Introduction
Clinically, peritoneal carcinomatosis represents an
extreme form of cancer progression with a poor prognosis that is
common in ovarian, colon and stomach cancer (1). Ovarian carcinoma is the fifth cause of
mortality from gynecological cancer in women. Furthermore, ~70% of
patients are diagnosed with peritoneal carcinomatosis at an
advanced disease stage (2,3). In colon cancer, ~8% of patients have
isolated peritoneal seeding at the time of the primary surgery, and
25% of patients with recurrence have been found to exhibit
peritoneal cavity metastasis (4,5). A
previous study showed that systemic chemotherapy or intraperitoneal
(i.p.) chemotherapy alone had no significant effects on patient
survival in the clinic (6).
Therefore, novel therapeutic approaches are required to improve the
therapeutic effects on peritoneal carcinomatosis.
An i.p. xenograft experimental model in nude mice
simulates the process of peritoneal dissemination in
intra-abdominal cancers. This model could be used to verify the
effects of novel therapeutic strategies for peritoneal
carcinomatosis in preclinical trials. A number of i.p.
carcinomatosis mouse models had been established to study the
processes of peritoneal dissemination and to verify the effects of
novel therapeutic approaches since the first report of subcutaneous
(s.c.) heterologous growth of human ovarian cancer tissue in nude
mice (7,8). In a number of these studies, human tumor
cells were used for s.c injection. In other studies, following
growth of the tumor to a suitable size, according to the experiment
employed, the tumor was removed and cut into pieces or cell
clusters for i.p. injection into nude mice (8,9). Certain
models were established by surgical implantation of fresh human
tumor tissues or in situ injection of tumor cells obtained
from homogenized human tumor tissues (10,11),
whereas other models were established by i.p. injection of specific
human cancer cell lines directly or using depth immunodeficiency
mice (12,13). However, these aforementioned methods
were complicated. A low success rate, a long latency period to
develop palpable i.p. carcinomatosis and the requirement for
surgery are the main obstacles in developing these models. The
easiest method is to implant the usual human cancer cell lines
directly into nude mice by i.p injection. However, it is difficult
to achieve heterologous growth of human cancer cell lines in the
abdominal cavity of nude mice by direct i.p. injection due to the
non-specific immune response in the nude mice and the change of
survival microenvironment for the human cancer cells. In our
previous study, 1×107 SKOV-3 and 6×106
HCT-116 cells were directly implanted in order to establish ovarian
peritoneal carcinomatosis and colorectal peritoneal carcinomatosis
(CRPC) in the nude mice by i.p. injection. However, only a small
number of tumor nodes were found in the mouse abdominal cavities in
the CRPC model group. Also, mice in the ovarian peritoneal
carcinomatosis group demonstrated a long latency period to develop
little palpable i.p. carcinomatosis (unpublished data). These
issues called for a more efficient way to establish a peritoneal
carcinomatosis experimental model in the nude mice.
Numerous studies have highlighted that the tumor
microenvironment, which is composed of structural [extracellular
matrix (ECM)] and soluble extracellular substances (cytokines,
proteases and hormones), as well as cellular components, including
tumor cells, inflammatory cells, fibroblasts, and vascular and
lymphatic endothelial cells. The microenvironment has a critical
function in the pathogenesis of tumors, including tumor invasion
and metastasis (14–16). Therefore, it is hypothesized that the
establishment of peritoneal carcinomatosis in nude mice by direct
i.p. injection of human cancer cells could become easier if the
peritoneal cancer cell microenvironment was accustomed to the
growth of human tumor cells.
ECM gel is a temperature-sensitive and reconstituted
nutritional compound that has been widely used in cell cultures,
tumor cell migration, angiogenesis and other biological experiments
(17–19). ECM gel is prepared from mouse
Engelbreth-Holm-Swarm sarcoma, which contains laminin as a major
component, and collagen type IV, heparan sulfate proteoglycans and
entactin as minor components (19,20). ECM
gel has been shown to enhance the s.c. tumor growth of nude mice
(21,22). We hypothesized that the ECM gel could
also promote the formation of tumor tissues in the abdominal cavity
of nude mice, since the ECM gel, which itself was obtained from
nude mice, could promote the growth of human tumor cells with no
immune rejection.
In the present study, liquid ECM gel solution was
used to suspend human ovarian cancer SKOV-3 and colon cancer
HCT-116 cells. Two stable and simple peritoneal tumor models were
established in nude mice through implanting these cell/ECM gel
suspensions into the abdominal cavity of the mice with high success
rates. Next, the i.p. chemotherapy effect of irinotecan (CPT-11),
an antitumor drug that is generally used for metastatic colon or
rectal cancer treatment, was evaluated using the colorectal i.p.
xenograft nude mouse model established by this method.
Materials and methods
Drugs and cell culture
CPT-11 was purchased from Prizer Co. (Shanghai,
China). The human ovarian cancer SKOV3 and colon cancer HCT-116
cell lines were obtained from the American Type Culture Collection
(Manassas, VA, USA) and cultured in Dulbecco's modified Eagle's
medium (Gibco Life Technologies, Carlsbad, CA, USA) supplemented
with 10% heat-inactivated fetal calf serum, 2 mM L-glutamine and
0.1 mg/ml amikacin. The cells were incubated in a humidified 5%
CO2 atmosphere at 37°C and harvested during the
logarithmic growth phase. The cells were then resuspended in
phosphate-buffered saline (PBS) or ECM gel for i.p. injection.
Animals
Female, 7–8-week-old, BALB/c athymic nude mice were
purchased from the Laboratory Animal Center of Sichuan University
(Chengdu, Sichuan, China) and acclimated for 1 week. The mice were
caged in groups of five in an air-filtered laminar flow cabinet and
fed with irradiated food and autoclaved reverse-osmosis treated
water ad libitum. All procedures were performed under
sterile conditions in a laminar flow hood. Animal experiments were
conducted under guidelines approved by the Institutional Animal
Care and Treatment Committee of Sichuan University (23), and the study was approved by the
animal experiment ethics committee of West China Hospital, Sichuan
University (Chengdu, China; approval no. 018).
Establishment of i.p. xenograft models
in nude mice
The suspended human ovarian cancer SKOV-3 cells were
injected into the abdominal cavities of 18 nude mice using a needle
with a length of 16 mm and a diameter of 0.45 mm. Specifically,
4×106 cells in 200 µl PBS for the control group and
4×106 cells in 200 µl cold liquid ECM gel (placed on ice
early) for the experimental group were injected into 9 control mice
and 9 experimental mice, respectively. In each group, 3 mice were
randomly selected and sacrificed by cervical dislocation on the
10th, 25th and 30th days after i.p. inoculation. A total of
3×106 human colon cancer HCT-116 cells were suspended in
200 µl PBS and 200 µl cold liquid ECM gel for the control and
experimental groups, respectively. Next, the solutions were
injected into the abdominal cavities of the 18 nude mice as
aforementioned. To investigate the progression and characteristics
of the i.p. xenografts, three mice were randomly selected for
sacrifice by cervical dislocation on the 7th, 14th and 28th days
after i.p. inoculation.
Necropsy
The mice were dissected and observed
macroscopically. Specifically, the sizes of the abdominal tumors
and tumor nodes, and the locations of the tumors were recorded.
Following this, the tumors were collected for histopathological
analysis.
Histopathological analysis
To establish the colorectal peritoneal
carcinomatosis (CRPC) model, the colonic tumor node, the apparent
masses in the abdominal cavity and the mesentery were stripped down
on the 7th, 14th and 28th days, respectively, and then frozen with
optimal cutting temperature (OCT) compound. The 5-µm frozen
sections were obtained from the optimal cross-sectional surface of
the masses. The sections were then stained with hematoxylin and
eosin (H&E) and examined under light microscopy. For the
ovarian peritoneal carcinomatosis model, when the mice were
sacrificed on the 25th and 30th days, the apparent masses in the
abdominal cavities of the nude mice were stripped down and frozen
with OCT compound. The frozen section and H&E analysis were
performed as aforementioned.
In vivo antitumor evaluation of CPT-11
by the CRPC nude mice model
A CRPC therapeutic nude mice model was established
and treated in a similar fashion. Briefly, 3×106 HCT-116
cells were injected into the abdominal cavities of nude mice using
a needle that was 16 mm in length, with a diameter of 0.45 mm. The
mice were randomly divided into groups of five for control and
experimental conditions after acclimation for 1 week (day 0). The
mice of the test group were injected with i.p. CPT-11 at a dose of
10 mg/kg/day, whereas the mice in the control group were injected
with i.p. normal saline (NS) at the same volume as the test group
on days 0, 4 and 8 (24,25). The body weights of all mice were
measured every 3 days. The mice were sacrificed by cervical
dislocation on the 21st day, when the tumor nodes and the weights
of the peritoneal tumors were measured.
Statistical analysis
Data are expressed as the mean ± standard deviation.
An unpaired two-tailed t-test was performed to calculate
significance differences using SPSS software (version 17.0; SPSS,
Inc., Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
Dynamic progression of the ovarian
i.p. xenografts
For the ovarian i.p. xenograft mice (Fig. 1), at 10 days post-i.p. injection, 3
mice from each group were randomly selected and sacrificed. The
mean number of tumor nodes was higher in the experimental group
(6.3±2.5) than the control group (1.0±1.0) (Table I). No ascites or macroscopic tumors
were found in the control or experimental subjects (Fig. 1A).
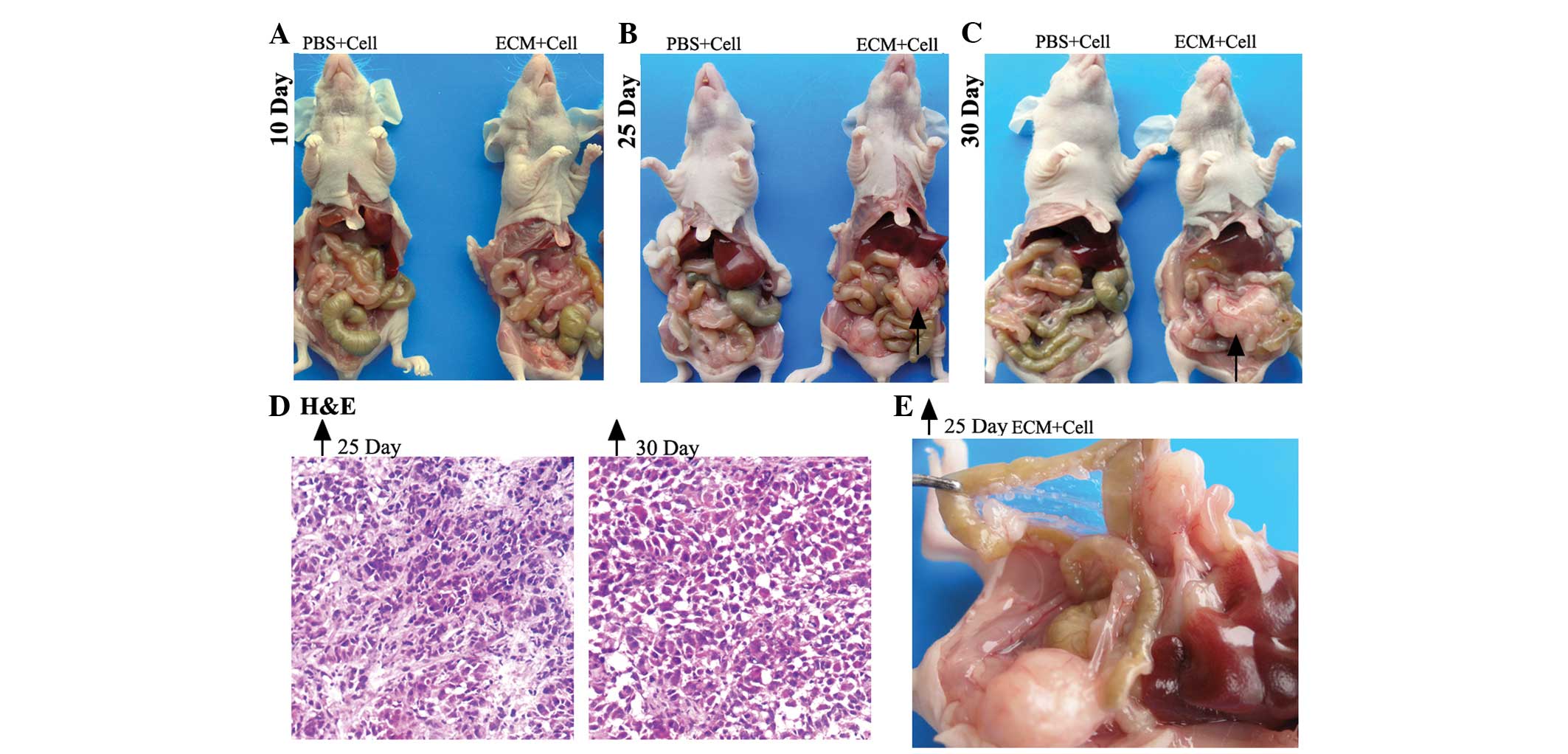 | Figure 1.Macroscopic and microscopic
observation of ovarian intraperitoneal xenografts in nude mice
sacrificed on the 10th, 25th and 30th days. Macroscopic and
representative images of mice from the PBS and ECM groups
sacrificed on (A) the 10th day, (B) the 25th day, and (C) the 30th
day, with black arrows indicating the representative tumor nodule.
(D) Microscopic observation of H&E staining of frozen sections
(×100 magnification) of the tumor nodules indicated by the black
arrows on the 25th and 30th day, respectively. (E) Magnified image
showing the peritoneal cavity of the mouse from the ECM group, as
indicated by the black arrow, on the 25th day. ECM, extracellular
matrix; PBS, phosphate-buffered saline; H&E, hematoxylin and
eosin. |
 | Table I.Dynamic progression of i.p. xenografts
of human ovarian and colorectal cancer in nude mice at different
time-points post-i.p. injection. |
Table I.
Dynamic progression of i.p. xenografts
of human ovarian and colorectal cancer in nude mice at different
time-points post-i.p. injection.
| Cell line | Day | Tumor nodes in ECM
group, na | Tumor nodes in PBS
group, na |
|---|
| SKOV-3 | 10 |
6.3±2.5b | 1.0±1.0 |
|
| 25 | 17.7±2.1b | 3.3±1.5 |
|
| 30 | 21.0±3.0b | 3.6±2.5 |
| HCT-116 | 7 |
8.0±2.6b | 1.7±0.8 |
|
| 14 |
13.0±2.0b | 3.0±1.0 |
|
| 28 | >50b | 5.0±1.5 |
At 25 days post-i.p. inoculation, no evident
abdominal distension was observed in either model. However, all the
mice in the experimental group (n=9) had developed palpable tumor
masses in the abdominal cavities. A total of 3 mice from each group
were randomly selected and sacrificed. The mean number of tumor
nodes was higher in the experimental group (17.7±2.1) than the
control group (3.3±1.5) (Table I).
The largest tumor node in the experimental group was up to 17 mm in
diameter, whereas in the control group, it was <7 mm. The
distribution of the tumor nodes was widespread, with locations that
included the mesentery, the omentum, the intestinal surface, the
retroperitoneum and around the pancreas (Fig. 1B). Partial enlargement of the abdomen
was also observed in the experimental subjects (Fig. 1E).
The remaining mice (n=6) were sacrificed at 30 days
post-i.p. inoculation. Despite the bigger palpable tumor masses
observed in the abdominal cavities of the experimental subjects,
the general characteristics of the abdomens in these two groups
were not significantly different compared with the mice sacrificed
on the 25th day. Again, the mean number of tumor nodes was higher
in the experimental group (21±3) compared with the control group
(3.6±2.5) (Table I). The tumor nodes
in the experimental subject had developed up to 24 mm in diameter
(Fig. 1C).
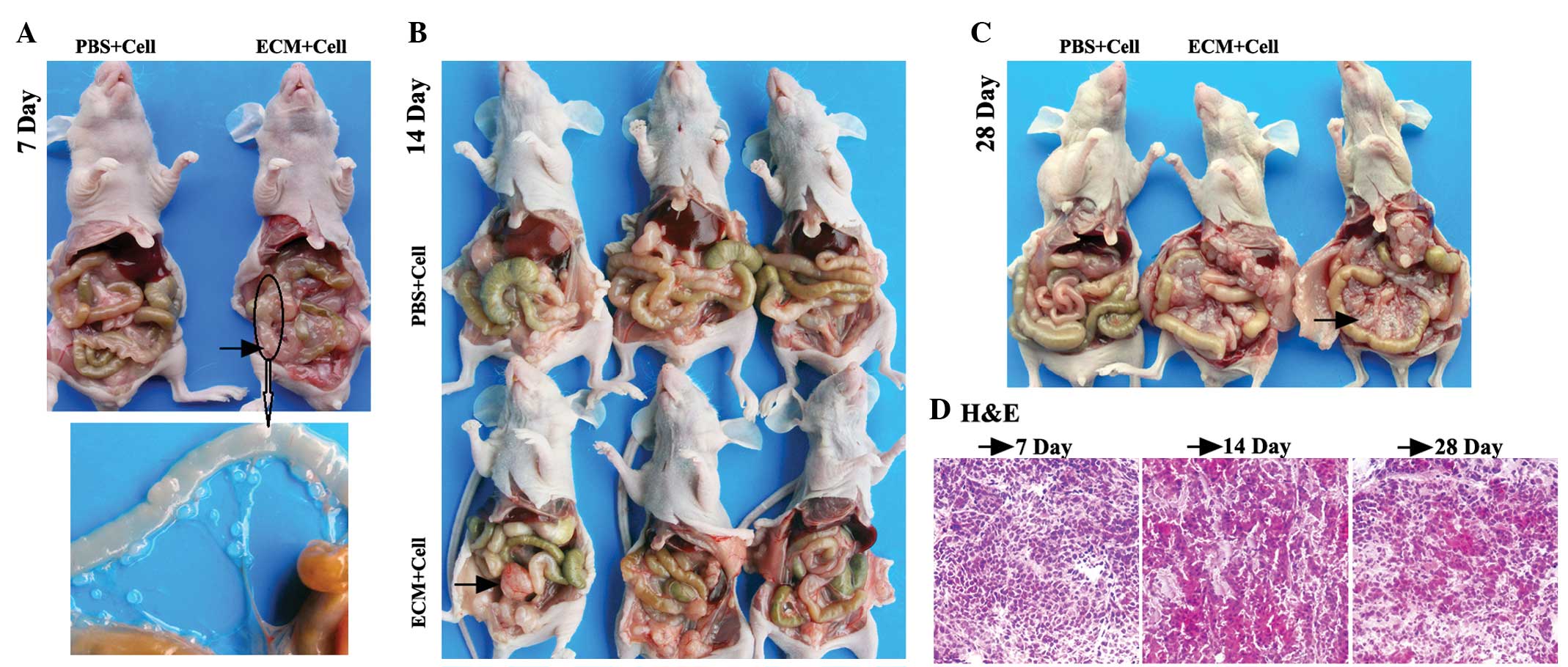
Dynamic progression characteristics of
colorectal i.p. xenografts
For the colorectal i.p. xenograft mice, 3 mice from
each group were randomly chosen and sacrificed at 7 days post-i.p.
inoculation. No ascites or macroscopic tumors were observed in the
mice of either group. However, several small tumor nodules of <1
mm diameter were found on the mesentery of one experimental subject
(Fig. 2A). The mean numbers of tumor
nodes are shown in Table I.
A total of 3 mice from each group were randomly
chosen and sacrificed at 14 days post-i.p. inoculation. Tumor
masses were observed in the abdominal cavities of two experimental
subjects (Fig. 2B). The mean numbers
of tumor nodes are shown in Table
I.
Marked abdominal distension was observed in the mice
of the experimental group at 28 days post-i.p. inoculation.
Furthermore, two of the mice had developed numerous bloody ascites.
Meanwhile, as expected, marked abdominal distension and bloody
ascites were not found in the control subjects (Fig. 2C).
Histopathological analysis
In order to determine whether the intra-abdominal
nodes observed were actually tumorous, H&E staining of the
frozen ovarian and colorectal i.p. xenograft tumor node sections
were performed. Microscopic analyses of the specimens were
conducted to determine the tumorous identity. For the CRPC model,
the tumor nodes on the colons of the mice of the experimental group
sacrificed on the 7th day, the apparent masses in the abdominal
cavities of the nude mice sacrificed on the 14th day and the masses
on the mesenteries of the nude mice sacrificed on the 28th day were
stripped down for the H&E staining of frozen sections. For the
ovarian peritoneal carcinomatosis model, when the mice were
sacrificed on the 25th and 30th days, the apparent masses in the
abdominal cavities of the nude mice in the experimental group were
stripped down for the H&E staining of frozen sections. The
microscopic observations of the H&E staining (×100
magnification) of these abdominal cavity masses are shown in
Fig. 1D and 2D. The staining analysis confirmed that
these intra-abdominal nodes in the ovarian and colorectal i.p.
xenografts were tumor tissue.
In vivo antitumor evaluation of CPT-11
in the CRPC nude mice model
To determine whether the i.p. xenograft nude mouse
models could be efficient as preclinical therapeutic models, an
additional experiment using the colorectal i.p. xenograft nude
mouse model was performed to evaluate the i.p. chemotherapy effect
of CPT-11. CPT-11 was most active against this i.p. xenograft nude
mouse model at a dose of 10 mg/kg/day, administered 3 times every 4
days (Fig. 3). The size of the tumor
nodes in the CPT-11 treatment group was significantly smaller than
that of the NS group (Fig. 3A and B).
The total weight of the peritoneal tumor nodes in the CPT-11
treatment group (0.81±0.16) was significantly less than that of the
NS group (2.18±0.21; P<0.05; Fig.
3C). All the tumor nodes in the two groups were counted and
marked as shown in Fig. 3E. The mouse
body weights were monitored every 4 days, and there was a
significant difference in weight between the NS and CPT-11 groups
(P<0.05; Fig. 3D).
Discussion
Peritoneal carcinomatosis is a secondary cancer that
occurs when cancer cells metastasize from other areas of the body
and implant into the abdominal cavity. Conventional chemotherapy
methods, including systemic chemotherapy or i.p. chemotherapy
alone, exhibit no significant effects on survival in the clinic
(26). Novel drugs and chemotherapy
approaches are required to improve the therapeutic effects of
peritoneal carcinomatosis. Nude mouse i.p. xenograft experimental
models simulate the process of peritoneal dissemination in
intra-abdominal cancers and could be used to verify the therapeutic
effects of novel drugs and treatment approaches for pre-clinical
evaluations. The easiest method for establishing the i.p. xenograft
experimental model in nude mice is to directly implant the human
cancer cell lines into the abdominal cavity of nude mice. However,
the heterologous growth of human single cancer cells in the
abdominal cavity of nude mice is difficult to achieve, with
obstacles such as the residual non-specific immune function in nude
mice that are difficult to overcome (27). One previous study indicated that a
tumor tissue mass could grow well in the abdominal cavity of nude
mice, but would require surgery and wounding of the mice (28). In the present study, to enable the
growth of the cells and the progression into a tumor tissue mass,
liquid ECM gel was used to suspend the human ovarian cancer SKOV-3
cells and the human colon cancer HCT-116 cells. Two nude mouse
peritoneal tumor models were established by i.p. injection to
implant these ECM gel cell suspensions into the abdominal cavities
of the nude mice (Figs. 1 and
2).
Liquid ECM gel (2–8°C) functions in a similar manner
as PBS to suspend cells sufficiently. When the cell suspensions are
injected into the abdominal cavities of nude mice, the PBS cell
suspension is distributed in the form of single cells within the
abdominal cavity, whereas the ECM gel cell suspension is
distributed in the form of a cell mass due to the fact that the ECM
gel forms into a jelly at 37°C (29).
ECM gel is a mixture of ECM proteins produced by a mouse sarcoma
cell line in vivo. The ECM gel is rich in laminin and
collagen IV, which could stimulate tumor cell adhesion and
motility. Therefore, cells in the ECM jelly could adhere to the
surface of the mouse intestines and peritoneum more easily than
when in the form of single cells. As they are surrounded by an
outer layer of ECM gel, cells in the ECM jelly could more easily
avoid the attacks from natural killer cells, monocytes and
macrophages.
ECM gel also provides a good microenvironment for
tumor growth. Liquid ECM gel cell suspension undergoes
thermal-activated polymerization at 20–40°C when injected into the
abdominal cavities of nude mice to form a reconstituted jelly
anchoring a high density of tumor cells. Cells that are in contact
with each other within the ECM gel are conducive to cells growth
and signal transduction. The ECM jelly, including laminin as a
major component, and collagen type IV, heparan sulfate
proteoglycans and entactin as minor components, is also a rich
store of angiogenic and tumor growth factors (30). For example, certain fragments of
laminin-1, collagen IV and other matrix proteins can increase
angiogenesis, tumor growth and metastasis (31,32).
Currently, the CRPC mouse model, established by the
injection of the mouse colon cancer CT-26 cell line into the
abdominal cavity of BALB/c mice, is widely used to evaluate the
i.p. chemotherapy effect of novel i.p. chemotherapy strategies
(33). However, the application of a
CRPC nude mouse model established using human cancer cells is rare.
In the present study, an additional experiment was performed using
the colorectal i.p. xenograft nude mouse model to evaluate the i.p.
chemotherapy effect of CPT-11. Our previous study using the ovarian
i.p. xenograft nude mouse model established by this method was also
performed to evaluate the anticancer effect of T-DM1, an antibody
drug conjugate (34). These results
also confirmed that the i.p. xenograft nude mouse models
established in this method are efficient and available preclinical
therapeutic models for intra-abdominal cancers.
Acknowledgements
The authors are grateful to Mr. Shijie Zhou, Dr Ping
Tang, Dr Rui Zhou and Mr. Cong Ma of the State Key Laboratory of
Biotherapy and Cancer Center/Collaborative Innovation Center for
Biotherapy (West China Hospital, Chengdu, China) Sichuan University
for providing excellent technical assistance. This study was
supported by the National Science and Technology Major Projects of
New Drugs (grant no. 2012ZX09103301-037).
References
|
1
|
Kusamura S, Baratti D, Zaffaroni N, Villa
R, Laterza B, Balestra MR and Deraco M: Pathophysiology and biology
of peritoneal carcinomatosis. World J Gastrointest Oncol. 2:12–18.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Muñoz-Casares FC, Rufián S, Arjona-Sánchez
Á, Rubio MJ, Díaz R, Casado Á, Naranjo Á, Díaz-Iglesias CJ, Ortega
R, Muñoz-Villanueva MC, et al: Neoadjuvant intraperitoneal
chemotherapy with paclitaxel for the radical surgical treatment of
peritoneal carcinomatosis in ovarian cancer: A prospective pilot
study. Cancer Chemother Pharmacol. 68:267–274. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Guardiola E, Delroeux D, Heyd B, Combe M,
Lorgis V, Demarchi M, Stein U, Royer B, Chauffert B and Pivot X:
Intra-operative intra-peritoneal chemotherapy with cisplatin in
patients with peritoneal carcinomatosis of ovarian cancer. World J
Surg Oncol. 7:142009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Macrì A, Saladino E, Bartolo V, Adamo V,
Altavilla G, Mondello E, Condemi G, Sinardi A and Famulari C:
Peritoneal carcinomatosis of colorectal origin. World J
Gastrointest Oncol. 2:98–101. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sadeghi B, Arvieux C, Glehen O, Beaujard
AC, Rivoire M, Baulieux J, Fontaumard E, Brachet A, Caillot JL,
Faure JL, et al: Peritoneal carcinomatosis from non-gynecologic
malignancies: Results of the EVOCAPE 1 multicentric prospective
study. Cancer. 88:358–363. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yan TD, Stuart OA, Yoo D and Sugarbaker
PH: Perioperative intraperitoneal chemotherapy for peritoneal
surface malignancy. J Transl Med. 4:172006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Davy M, Mossige J and Johannessen JV:
Heterologous growth of human ovarian cancer. A new in vivo testing
system. Acta Obstet Gynecol Scand. 56:55–59. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mei LJ, Yang XJ, Tang L, Hassan AH,
Yonemura Y and Li Y: Establishment and identification of a rabbit
model of peritoneal carcinomatosis from gastric cancer. BMC Cancer.
10:1242010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Massazza G, Tomasoni A, Lucchini V,
Allavena P, Erba E, Colombo N, Mantovani A, D'Incalci M, Mangioni C
and Giavazzi R: Intraperitoneal and subcutaneous xenografts of
human ovarian carcinoma in nude mice and their potential in
experimental therapy. Int J Cancer. 44:494–500. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ward BG, Wallace K, Shepherd JH and
Balkwill FR: Intraperitoneal xenografts of human epithelial ovarian
cancer in nude mice. Cancer Res. 47:2662–2667. 1987.PubMed/NCBI
|
|
11
|
Fidler IJ: Critical factors in the biology
of human cancer metastasis: Twenty-eighth G.H.A. Clowes memorial
award lecture. Cancer Res. 50:6130–6138. 1990.PubMed/NCBI
|
|
12
|
Santoro L, Boutaleb S, Garambois V,
Bascoul-Mollevi C, Boudousq V, Kotzki PO, Pèlegrin M,
Navarro-Teulon I, Pèlegrin A and Pouget JP: Noninternalizing
monoclonal antibodies are suitable candidates for 125I
radioimmunotherapy of small-volume peritoneal carcinomatosis. J
Nucl Med. 50:2033–2041. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xu Y, Silver DF, Yang NP, Oflazoglu E,
Hempling RE, Piver MS and Repasky EA: Characterization of human
ovarian carcinomas in a SCID mouse model. Gynecol Oncol.
72:161–170. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhong L, Roybal J, Chaerkady R, Zhang W,
Choi K, Alvarez CA, Tran H, Creighton CJ, Yan S, Strieter RM, et
al: Identification of secreted proteins that mediate cell-cell
interactions in an in vitro model of the lung cancer
microenvironment. Cancer Res. 68:7237–7245. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gout S and Huot J: Role of cancer
microenvironment in metastasis: Focus on colon cancer. Cancer
Microenviron. 1:69–83. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fidler IJ, Kim SJ and Langley RR: The role
of the organ microenvironment in the biology and therapy of cancer
metastasis. J Cell Biochem. 101:927–936. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xu X and Prestwich GD: Inhibition of tumor
growth and angiogenesis by a lysophosphatidic acid antagonist in an
engineered three-dimensional lung cancer xenograft model. Cancer.
116:1739–1750. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nicosia RF and Ottinetti A: Modulation of
microvascular growth and morphogenesis by reconstituted basement
membrane gel in three-dimensional cultures of rat aorta: A
comparative study of angiogenesis in matrigel, collagen, fibrin and
plasma clot. Vitro Cell Dev Biol. 26:119–128. 1990. View Article : Google Scholar
|
|
19
|
Kleinman HK and Martin GR: Matrigel:
Basement membrane matrix with biological activity. Semin Cancer
Biol. 15:378–386. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Carey DJ, Todd MS and Rafferty CM: Schwann
cell myelination: Induction by exogenous basement membrane-like
extracellular matrix. J Cell Biol. 102:2254–2263. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Akbasak A, Toevs CC and Laske DW:
Reconstituted basement membrane (matrigel) enhances the growth of
human glioma cell lines in nude mice. J Neurooncol. 27:23–30. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ishii E, Greaves A, Grunberger T, Freedman
MH and Letarte M: Tumor formation by a human pre-B leukemia cell
line in scid mice is enhanced by matrigel and is associated with
induction of CD10 expression. Leukemia. 9:175–184. 1995.PubMed/NCBI
|
|
23
|
Wang X, Duan X, Yang G, Zhang X, Deng L,
Zheng H, Deng C, Wen J, Wang N, Peng C, et al: Honokiol Crosses BBB
and BCSFB and inhibits brain tumor growth in rat 9L intracerebral
gliosarcoma model and human U251 xenograft glioma model. PLoS One.
6:e184902011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sumitomo M, Koizumi F, Asano T, Horiguchi
A, Ito K, Asano T, Kakizoe T, Hayakawa M and Matsumura Y: Novel
SN-38-incorporated polymeric micelle, NK012, strongly suppresses
renal cancer progression. Cancer Res. 68:1631–1635. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nagano T, Yasunaga M, Goto K, Kenmotsu H,
Koga Y, Kuroda J, Nishimura Y, Sugino T, Nishiwaki Y and Matsumura
Y: Synergistic antitumor activity of the SN-38-incorporating
polymeric micelles NK012 with S-1 in a mouse model of non-small
cell lung cancer. Int J Cancer. 127:2699–2706. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Micames C, Jowell PS, White R, Paulson E,
Nelson R, Morse M, Hurwitz H, Pappas T, Tyler D and McGrath K:
Lower frequency of peritoneal carcinomatosis in patients with
pancreatic cancer diagnosed by EUS-guided FNA vs. percutaneous FNA.
Gastrointest Endosc. 58:690–695. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sasiak AB, Sebesteny A, Hrivnak G and
Lloyd DH: Experimental dermatophilosis in murine models of
immunodeficiency. Rev Elev Med Vet Pays Trop. 46:263–269.
1993.PubMed/NCBI
|
|
28
|
Zeng QL, Chu ZH, Zhou K and Luo XJ: Effect
of Endostatin and SU6668 combined with 5-FU on human colon cancer
xenograft in nude mice. Zhonghua Wei Chang Wai Ke Za Zhi.
11:376–378. 2008.(In Chinese). PubMed/NCBI
|
|
29
|
Frisk T, Rydholm S, Andersson H, Stemme G
and Brismar H: A concept for miniaturized 3-D cell culture using an
extracellular matrix gel. Electrophoresis. 26:4751–4758. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Engbring JA and Kleinman HK: The basement
membrane matrix in malignancy. J Pathol. 200:465–470. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kleinman HK, Philp D and Hoffman MP: Role
of the extracellular matrix in morphogenesis. Curr Opin Biotechnol.
14:526–532. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ponce ML, Nomizu M, Delgado MC, Kuratomi
Y, Hoffman MP, Powell S, Yamada Y, Kleinman HK and Malinda KM:
Identification of endothelial cell binding sites on the laminin
gamma 1 chain. Circ Res. 84:688–694. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang Y, Gong C, Yang L, Wu Q, Shi S, Shi
H, Qian Z and Wei Y: 5-FU-hydrogel inhibits colorectal peritoneal
carcinomatosis and tumor growth in mice. BMC Cancer. 10:4022010.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yu L, Wang Y, Yao Y, Li W, Lai Q, Li J,
Zhou Y and Kang T: Eradication of growth of HER2-positive ovarian
cancer with trastuzumab-DM1, an antibody-cytotoxic drug conjugate
in mouse xenograft model. Int J Gynecol Cancer. 24:1158–1164. 2014.
View Article : Google Scholar : PubMed/NCBI
|