Introduction
Gastric cancer (GC) is the fourth most common cancer
and the second cause of cancer-related mortalities worldwide
(1,2).
Countries in East Asia (including China, Japan and Korea) have a
high incidence of GC (>40 cases/100,000 men). Data for
individual countries have shown that GC is the most common cancer
type in Japan and the second most common in China and Korea
(3). Although early diagnosis and
treatment improved prognosis significantly (4,5), the
5-year survival rate remains at only 10–15% among individuals with
advanced disease (6).
The systemic inflammatory response (SIR) is
associated with the outcome of several types of cancer (7). It has been shown that neutrophils,
lymphocytes and platelets are important in tumor-induced SIR
(8). Based on this hypothesis, the
quantification of these blood counts has been investigated in
various malignant tumors as markers of SIR (9,10).
Of these markers, neutrophil to lymphocyte ratio
(NLR) and platelet to lymphocyte ratio (PLR) have been identified
as a promising diagnostic prospect. Investigations have
demonstrated that NLR and PLR are highly repeatable, cost-effective
and widely available (11). NLR is
diagnostically valuable in certain pathologies characterized by
systemic or local inflammatory response, such as diabetes mellitus,
coronary artery disease, ulcerative colitis, inflammatory arthritis
(12–14), as well as various types of cancer,
including colon cancer, gastric cancer, esophageal cancer, ovarian
cancer, lung cancer and breast cancer (15–20). PLR
is considered to be a marker of endogenous residual anticancer
preinflammatory and precoagulative response that arises in
malignancies. These biomarkers combine the evident preinflammatory
and precoagulative status in cancer with the endogenous residual
anticancer ability (21).
The aim of the present study was to examine whether
NLR and PLR served as sensitive markers and prognostic factors in
patients with unresectable GC. In addition, the relationship
between the changes of NLR and PLR following chemotherapy and
prognosis was investigated.
Materials and methods
Subjects and inclusion criteria
The study was conducted as a retrospective
investigation of GC patients that had been referred to the First
Affiliated Hospital of Soochow University (Jiangsu, China) between
June 2010 and 2011. Approval for the study was granted by the
Medical Ethics Committees of the First Affiliated Hospital of
Soochow University. Clinical and pathological records of all the
patients participating in the study were reviewed periodically.
In total, 120 unresectable GC patients were included
in this study. Patient characteristics are provided in Tables I and II. The mean age of the 120 patients was 68
years (range, 32–82 years), and 75 patients were male and 45 were
female. The inclusion criteria were: i) patients with
histologically or cytologically confirmed recurrent or metastatic
GC; ii) age >18 years; iii) Karnofsky performance status (KPS)
score of ≥70; iv) patients with a predicted survival of ≥3 months;
v) naive to antitumor treatment or the post-operative adjuvant
chemotherapy was performed ≥6 months after the previous dose of
chemotherapy; vi) in the case of patients scheduled for
radiotherapy on the target lesion, radiotherapy was required to
have been terminated for at ≥3 months; vii) patients with at least
one measurable lesion (at least 10×10 mm on CT or MRI); and viii)
patients who met the following laboratory criteria: white blood
cells (WBC) ≥4.0×109/l, absolute neutrophil count (ANC)
≥1.5×109/l, platelet (PLT) ≥100×109/l, serum
bilirubin ≤ upper limit of normal (ULN), ALT, AST and ALP ≤ ULN
×2.5 (if without liver metastasis) or ≤ ULN ×5 (if with liver
metastasis), urea nitrogen ≤ ULN ×1.25, and creatinine ≤ ULN
×1.25.
 | Table I.Relationship between baseline NLR
level and clinicopathological characteristics. |
Table I.
Relationship between baseline NLR
level and clinicopathological characteristics.
|
| NLR |
|---|
|
|
|
|---|
| Clinicopathological
characteristics | n | Low (n) | High (n) | χ2 | P-value |
|---|
| Gender |
|
|
|
|
|
|
Men | 75 | 37 | 38 | 0.0356 | 0.850 |
|
Women | 45 | 23 | 22 |
|
|
| Age (years) |
|
|
|
|
|
|
<65 | 67 | 31 | 36 | 0.8448 | 0.358 |
|
≥65 | 53 | 29 | 24 |
|
|
| Tumor size
(cm) |
|
|
|
|
|
|
<5 | 82 | 47 | 35 | 5.5456 | 0.018a |
| ≥5 | 38 | 13 | 25 |
|
|
| Lauren type |
|
|
|
|
|
|
Intestinal type | 68 | 35 | 33 | 0.1357 | 0.713 |
| Diffuse
type | 52 | 25 | 27 |
|
|
| Distant
metastasis |
|
|
|
|
|
| No | 31 | 26 | 5 | 19.1809 |
<0.001b |
|
Yes | 89 | 34 | 55 |
|
|
| Degree of
differentiation |
|
|
|
|
|
| Highly
differentiated | 34 | 23 | 11 | 5.9097 | 0.015a |
|
Moderately and poorly
differentiated | 86 | 37 | 49 |
|
|
| HER-2 |
|
|
|
|
|
|
0-+ | 78 | 39 | 39 | 0.000 | 1.000 |
|
++-+++ | 42 | 21 | 21 |
|
|
| Ki-67 |
|
|
|
|
|
|
<15% | 67 | 36 | 31 | 0.3580 | 0.845 |
|
≥15% | 53 | 24 | 29 |
|
|
 | Table II.Relationship between baseline PLR
level and clinicopathological characteristics. |
Table II.
Relationship between baseline PLR
level and clinicopathological characteristics.
|
| PLR |
|---|
|
|
|
|---|
| Clinicopathological
characteristics | n | Low (n) | High (n) | χ2 | P-value |
|---|
| Gender |
|
|
|
|
|
|
Men | 75 | 39 | 36 | 0.320 | 0.572 |
|
Women | 45 | 21 | 24 |
|
|
| Age (years) |
|
|
|
|
|
|
<65 | 67 | 36 | 31 | 0.8448 | 0.358 |
|
≥65 | 53 | 24 | 29 |
|
|
| Tumor size
(cm) |
|
|
|
|
|
|
<5 | 82 | 38 | 44 | 2.9405 | 0.086 |
| ≥5 | 38 | 24 | 14 |
|
|
| Lauren type |
|
|
|
|
|
|
Intestinal type | 68 | 32 | 36 | 0.5430 | 0.461 |
| Diffuse
type | 52 | 28 | 24 |
|
|
| Distant
metastasis |
|
|
|
|
|
| No | 31 | 4 | 27 | 23.0083 |
<0.001a |
|
Yes | 89 | 56 | 33 |
|
|
| Degree of
differentiation |
|
|
|
|
|
| Highly
differentiated | 34 | 14 | 20 | 2.0906 | 0.148 |
|
Moderately and poorly
differentiated | 86 | 48 | 38 |
|
|
| HER-2 |
|
|
|
|
|
|
0-+ | 78 | 40 | 38 | 0.1465 | 0.702 |
|
++-+++ | 42 | 20 | 22 |
|
|
| Ki-67 |
|
|
|
|
|
|
<15% | 67 | 32 | 35 | 0.5813 | 0.304 |
|
≥15% | 53 | 28 | 25 |
|
|
Blood samples
Peripheral venous blood (5–7 ml) was collected into
a sterile ethylenediaminetetraacetic acid (EDTA) tube. The blood
samples were obtained between 6:30 and 7:30 a.m. in order to
standardize the known impact of circulating hormones (circadian
rhythm) on the number and subtype distribution of the various white
blood cell indices. Hematological parameters were analyzed within
30 min after collection using a hematology analyser (Sysmex
XE-2100; Sysmex, Kobe, Japan). Neutrophil (103/µl), lymphocyte
(103/µl) and platelet (103/µl) counts were recorded. The results
were expressed in 103/µl. NLR and PLR were calculated as the ratio
of the neutrophils and platelets to lymphocytes, respectively. Mean
value was used for NLR and PLR as normal distribution was absent.
The patients were divided into two groups according to the mean
value of NLR or PLR [(NLR low, <4.62 or NLR high, ≥4.62; and PLR
low, <235 or PLR high, ≥235, respectively].
Chemotherapy and evaluation
Patients received first-line chemotherapy as
outlined in the National Comprehensive Cancer Network (NCCN)
clinical practice guideline for GC (2006, first edition).
5-FU/leucovorin (LV), 5-FU-based, cisplatin (CDDP)-based,
oxaliplatin (L-OHP)-based, taxane-based and irinotecan
(CPT-11)-based, ECF treatments were employed. Computed tomography
(CT) scan was performed for the assessment of response every 2
months and evaluated according to the criteria of Response
Evaluation Criteria in Solid Tumors (RECIST) 1.1 (22).
The responses to chemoradiotherapy including
complete remission, regression, stable disease and disease
progression, as well as overall and disease-free survival were
recorded. Survival time was measured from the date of
chemoradiotherapy until the patient succumbed or last clinical
evaluation. Following first-line chemotherapy, disease progression
after chemoradiotherapy (n=44) was defined as lack of response to
chemoradiotherapy. By contrast, stable disease, complete response
or disease regression following chemoradiotherapy was defined as
response to chemoradiotherapy (n=76).
Follow-up
The responses to chemotherapy including complete
remission, regression, stable disease and disease progression, as
well as overall and progression-free survival were recorded.
Survival time was defined as the time from the date of
chemoradiotherapy until the patient succumbed or last clinical
evaluation. Patients were followed up regularly for 40 months. The
prognostic analyses were performed to determine the
progression-free survival (PFS) and overall survival (OS).
Statistical analysis
Multivariate Cox regression analysis was performed
for each outcome parameter, using a backwards elimination technique
to derive a potentially suitable set of predictors. The association
between NLR or PLR levels and chemotherapeutic efficacy was
examined and assessed using the χ2 tests. For the analysis of
survival data, Kaplan-Meier curves were constructed, and
statistical analysis was carried out using the log-rank test. OS
was defined as the time from the initiation of chemotherapy to the
patient succumbing to any cause. P<0.05 was considered to
indicate statistically significant results. Statistical analyses
were performed using SPSS 19.0 software (SPSS, Inc., Chicago, IL,
USA).
Results
Relationship of baseline NLR and PLR
levels and clinicopathologic characteristics
A low baseline NLR level correlated with improved
clinicopathological characteristics, including smaller tumor size
(χ2=5.5456, P=0.018), high differentiation (χ2=5.9097, P=0.015) and
less metastasis (χ2=19.1809, P<0.001) (Table I). A low baseline PLR level was also
associated with less metastasis (χ2=23.0083, P<0.001) (Table II).
Baseline NLR and PLR levels predict
chemotherapeutic efficacy
The associations between the baseline NLR or PLR
level and chemotherapeutic efficacy are shown in Tables III and IV, respectively. Patients with a low
baseline level of NLR or PLR had improved response to chemotherapy
(χ2=5.167, P=0.023; χ2=7.033, P=0.008), suggesting the baseline
level of NLR or PLR predicts response to chemotherapy.
 | Table III.Relationship between NLR baseline
levels and chemotherapeutic efficacy. |
Table III.
Relationship between NLR baseline
levels and chemotherapeutic efficacy.
| NLR levels | PR + SD (n=76) | PD (n=44) | χ2 | P-value |
|---|
| Low (n=60) | 44 | 16 | 5.167 | 0.023 |
| High (n=60) | 32 | 28 |
|
|
 | Table IV.Relationship between PLR baseline
levels and chemotherapeutic efficacy. |
Table IV.
Relationship between PLR baseline
levels and chemotherapeutic efficacy.
| PLR levels | PR + SD (n=76) | PD (n=44) | χ2 | P-value |
|---|
| Low (n=60) | 45 | 15 | 7.033 | 0.008 |
| High (n=60) | 31 | 29 |
|
|
Baseline NLR and PLR levels predict
outcomes
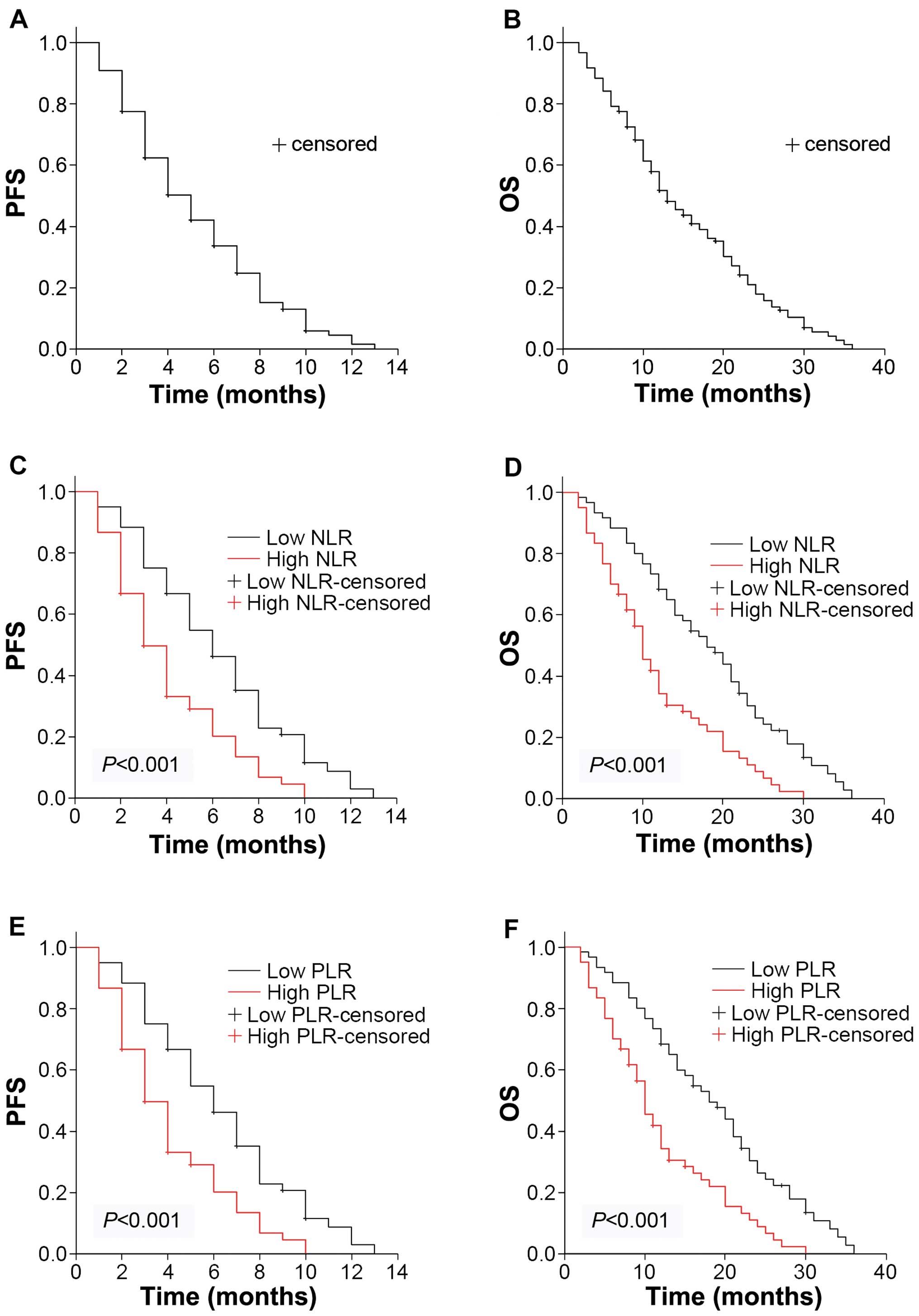
Median OS for all the patients was 13 months
(10.45–15.55 months) with a median progression-free survival (PFS)
of 5 months (4.11–5.89) (Fig. 1A and
B). Follow-up for survivors was 40 months. The Kaplan-Meier
plots were used to determine the effect of NLR and PLR status on OS
and PFS (Fig. 1C–F). The median OS
and PFS of the high NLR group were 10 months (8.23–11.77) and 3
months (2.24–3.76), respectively, while that of the low NLR group
were 18 months (13.53–22.47) and 6 months (4.79–7.21). Significant
differences were identified between the OS and PFS of the two
groups (P<0.001). Similarly, the median OS was 10 months
(8.23–11.77) in the high PLR group and 18 months (13.53–22.48) in
the low PLR group (P<0.001). The median PFS was 3 months
(2.24–3.76) in the high PLR group and 6 months (4.79–7.21) in the
low PLR group (P<0.001). Thus, the patients with higher baseline
NLR and PLR levels had decreased survival ratios.
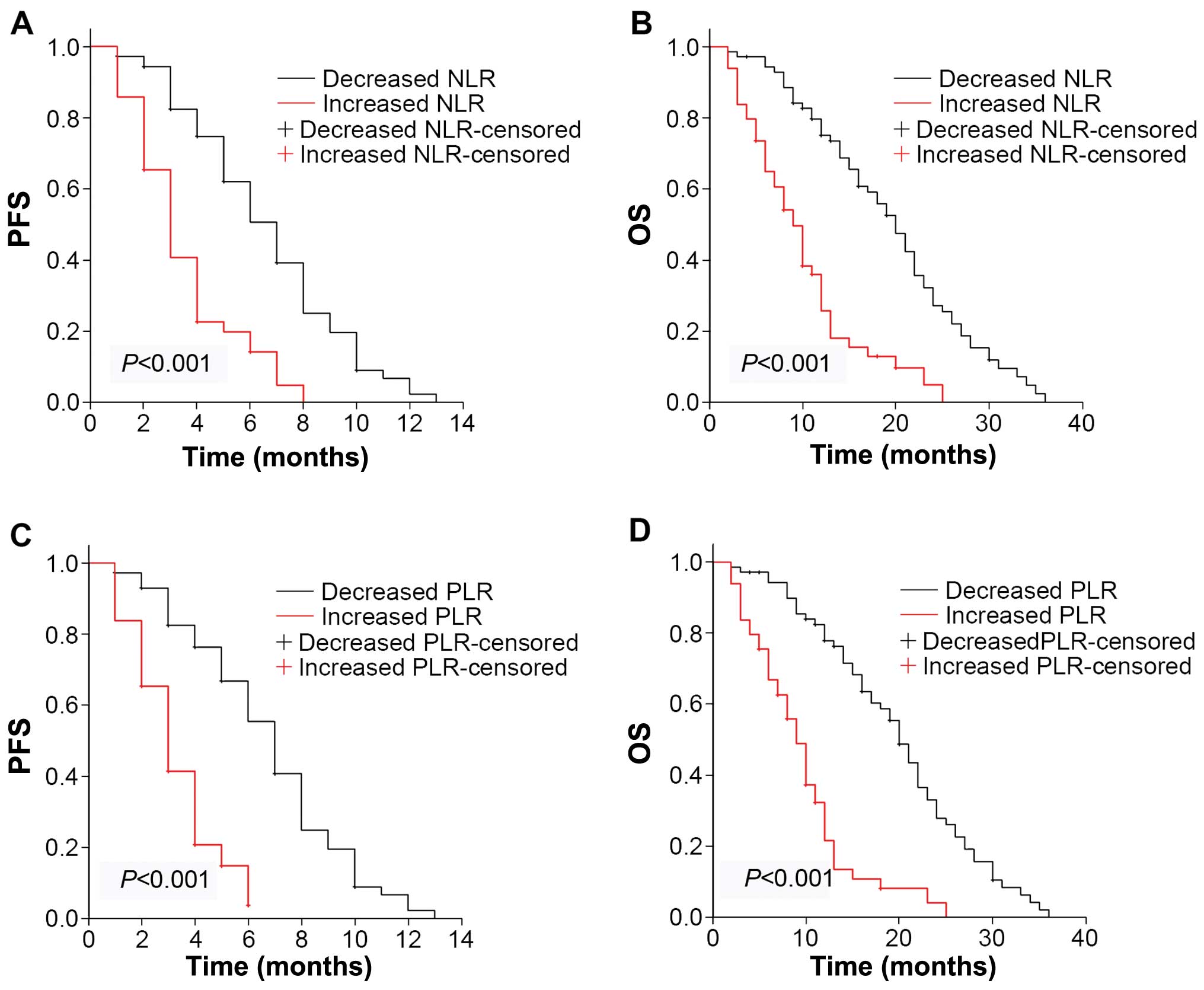
Changes in NLR and PLR levels are
associated with chemotherapeutic efficacy
To determine the association between changes in the
NLR or PLR level and chemotherapeutic efficacy, the blood samples
were obtained and CT evaluation was performed simultaneously after
the first-line chemotherapy. Forty-seven patients with a low
baseline NLR level remained in this group subsequent to first-line
chemotherapy (Table V). By contrast,
13 patients from this group were transferred to the high NLR level
group. Twenty-four patients with a high baseline NLR level retained
this level after first-line chemotherapy. By contrast, 36 patients
with a high baseline NLR level were transferred to the low NLR
level group. Patients who remained in or were transferred to the
low NLR level subgroup following first-line chemotherapy exhibited
improved response, compared to patients who remained in or were
transferred to the high NLR level group. Similar results were
observed when the PLR level was investigated (Table VI).
 | Table V.Relationship between changes in the
NLR level and chemotherapeutic efficacy. |
Table V.
Relationship between changes in the
NLR level and chemotherapeutic efficacy.
|
Pre-chemotherapy |
Post-chemotherapy | PR + SD (n=76) | PD (n=44) | χ2 | P-value |
|---|
| Low (n=60) | Low (n=47) | 39 | 8 |
4.831 | 0.0279 |
|
| High (n=13) | 7 | 6 |
|
|
| High (n=60) | Low (n=24) | 18 | 6 | 10.00 | 0.0016 |
|
| High (n=36) | 12 | 24 |
|
|
 | Table VI.Relationship between changes in the
PLR level and chemotherapeutic efficacy. |
Table VI.
Relationship between changes in the
PLR level and chemotherapeutic efficacy.
|
Pre-chemotherapy |
Post-chemotherapy | PR + SD (n=76) | PD (n=44) | χ2 | P-value |
|---|
| Low (n=60) | Low (n=48) | 42 | 6 | 15.745 | <0.001 |
|
| High (n=12) | 4 | 8 |
|
|
| High (n=60) | Low (n=23) | 17 | 6 |
8.531 | 0.0035 |
|
| High (n=37) | 13 | 24 |
|
|
Changes in NLR and PLR levels predict
outcomes
The Kaplan-Meier plots were used to determine the
effect of changes in the NLR and PLR status for OS and PFS
(Fig. 2). The median OS and PFS of
patients whose NLR levels increased following first-line
chemotherapy were 9 months (7.07–10.93) and 3 months (2.43–3.57),
respectively, while that of patients with decreased NLR were 20
months (17.25–22.75) and 7 months (5.94–8.05), respectively.
Significant differences were identified for OS and PFS for the two
groups (P<0.001). Similarly, the median OS was 9 months
(7.45–10.55) in the PLR-increased group and 20 months (17.93–22.07)
in the PLR-decreased group (P<0.001). Median PFS was 3 months
(2.41–3.59) in the PLR-increased group and 7 months (6.97–9.03) in
the PLR-decreased group (P<0.001). Thus, the patients with
increased NLR and PLR levels had decreased survival ratios.
Discussion
Inflammation, recognized as the ‘seventh hallmark of
cancer’, contributes to tumor proliferation, angiogenesis,
metastasis, and resistance to hormonal and chemotherapy (23). Clinical and epidemiological studies
have shown the connection between GC and chronic inflammation;
thus, the pathogenesis of GC is an inflammation-driven malignancy
(24–26).
Although the precise pathophysiological mechanisms
involved in the association between inflammation and tumor cells
remain unclear, there is growing interest in a clinical
interpretation of these interactions, resulting in the
establishment of novel biomarkers of cancer (27). Of these, biomarkers obtained from
routine blood tests have been recently developed (15–20,28).
Increased NLR and PLR levels have been shown to be correlated with
the increase in cancer-associated SIR, and indicate advanced stage
in several types of malignancy (29).
Elevated NLR has also been shown to predict poor outcomes in
colorectal cancer patients undergoing primary resection and in
patients undergoing hepatectomy for liver metastases (15,30). A
number of studies do not support the utilization of PLR level as an
independent prognostic factor of survival in contrast to NLR, which
is widely accepted in the majority of relevant studies. This
discrepancy may be due to the prethrombotic profile of the
underlying cancer type. For cancers associated with high thrombotic
risk, such as pancreatic cancer, PLR is a potential prognostic
factor. However, the use of PLR as a prognostic factor may not be
precise with regard to relatively hypocoagulative cancer types,
such as breast cancer (11). Previous
results have demonstrated that GC was hypercoagulative (31). Thus, based on previous and the present
results, baseline NLR and PLR levels may be applied as biomarkers
in GC. In the present study, we observed that changes of the NLR
and PLR levels were consistent with chemotherapeutic efficacy and
prognosis, suggesting changes in the NLR and PLR levels following
treatment may also provide valuable prognostic information.
Neutrophils are actively involved in systemic and
local inflammatory response via multiple mechanisms, such as
releasing pro-inflammatory factors. They can promote tumor growth
and metastasis by remodeling the extracellular matrix. They release
reactive oxygen species (ROS), nitric oxide (NO), arginase, and
suppress the T-cell response (32).
Increased neutrophil count and low serum albumin levels have been
shown to be the independent predictors of outcome following hepatic
resection for metastatic colorectal cancer (33). Preclinical studies have also indicated
that neutrophils may act as tumor-promoting leukocytes by the
transforming growth factor-β-induced signaling pathway (34).
Platelets play an important and multifaceted role in
cancer progression (35). During
hematogenous dissemination, the ability of circulating tumor cells
to interact with platelets is believed to promote tumor cell
survival within the circulation and increase the arrest of tumor
cell emboli within the microcirculation (36,37),
thereby facilitating metastasis. Interaction with platelet is
currently gaining acceptance as a key intermediate step in the
process of blood-borne metastasis (35). Pre-clinical animal models have
demonstrated that pharmacologically- or genetically-induced
thrombocytopenia and platelet function defects are associated with
reduced metastasis (38,39). These observations have led to the use
of antiplatelet and anticoagulation agents to prevent metastasis in
experimental models and human cancer patients (35).
Besides the effects of platelets on cancer
progression, counts and function of platelets may also be affected
in many types of cancer. The release of pro-inflammatory cytokines
by cancer cells, such as interleukin-1 (IL-1), IL-3 and IL-6,
clearly promote the proliferation and differentiation of early
progenitor cells, such as megakaryocyte progenitors, resulting in
the gradual establishment of thrombocytosis (40,41).
Elevated serum concentrations of IL-6 were significantly higher in
individuals with gastric, colon and prostate cancers (42,43).
Additionally, high levels of IL-6 and C-reactive protein play
important roles in the growth process and progression of GC
(44). The stimulation from
cancer-released cytokines led to an increased detection of more
primitive types of circulating platelets (45). Thus, the evaluation of the platelet
count and functional status is consistent with the progression of
malignancy (46,47).
On the other hand, lymphocytes, usually
CD3+ T cells and NK cells, possess potent anti-cancer
activities that potentially affect growth and/or metastasis in
various types of cancer (48). The
number of lymphocytes is an undisputed prognostic marker in
surgical oncology, reflecting the endogenous anticancer ability of
the immune system (49,50). Tumor-infiltrating lymphocytes in
particular have been extensively studied, and appear to have an
anti-tumorigenic role in colorectal cancer (51,52). A
high density of CD8+ T-cell infiltration independently
predicted superior survival (53).
In summary, the results of the present study explain
the reason for elevated NLR and PLR enhancing malignant
progression, and low levels of NLR and PLR conferring a more
favorable prognosis. The normalization of NLR and PLR values after
treatment may indicate resolution of intestinal inflammation. The
present findings suggest that the baseline NLR and PLR levels may
be used in the prediction of a chemotherapeutic response and
prognosis in unresectable GC. Furthermore, changes in the NLR and
PLR levels were consistent with the chemotherapeutic response and
may also be useful in predicting outcomes. Considering the high GC
morbidity and less developed economic condition in China, these
non-invasive, convenient and inexpensive biomarkers may be
beneficial with regard to the treatment of GC.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81472296, 81101867,
81272542, 81200369 and 81372443), the China International Medical
Foundation (grant no. CIMF-F-H001-057), the Special Foundation of
Clinical Medicine of Jiangsu Provincial Bureau of Science and
Technology (grant no. BL2014039), the Scientific Research Project
of Jiangsu Provincial Bureau of Traditional Chinese Medicine (grant
no. L213236), the Medical Scientific Research Project of Jiangsu
Provincial Bureau of Health (grant no. Z201206), the Special
Foundation of Wu Jieping Medical Foundation for Clinical Scientific
Research (grant nos. 320.6753.1225 and 320.6750.12242), the Science
and Education for Health Foundation of Suzhou for Youth (grant nos.
SWKQ1003 and SWKQ1011), the Science and Technology Project
Foundation of Suzhou (grant nos. SYS201112, SYSD2012137 and
SYS201335), the Science and Technology Foundation of Suzhou
Xiangcheng (grant nos. SZXC2012–70 and XJ201451) and a Project
Founded by the Priority Academic Program Development of Jiangsu
Higher Education Institutions
References
|
1
|
Jiang HB, Yang TJ, Lu P and Ma YJ: Gene
expression profiling of gastric cancer. Eur Rev Med Pharmacol Sci.
18:2109–2115. 2014.PubMed/NCBI
|
|
2
|
Li T, Chen J, Liu QL, Huo ZH and Wang ZW:
Meta-analysis: E-cadherin immunoexpression as a potential prognosis
biomarker related to gastric cancer metastasis in Asian patients.
Eur Rev Med Pharmacol Sci. 18:2693–2703. 2014.PubMed/NCBI
|
|
3
|
Leung WK, Wu MS, Kakugawa Y, Kim JJ, Yeoh
KG, Goh KL, Wu KC, Wu DC, Sollano J, Kachintorn U, et al: Asia
Pacific Working Group on Gastric Cancer: Screening for gastric
cancer in Asia: Current evidence and practice. Lancet Oncol.
9:279–287. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Popiela T, Kulig J, Kolodziejczyk P and
Sierzega M: Polish Gastric Cancer Study Group: Long-term results of
surgery for early gastric cancer. Br J Surg. 89:1035–1042. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Forman D, Morris E, Eastwood A and
Kleijnen J: Guidelines for treatment of upper gastrointestinal
cancer. Lancet. 361:802003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang WH, Huang JQ, Zheng GF, Lam SK,
Karlberg J and Wong BC: Non-steroidal anti-inflammatory drug use
and the risk of gastric cancer: A systematic review and
meta-analysis. J Natl Cancer Inst. 95:1784–1791. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
McMillan DC: Systemic inflammation,
nutritional status and survival in patients with cancer. Curr Opin
Clin Nutr Metab Care. 12:223–226. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Schreiber RD, Old LJ and Smyth MJ: Cancer
immunoediting: Integrating immunity's roles in cancer suppression
and promotion. Science. 331:1565–1570. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Carruthers R, Tho LM, Brown J, Kakumanu S,
McCartney E and McDonald AC: Systemic inflammatory response is a
predictor of outcome in patients undergoing preoperative
chemoradiation for locally advanced rectal cancer. Colorectal Dis.
14:e701–707. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kemal Y, Demirağ G, Ekiz K and Yücel I:
Mean platelet volume could be a useful biomarker for monitoring
epithelial ovarian cancer. J Obstet Gynaecol. 34:515–518. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Seretis C, Seretis F, Lagoudianakis E,
Politou M, Gemenetzis G and Salemis NS: Enhancing the accuracy of
platelet to lymphocyte ratio after adjustment for large platelet
count: a pilot study in breast cancer patients. Int J Surg Oncol.
2012:6536082012.PubMed/NCBI
|
|
12
|
Celikbilek M, Dogan S, Ozbakır O, Zararsız
G, Kücük H, Gürsoy S, Yurci A, Güven K and Yücesoy M:
Neutrophil-lymphocyte ratio as a predictor of disease severity in
ulcerative colitis. J Clin Lab Anal. 27:72–76. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Imtiaz F, Shafique K, Mirza SS, Ayoob Z,
Vart P and Rao S: Neutrophil lymphocyte ratio as a measure of
systemic inflammation in prevalent chronic diseases in Asian
population. Int Arch Med. 5:22012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tousoulis D, Antoniades C, Koumallos N and
Stefanadis C: Pro-inflammatory cytokines in acute coronary
syndromes: From bench to bedside. Cytokine Growth Factor Rev.
17:225–233. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Walsh SR, Cook EJ, Goulder F, Justin TA
and Keeling NJ: Neutrophil-lymphocyte ratio as a prognostic factor
in colorectal cancer. J Surg Oncol. 91:181–184. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gwak MS, Choi SJ, Kim JA, Ko JS, Kim TH,
Lee SM, Park JA and Kim MH: Effects of gender on white blood cell
populations and neutrophil-lymphocyte ratio following gastrectomy
in patients with stomach cancer. J Korean Med Sci. 22(Suppl):
S104–S108. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sharaiha RZ, Halazun KJ, Mirza F, Port JL,
Lee PC, Neugut AI, Altorki NK and Abrams JA: Elevated preoperative
neutrophil: lymphocyte ratio as a predictor of postoperative
disease recurrence in esophageal cancer. Ann Surg Oncol.
18:3362–3369. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Thavaramara T, Phaloprakarn C,
Tangjitgamol S and Manusirivithaya S: Role of neutrophil to
lymphocyte ratio as a prognostic indicator for epithelial ovarian
cancer. J Med Assoc Thai. 94:871–877. 2011.PubMed/NCBI
|
|
19
|
Kemal Y, Yucel I, Ekiz K, Demirag G,
Yilmaz B, Teker F and Ozdemir M: Elevated serum neutrophil to
lymphocyte and platelet to lymphocyte ratios could be useful in
lung cancer diagnosis. Asian Pac J Cancer Prev. 15:2651–2654. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Azab B, Bhatt VR, Phookan J, Murukutla S,
Kohn N, Terjanian T and Widmann WD: Usefulness of the
neutrophil-to-lymphocyte ratio in predicting short- and long-term
mortality in breast cancer patients. Ann Surg Oncol. 19:217–224.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Proctor MJ, Morrison DS, Talwar D, Balmer
SM, Fletcher CD, O'Reilly DS, Foulis AK, Horgan PG and McMillan DC:
A comparison of inflammation-based prognostic scores in patients
with cancer. A Glasgow Inflammation Outcome Study. Eur J Cancer.
47:2633–2641. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wu Y and Zhou BP: Inflammation: A driving
force speeds cancer metastasis. Cell Cycle. 8:3267–3273. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ilhan N, Ilhan N, Ilhan Y, Akbulut H and
Kucuksu M: C-reactive protein, procalcitonin, interleukin-6,
vascular endothelial growth factor and oxidative metabolites in
diagnosis of infection and staging in patients with gastric cancer.
World J Gastroenterol. 10:1115–1120. 2004.PubMed/NCBI
|
|
25
|
Lochhead P and El-Omar EM: Gastric cancer.
Br Med Bull. 85:87–100. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hussain SP and Harris CC: Inflammation and
cancer: an ancient link with novel potentials. Int J Cancer.
121:2373–2380. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Aliustaoglu M, Bilici A, Ustaalioglu BB,
Konya V, Gucun M, Seker M and Gumus M: The effect of peripheral
blood values on prognosis of patients with locally advanced gastric
cancer before treatment. Med Oncol. 27:1060–1065. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Smith RA, Bosonnet L, Raraty M, Sutton R,
Neoptolemos JP, Campbell F and Ghaneh P: Preoperative
platelet-lymphocyte ratio is an independent significant prognostic
marker in resected pancreatic ductal adenocarcinoma. Am J Surg.
197:466–472. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Raungkaewmanee S, Tangjitgamol S,
Manusirivithaya S, Srijaipracharoen S and Thavaramara T: Platelet
to lymphocyte ratio as a prognostic factor for epithelial ovarian
cancer. J Gynecol Oncol. 23:265–273. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Malik HZ, Prasad KR, Halazun KJ, Aldoori
A, Al-Mukhtar A, Gomez D, Lodge JP and Toogood GJ: Preoperative
prognostic score for predicting survival after hepatic resection
for colorectal liver metastases. Ann Surg. 246:806–814. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Heras P, Hatzopoulos A, Kritikos N and
Kritikos K: Platelet count and tumor progression in gastric cancer
patients. Scand J Gastroenterol. 45:1005–1006. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
De Larco JE, Wuertz BR and Furcht LT: The
potential role of neutrophils in promoting the metastatic phenotype
of tumors releasing interleukin-8. Clin Cancer Res. 10:4895–4900.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Neal CP, Mann CD, Sutton CD, Garcea G, Ong
SL, Steward WP, Dennison AR and Berry DP: Evaluation of the
prognostic value of systemic inflammation and socioeconomic
deprivation in patients with resectable colorectal liver
metastases. Eur J Cancer. 45:56–64. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Suzuki K, Kachala SS, Kadota K, Shen R, Mo
Q, Beer DG, Rusch VW, Travis WD and Adusumilli PS: Prognostic
immune markers in non-small cell lung cancer. Clin Cancer Res.
17:5247–5256. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lian L, Li W, Li ZY, Mao YX, Zhang YT,
Zhao YM, Chen K, Duan WM and Tao M: Inhibition of MCF-7 breast
cancer cell-induced platelet aggregation using a combination of
antiplatelet drugs. Oncol Lett. 5:675–680. 2013.PubMed/NCBI
|
|
36
|
Egan K, Crowley D, Smyth P, O'Toole S,
Spillane C, Martin C, Gallagher M, Canney A, Norris L, Conlon N, et
al: Platelet adhesion and degranulation induce pro-survival and
pro-angiogenic signalling in ovarian cancer cells. PLoS One.
6:e261252011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Tsuruo T and Fujita N: Platelet
aggregation in the formation of tumor metastasis. Proc Jpn Acad,
Ser B, Phys Biol Sci. 84:189–198. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Camerer E, Qazi AA, Duong DN, Cornelissen
I, Advincula R and Coughlin SR: Platelets, protease-activated
receptors, and fibrinogen in hematogenous metastasis. Blood.
104:397–401. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Jain S, Zuka M, Liu J, Russell S, Dent J,
Guerrero JA, Forsyth J, Maruszak B, Gartner TK, Felding-Habermann
B, et al: Platelet glycoprotein Ib alpha supports experimental lung
metastasis. Proc Natl Acad Sci USA. 104:9024–9028. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Klinger MH and Jelkmann W: Role of blood
platelets in infection and inflammation. J Interferon Cytokine Res.
22:913–922. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Alexandrakis MG, Passam FH, Moschandrea
IA, Christophoridou AV, Pappa CA, Coulocheri SA and Kyriakou DS:
Levels of serum cytokines and acute phase proteins in patients with
essential and cancer-related thrombocytosis. Am J Clin Oncol.
26:135–140. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Galizia GI, Lieto E, De Vita F, Romano C,
Orditura M, Castellano P, Imperatore V, Infusino S, Catalano G and
Pignatelli C: Circulating levels of interleukin-10 and
interleukin-6 in gastric and colon cancer patients before and after
surgery: relationship with radicality and outcome. J Interferon
Cytokine Res. 22:473–482. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Shariat SF, Andrews B, Kattan MW, Kim J,
Wheeler TM and Slawin KM: Plasma levels of interleukin-6 and its
soluble receptor are associated with prostate cancer progression
and metastasis. Urology. 58:1008–1015. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kim DK, Oh SY, Kwon HC, Lee S, Kwon KA,
Kim BG, Kim SG, Kim SH, Jang JS, Kim MC, et al: Clinical
significances of preoperative serum interleukin-6 and C-reactive
protein level in operable gastric cancer. BMC Cancer. 9:1552009.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Khandekar MM, Khurana AS, Deshmukh SD,
Kakrani AL, Katdare AD and Inamdar AK: Platelet volume indices in
patients with coronary artery disease and acute myocardial
infarction: An Indian scenario. J Clin Pathol. 59:146–149. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Hurst NJ Jr, Najy AJ, Ustach CV, Movilla L
and Kim HR: Platelet-derived growth factor-C (PDGF-C) activation by
serine proteases: Implications for breast cancer progression.
Biochem J. 441:909–918. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Bambace NM and Holmes CE: The platelet
contribution to cancer progression. J Thromb Haemost. 9:237–249.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Ohashi R, Takahashi K, Miura K, Ishiwata
T, Sakuraba S and Fukuchi Y: Prognostic factors in patients with
inoperable non-small cell lung cancer - an analysis of long-term
survival patients. Gan To Kagaku Ryoho. 33:1595–1602.
2006.PubMed/NCBI
|
|
49
|
Milne K, Alexander C, Webb JR, Sun W,
Dillon K, Kalloger SE, Gilks CB, Clarke B, Köbel M and Nelson BH:
Absolute lymphocyte count is associated with survival in ovarian
cancer independent of tumor-infiltrating lymphocytes. J Transl Med.
10:332012. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Kobayashi N, Usui S, Kikuchi S, Goto Y,
Sakai M, Onizuka M and Sato Y: Preoperative lymphocyte count is an
independent prognostic factor in node-negative non-small cell lung
cancer. Lung Cancer. 75:223–227. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Ohtani H: Focus on TILs: Prognostic
significance of tumor infiltrating lymphocytes in human colorectal
cancer. Cancer Immun. 7:42007.PubMed/NCBI
|
|
52
|
Galon J, Costes A, Sanchez-Cabo F,
Kirilovsky A, Mlecnik B, Lagorce-Pagès C, Tosolini M, Camus M,
Berger A, Wind P, et al: Type, density, and location of immune
cells within human colorectal tumors predict clinical outcome.
Science. 313:1960–1964. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Mlecnik B, Tosolini M, Kirilovsky A,
Berger A, Bindea G, Meatchi T, Bruneval P, Trajanoski Z, Fridman
WH, Pagès F, et al: Histopathologic-based prognostic factors of
colorectal cancers are associated with the state of the local
immune reaction. J Clin Oncol. 29:610–618. 2011. View Article : Google Scholar : PubMed/NCBI
|