or neutralizing antibodies has not been
evaluated in human cancer. The current results suggest that PDGF-D
may be a therapeutic target for advanced or metastatic gastric
cancer. Furthermore, PDGF-D overexpression was detected in 85% of
advanced gastric cancers in the present study, indicating that
antagonizing PDGF-D may be a useful therapeutic strategy.
PDGF-C is also associated with tumor growth, and a
number of studies have demonstrated its role in tumor growth to
date (22,36,37).
Lokker et al (22) reported
that PDGF-C autocrine signaling may play a role in the progression
of brain tumors, such as glioblastoma and medulloblastoma.
Anderberg et al (35) reported
that that paracrine signaling of PDGF-C accelerated tumor growth
through recruitment and activation of cancer-associated fibroblasts
in malignant melanoma. These findings indicate that overexpression
of PDGF-C accelerates tumor growth through autocrine and paracrine
signaling. In fact, Yamauchi et al (37) reported that PDGF-C overexpression in
colorectal cancer was associated with significantly poorer overall
survival and RFS, and was an independent risk factor for
recurrence. However, in the present study, PDGF-C overexpression in
gastric cancer showed no significant correlation with tumor growth,
distant metastasis and recurrence, in contrast to PDGF-D
overexpression. This result indicates that the role of PDGF-C
overexpression may be less important than that of PDGF-D in the
progression of gastric cancer. However, further investigation of
the molecular function of PDGF-C in gastric cancer is required.
PDGFR-β is a receptor for PDGF-C and PDGF-D. Guo
et al (38) reported that
PDGFR-β was overexpressed predominantly in tumor stromal cells and
was positively correlated with tumor depth, lymph node metastasis
and tumor stage in gastric cancer. Considering the results of the
current study, it is possible that PDGF-D accelerates tumor growth
through the activation of adjacent stromal cells; however, further
studies are necessary to clarify this.
In conclusion, high PDGF-C and PDGF-D expression
were associated with tumor progression and poor survival in
patients with gastric cancer. In particular, PDGF-D was frequently
expressed in gastric cancer and was associated with tumor
progression and poor prognosis. PDGF-D signaling pathways may be a
prognostic factor related to recurrence following curative surgery,
and could serve as a novel target for the treatment of gastric
cancer.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Catalano V, Labianca R, Beretta GD, Gatta
G, de Braud F and Van Cutsem E: Gastric cancer. Crit Rev Oncol
Hematol. 71:127–164. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Koizumi W, Narahara H, Hara T, Takagane A,
Akiya T, Takagi M, Miyashita K, Nishizaki T, Kobayashi O, Takiyama
W, et al: S-1 plus cisplatin versus S-1 alone for first-line
treatment of advanced gastric cancer (SPIRITS trial): A phase III
trial. Lancet Oncol. 9:215–221. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Terashima M, Kitada K, Ochiai A, Ichikawa
W, Kurahashi I, Sakuramoto S, Katai H, Sano T, Imamura H and Sasako
M: ACTS-GC group: Impact of expression of human epidermal growth
factor receptors EGFR and ERBB2 on survival in stage II/III gastric
cancer. Clin Cancer Res. 18:5992–6000. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lieto E, Ferraraccio F, Orditura M,
Castellano P, Mura AL, Pinto M, Zamboli A, De Vita F and Galizia G:
Expresison of vascular endothelial growth factor (VEGF) and
epidermal growth factor receptor (EGFR) is an independent
prognostic indicator of worse outcome in gastric cancer patients.
Ann Surg Oncol. 15:69–79. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hayashi M, Inokuchi M, Takagi Y, Yamada H,
Kojima K, Kumagai J, Kawano T and Sugihara K: High expression of
HER3 is associated with a decreased survival in gastric cancer.
Clin Cancer Res. 14:7843–7849. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Begnami MD, Fukuda E, Fregnani JH,
Nonogaki S, Montagnini AL, da Costa WL Jr and Soares FA: Prognostic
implications of altered human epidermal growth factor receptors
(HERs) in gastric carcinomas: HER2 and HER3 are predictors of poor
outcome. J Clin Oncol. 29:3030–3036. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kim MA, Lee HS, Lee HE, Jeon YK, Yang HK
and Kim WH: EGFR in gastric carcinomas: Prognostic significance of
protein overexpression and high gene copy number. Histopathology.
52:738–746. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
García I, Vizoso F, Martín A, Sanz L,
Abdel-Lah O, Raigoso P and García-Muñiz JL: Clinical significance
of the epidermal growth factor receptor and HER2 receptor in
resectable gastric cancer. Ann Surg Oncol. 10:234–241. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hirashima Y, Yamada Y, Matsubara J,
Takahari D, Okita N, Takashima A, Kato K, Hamaguchi T, Shirao K,
Shimada Y, et al: Impact of vascular endothelial growth factor
receptor 1, 2 and 3 expression on the outcome of patients with
gastric cancer. Cancer Sci. 100:310–315. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jüttner S, Wissmann C, Jöns T, Vieth M,
Hertel J, Gretschel S, Schlag PM, Kemmner W and Höcker M: Vascular
endothelial growth factor-D and its receptor VEGFR-3: Two novel
independent prognostic markers in gastric adenocarcinoma. J Clin
Oncol. 24:228–240. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ohtsu A, Shah MA, Van Cutsem E, Rha SY,
Sawaki A, Park SR, Lim HY, Yamada Y, Wu J, Langer B, et al:
Bevacizumab in combination with chemotherapy as first-line therapy
in advanced gastric cancer: A randomized, double-blind,
placebo-controlled phase III study. J Clin Oncol. 29:3968–3976.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Waddell T, Chau I, Cunningham D, Gonzalez
D, Okines AF, Okines C, Wotherspoon A, Saffery C, Middleton G,
Wadsley J, et al: Epirubicin, oxaliplatin, and capecitabine with or
without panitumumab for patients with previously untreated advanced
oesophagogastric cancer (REAL3): A randomised, open-label phase 3
trial. Lancet Oncol. 14:481–489. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lordick F, Kang YK, Chung HC, Salman P, Oh
SC, Bodoky G, Kurteva G, Volovat C, Moiseyenko VM, Gorbunova V, et
al: Arbeitsgemeinschaft Internistische Onkologie and EXPAND
Investigators: Capecitabine and cisplatin with or without cetuximab
for patients with previously untreated advanced gastric cancer
(EXPAND): A randomised, open-label phase 3 trial. Lancet Oncol.
14:490–499. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bang YJ, Van Cutsem E, Feyereislova A,
Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T,
et al: Trastuzumab in combination with chemotherapy versus
chemotherapy alone for treatment of HER-2 positive advanced gastric
or gastro-oseophageal junction cancer (ToGA): A phase 3,
open-label, randomized controlled trial. Lancet. 376:687–697. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tanner M, Hollmén M, Junttila TT, Kapanen
AI, Tommola S, Soini Y, Helin H, Salo J, Joensuu H, Sihvo E, et al:
Amplification of HER-2 in gastric carcinoma: Association with
topoisomerase IIalpha gene amplification, intestinal type, poor
prognosis and sensitivity to trastuzumab. Ann Oncol. 16:273–278.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yano T, Doi T, Ohtsu A, Boku N, Hashizume
K, Nakanishi M and Ochiai A: Comparison of HER2 gene amplification
assessed by fluorescence in situ hybridization and HER2 protein
expression assessed by immunohistochemistry in gastric cancer.
Oncol Rep. 15:65–71. 2006.PubMed/NCBI
|
|
18
|
Ustach CV, Taube ME, Hurst NJ Jr, Bhagat
S, Bonfil RD, Cher ML, Schuger L and Kim HR: A potential oncogenic
activity of platelet-derived growth factor d in prostate cancer
progression. Cancer Res. 64:1722–1729. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
LaRochelle WJ, Jeffers M, McDonald WF,
Chillakuru RA, Giese NA, Lokker NA, Sullivan C, Boldog FL, Yang M,
Vernet C, et al: PDGF-D, new protease-actovated growth factor. Nat
Cell Biol. 3:517–521. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Andrae J, Gallini R and Betsholtz C: Role
of platelet-derived growth factors in physiology and medicine.
Genes Dev. 22:1276–1312. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
LaRochelle WJ, Jeffers M, Corvalan JR, Jia
XC, Feng X, Vanegas S, Vickroy JD, Yang XD, Chen F, Gazit G, et al:
Platelet-derived growth factor D: Tumorigenicity in mice and
dysregulated expression in human cancer. Cancer Res. 62:2468–2473.
2002.PubMed/NCBI
|
|
22
|
Lokker NA, Sullivan CM, Hollenbach SJ,
Israel MA and Giese NA: Platelet-derived growth factor (PDGF)
autocrine signaling regulates survival and mitogenic pathways in
glioblastoma cells: Evidence that the novel PDGF-C and PDGF-D
ligands may play a role in the development of brain tumors. Cancer
Res. 62:3729–3735. 2002.PubMed/NCBI
|
|
23
|
Zwerner JP and May WA: Dominant negative
PDGF-C inhibits growth of ewing family tumor cell lines. Oncogene.
21:3847–3854. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tejada ML, Yu L, Dong J, Jung K, Meng G,
Peale FV, Frantz GD, Hall L, Liang X, Gerber HP and Ferrara N:
Tumor-driven paracrine platelet-derived growth factor receptor
alpha signaling is a key determinant of stromal cell recruitment in
a model of human lung carcinoma. Clin Cancer Res. 12:2676–2688.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kong D, Banerjee S, Huang W, Li Y, Wang Z,
Kim HR and Sarkar FH: Mammalian target of rapamycin repression by
3,3′-diindolylmethane inhibits invasion and angiogenesis in
platelet-derived growth factor-D-overexpressing PC3 cells. Cancer
Res. 68:1927–1934. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang Z, Kong D, Banerjee S, Li Y, Adsay
NV, Abbruzzese J and Sarkar FH: Down-regulation of platelet-derived
growth factor-D inhibits cell growth and angiogenesis through
inactivation of Notch-1 and nuclear factor-kappaB signaling. Cancer
Res. 67:11377–11385. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ustach CV, Taube ME, Hurst NJ Jr, Bhagat
S, Bonfil RD, Cher ML, Schuger L and Kim HR: A potential oncogenic
activity of platelet-derived growth factor d in prostate cancer
progression. Cancer Res. 64:1722–1729. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ustach CV and Kim HR: Platelet-derived
growth factor D is activated by urokinase plasminogen activator in
prostate carcinoma cells. Mol Cell Biol. 25:6279–6288. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Xu L, Tong R, Cochran DM and Jain RK:
Blocking platelet-derived growth factor-D/platelet-derived growth
factor receptor beta signaling inhibits human renal cell carcinoma
progression in an orthotopic mouse model. Cancer Res. 65:5711–5719.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yang S, Shin J, Park KH, Jeung HC, Rha SY,
Noh SH, Yang WI and Chung HC: Molecular basis of the differences
between normal and tumor tissues of gastric cancer. Biochim Biophys
Acta. 1772:1033–1040. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sobin LH, Gospodarowicz MK and Wittekind
C: TNM Classification of Malignant Tumours (7th). Wiley-Blackwell.
2009.
|
|
32
|
Kong D, Wang Z, Sarkar SH, Li Y, Banerjee
S, Saliganan A, Kim HR, Cher ML and Sarkar FH: Platelet-derived
growth factor-D overexpression contributes to
epithelial-mesenchymal transition of PC3 prostate cancer cells.
Stem Cells. 26:1425–1435. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kong D, Li Y, Wang Z, Banerjee S, Ahmad A,
Kim HR and Sarkar FH: miR-200 regulates PDGF-D-mediated
epithelial-mesenchymal transition, adhesion and invasion of
prostate cancer cells. Stem Cells. 27:1712–1721. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang Z, Ali S, Banerjee S, Bao B, Li Y,
Azmi AS, Korc M and Sarkar FH: Activated K-Ras and INK4a/Arf
deficiency promote aggressiveness of pancreatic cancer by induction
of EMT consistent with cancer stem cell phenotype. J Cell Physiol.
228:556–562. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhao L, Zhang C, Liao G and Long J:
RNAi-mediated inhibition of PDGF-D leads to decreased cell growth,
invasion and angiogenesis in the SGC-7901 gastric cancer xenograft
model. Cancer Biol Ther. 9:42–48. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Anderberg C, Li H, Fredriksson L, Andrae
J, Betsholtz C, Li X, Eriksson U and Pietras K: Paracrine signaling
by platelet-derived growth factor-CC promotes tumor growth by
recruitment of cancer-associated fibroblasts. Cancer Res.
69:369–378. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yamauchi S, Iida S, Ishiguro M, Ishikawa
T, Uetake H and Sugihara K: Clinical significance of
platelet-derived growth factor-C expression in colorectal cancer. J
Cancer Ther. 5:11–20. 2014. View Article : Google Scholar
|
|
38
|
Guo Y, Yin J, Zha L and Wang Z:
Clinicopathological significance of platelet-derived growth factor
B, platelet-derived growth factor receptor-β and E-cadherin
expression in gastric carcinoma. Contemp Oncol (Pozn). 17:150–155.
2013.PubMed/NCBI
|