Introduction
Gastric cancer is the fourth most common malignant
disease worldwide and the second most common cause of mortality
from cancer (1). Countries in East
Asia have a higher incidence of gastric cancer (i.e., >40 cases
per 100,000). Data for individual countries have shown that gastric
cancer is the most common cancer in Japan and the second most
common in China and Korea (2).
Although early diagnosis and treatment of gastric cancer can
significantly improve prognosis, the 5-year survival rate is only
10–15% in individuals with advanced disease (3). This poor outcome may be due to the high
incidence of serosal invasion, direct invasion into the adjacent
organs and early metastasis (4,5).
Biomarkers, such as carbohydrate antigen 19-9 and carcinoembryonic
antigen are unsatisfactory due to their low sensitivity (5,6).
Therefore, identification of novel biomarkers for the diagnosis and
follow up of gastric cancer is essential.
Platelets (PLTs) play an important and multifaceted
role in cancer progression (7).
Firstly, PLTs facilitate metastasis (8). During hematogenous dissemination, the
interaction between circulating tumor cells and PLTs is believed to
promote tumor cell survival within the circulation (9) and increase the arrest of tumor cell
emboli within the microcirculation (10). Secondly, various studies demonstrated
that the release of pro-inflammatory cytokines by cancer, such as
interleukin (IL)-1, IL-3, and IL-6, promotes the proliferation of
megakaryocytes, leading to the gradual establishment of
thrombocytosis (11). Considering the
close relationship between PLTs and cancer, biomarkers derived from
PLTs are important. Elevated mean platelet volume (MPV) detected in
peripheral blood has been identified in various types of cancer,
including hepatocellular carcinoma (12), ovarian cancer (13), colon cancer (14), lung cancer and breast cancer (15), suggesting that PLT-associated markers
serve as potential candidates for the diagnosis and follow up of
gastric cancer.
In the present study, we investigated whether MPV
provided beneficial diagnostic and prognostic information for
patients with unresectable gastric cancer.
Materials and methods
Subjects and inclusion criteria
The study was conducted as a retrospective
investigation of gastric cancer patients who had been referred to
the First Affiliated Hospital of Soochow University (Suzhou, China)
between June 2010 and June 2011. Approval for the study was granted
by the Medical Ethics Committees of the First Affiliated Hospital
of Soochow University.
In total, 128 inoperable gastric cancer patients
were recruited in this study. Of the 128 patients, 53 patients were
locally advanced and the remaining 75 patients were relapsed or
metastastic. Patient characteristics are detailed in Table I. The mean age of the 128 patients was
68 years (range, 32–82 years). The inclusion criteria were as
follows: a) those with histologically or cytologically confirmed
recurrent or metastatic gastric cancer; b) age >18 years; c)
Karnofsky performance status score of ≥70; d) those with a
predicted survival of ≥3 months; e) either naive to antitumor
treatment or the postoperative adjuvant chemotherapy was performed
≥6 months after the last dose of chemotherapy; f) in case of
patients who were scheduled for radiotherapy on the target lesion,
radiotherapy was required to have been finished for ≥3 months; g)
those with ≥1 measurable lesion [minimum 10×10 mm on computed
tomography (CT) or magnetic resonance imaging]; and h) those who
met the following laboratory criteria: white blood cells (WBC)
≥4.0×109/l; absolute neutrophil count
≥1.5×109/l; PLT ≥100×109/l; serum bilirubin ≤
upper limit of normal (ULN); alanine aminotransferase, aspartate
aminotransferase and alkaline phosphatase ≤ ULN×2.5 (if without
liver metastasis) or ≤ ULN×5 (if with liver metastasis); urea
nitrogen ≤ ULN×1.25; and creatinine ≤ ULN×1.25.
 | Table I.Relationship between MPV and
clinicopathological characteristics. |
Table I.
Relationship between MPV and
clinicopathological characteristics.
|
|
| MPV |
|
|
|---|
|
|
|
|
|
|
|---|
| Clinicopathological
characteristics | No. | High (no.) | Low (no.) | χ2
test | P-value |
|---|
| Gender |
|
|
| 0.298 | 0.585 |
| Male | 79 | 41 | 38 |
|
|
|
Female | 49 | 23 | 26 |
|
|
| Age, years |
|
|
|
|
|
|
<65 | 72 | 38 | 34 | 0.508 | 0.476 |
| ≥65 | 56 | 26 | 30 |
|
|
| Tumor size, cm |
|
|
| 1.276 | 0.259 |
|
<5 | 86 | 40 | 46 |
|
|
| ≥5 | 42 | 24 | 18 |
|
|
| Lauren type |
|
|
| 0.508 | 0.476 |
|
Intestinal | 72 | 34 | 38 |
|
|
|
Diffuse | 56 | 30 | 26 |
|
|
| Distant
metastasis |
|
|
| 20.802 |
<0.0001a |
| No | 35 | 6 | 29 |
|
|
|
Yes | 93 | 58 | 35 |
|
|
| Degree of
differentiation |
|
|
| 1.3474 | 0.2457 |
| Highly
differentiated | 38 | 16 | 22 |
|
|
|
Moderately and poorly
differentiated | 90 | 48 | 42 |
|
|
| HER-2 |
|
|
| 0.1357 | 0.7126 |
| ++ −
+++ | 46 | 22 | 24 |
|
|
| 0 −
+ | 82 | 42 | 40 |
|
|
| Ki-67 |
|
|
| 0.011 | 0.918 |
|
≥15% | 57 | 29 | 28 |
|
|
|
<15% | 71 | 35 | 36 |
|
|
Blood samples
Blood (5–7 ml) was collected in a sterile
ethylenediamime-N,N,N´,N´-tetraacetic acid tube. The blood samples
were obtained between 06:30 a.m. and 07:30 a.m. to standardize the
known impact of circulating hormones (circadian rhythm) on the
number and subtype distribution of the various WBC indices.
Hematological parameters were analyzed within 30 min after
collection using a hematology analyzer (XE2100; Sysmex Corp., Kobe,
Japan) and MPV levels were recorded.
Chemotherapy and evaluation
Patients were administered first-line chemotherapy
according to the clinical practice guideline for gastric cancer
(2006, the first edition) of National Comprehensive Cancer Network.
5-Fluorouracil (5-FU)/leucovorin, 5-FU-based, cisplatin-based,
oxaliplatin-based, taxane-based, and irinotecan-based, epirubicin,
cisplatin and fluorouracil were recommended. CT scanning was
performed for the assessment of response every 2 months and
evaluated according to the Response Evaluation Criteria in Solid
Tumors 1.1 criteria (16).
Follow up
The responses to chemoradiotherapy including
complete remission, regression, stable disease, and disease
progression, and overall and disease-free survival (DFS) were
recorded. Survival time was measured from the date of
chemoradiotherapy until death or last clinical evaluation.
Following first-line chemotherapy, disease progression after
chemoradiotherapy was defined as lack of response to
chemoradiotherapy. By contrast, stable disease, complete response
or disease regression after chemoradiotherapy was defined as
response to chemoradiotherapy. Patients were regularly followed up
for 36 months. The prognostic analyses were performed based on
progression-free survival (PFS) and overall survival (OS).
Statistical analysis
Statistical analyses were performed using SPSS 19.0
software (SPSS, Inc., Chicago, IL, USA). Multivariate Cox
regression was performed for each outcome parameter, using a
backwards elimination technique to derive a potentially suitable
set of predictors. The association between the MPV level and
clinicopathological characteristics or chemotherapeutic efficacy
was examined and assessed by the χ2 tests. For analysis
of survival data, Kaplan-Meier curves were constructed, and
statistical analysis was carried out using the log-rank test. OS
was defined as the time from the initiation of chemotherapy to the
patient succumbing due to any cause. P<0.05 was considered to
indicate a statistically significant difference.
Results
Relationship between the baseline MPV
level and clinicopathological characteristics
Patients were divided according to the median value
of baseline MPV (MPV low: <11.65 or MPV high: ≥11.65). The
relationships between the baseline MPV level and
clinicopathological characteristics were examined and assessed by
the χ2 tests. The results showed that a low baseline MPV
level was only correlated with reduced metastasis, but not with
other clinicopathological characteristics (Table I).
Baseline MPV level predicts the
chemotherapeutic efficacy
The association between the baseline MPV level and
chemotherapeutic efficacy is provided in Table II. Patients with low baseline level
of MPV had an improved response to chemotherapy, suggesting that
the baseline MPV level did not predict chemotherapeutic
efficacy.
 | Table II.Association between the MPV baseline
levels and chemotherapeutic efficacy. |
Table II.
Association between the MPV baseline
levels and chemotherapeutic efficacy.
| MPV levels | PR+SD (n=81) | PD (n=47) | χ2
test | P-value |
|---|
| Low, n=60 | 47 | 17 | 5.6822 | 0.0171a |
| High, n=60 | 34 | 30 |
|
|
Changes in MPV levels are associated
with the chemotherapeutic efficacy
To define the association between changes in the MPV
level with chemotherapeutic efficacy, blood samples were collected
at the same time the CT evaluation was performed after first-line
chemotherapy. The results showed that 51 patients with a low
baseline MPV level, remained in this group after first-line
chemotherapy (Table III). By
contrast, 13 patients from this group were transferred into the
high MPV level group. A total of 39 patients with a high baseline
MPV level retained a high MPV level following first-line
chemotherapy. By contrast, 25 patients with a high baseline MPV
level were transferred into the low MPV level group. Patients
remaining in or transferring into the low MPV level subgroup after
first-line chemotherapy had an improved chemotherapy response,
compared to those remaining in or transferring into the high level
group.
 | Table III.Association between changes in the
MPV level and chemotherapeutic efficacy. |
Table III.
Association between changes in the
MPV level and chemotherapeutic efficacy.
|
Pre-chemotherapy |
Post-chemotherapy | PR+SD (n=81) | PD (n=47) | χ2
test | P-value |
|---|
| Low (n=64) | Low (n=51) | 44 | 7 | 13.1977 | 0.0003a |
|
| High (n=13) | 5 | 8 |
|
|
| High (n=64) | Low (n=25) | 18 | 7 | 7.9426 | 0.0048a |
|
| High (n=39) | 14 | 25 |
|
|
MPV levels predict the outcomes
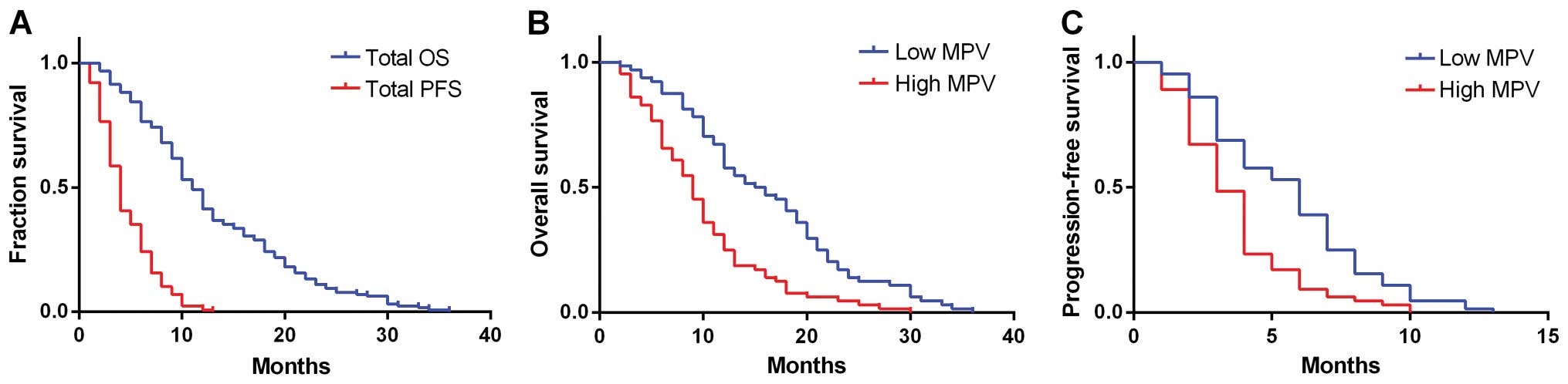
The median OS for all the patients was 11 months
with a median PFS of 4 months (Fig.
1A). Follow up for survivors was 36 months. Kaplan-Meier plots
showing the influence of MPV status on OS and PFS are shown in
Fig. 1B and C. The median OS and PFS
of the high MPV level group were 9 and 3 months, respectively,
whereas that for the low MPV level group were 15.5 and 6 months,
respectively. Significant differences were identified between the
OS and PFS of the two groups (P<0.001). Thus, the patients with
a higher MPV level had decreased survival.
Univariate and multivariate analyses
of risk factors for OS and DFS
Univariate and multivariate analyses were performed
to identify the risk factors associated with OS and PFS. As shown
in Table IV, the univariate analysis
revealed that 3 of the 10 risk factors affected OS and PFS,
including distant metastasis, chemotherapeutic efficacy and MPV.
The multivariate analysis confirmed that distant metastasis was the
prognostic factor affecting OS. By contrast, the chemotherapeutic
efficacy and MPV were prognostic factors affecting PFS.
 | Table IV.Univariate and multivariate analyses
of risk factors for the overall and disease-free survival. |
Table IV.
Univariate and multivariate analyses
of risk factors for the overall and disease-free survival.
|
| Overall
survival | Progression- free
survival |
|---|
|
|
|
|
|---|
|
| Univariate
analysis | Multivariate
analysis | Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|
|
|---|
| Risk factor | OR (95% CI) | P-value | OR (95% CI) | P-value | OR (95% CI) | P-value | OR (95% CI) | P-value |
|---|
| Gender |
|
|
|
|
|
|
|
|
| Male or
female | 1.21
(0.65–1.92) | 0.793 | – | – | 1.18
(0.69–1.92) | 0.887 | – | – |
| Age |
|
|
|
|
|
|
|
|
| <65
or ≥65 years | 1.19
(0.58–2.60) | 0.798 | – | – | 1.17
(0.68–1.94) | 0.848 | – | – |
| Tumor size |
|
|
|
|
|
|
|
|
| <5
or ≥5 cm | 1.22
(0.69–2.34) | 0.305 | – | – | 1.34
(0.89–2.58) | 0.276 | – | – |
| Lauren type |
|
|
|
|
|
|
|
|
|
Intestinal or diffuse
type | 1.37
(0.83–2.16) | 0.697 | – | – | 1.35
(0.52–2.59) | 0.784 | – | – |
| Distant
metastasis |
|
|
|
|
|
|
|
|
| No or
yes | 1.92
(1.29–3.36) | 0.025a | 1.93
(1.28–3.41) | 0.022a | 1.72
(1.41–2.96) | 0.028a | – | – |
| Degree of
differentiation |
|
|
|
|
|
|
|
|
| Highly
or moderately + poorly | 1.21
(0.77–1.91) | 0.856 | – | – | 1.19
(0.68–1.87) | 0.832 | – | – |
| HER-2 |
|
|
|
|
|
|
|
|
| ++ −
+++ or 0 − + | 1.25
(0.78–2.10) | 0.694 | – | – | 1.28
(0.78–2.35) | 0.722 | – |
|
| Ki-67 |
|
|
|
|
|
|
|
|
| (≥15 or
<15%) | 1.12
(0.71–1.62) | 0.847 | – | – | 1.21
(0.55–1.58) | 0.395 | – | – |
| Chemotherapeutic
efficacy |
|
|
|
|
|
|
|
|
| PR+SD
or PD | 1.98
(1.31–3.30) | 0.021a | – | – | 2.03
(1.62–3.05) | 0.041a | 2.16
(1.69–3.37) | 0.039a |
| MPV |
|
|
|
|
|
|
|
|
| Low or
high | 2.68
(1.70–3.48) | 0.001b | – | – | 2.64
(1.52–3.34) | 0.001b | 2.52
(1.39–3.50) | 0.001b |
Discussion
PLT activation is of paramount importance in the
progression of malignancy. Previous findings have shown that the
risk of cancer diagnosis is elevated after primary deep vein
thrombosis or pulmonary embolism (17). Additionally, experimental and clinical
data suggest that the activation of PLTs is a hallmark in the
natural course of cancer, by promoting neoangiogenesis, degradation
of the extracellular matrix, release of adhesion molecules, and
growth factors, all of which are essential components for further
tumor growth and metastatic spread (11).
Besides the impact that PLT activation has on
cancer, an elevated PLT count in combination with other abnormal
test results seems to be predictive for an underlying malignant
disease (18), suggesting the
potential of using PLT-associated factors as biomarkers for cancer
diagnosis and treatment.
Evidence has shown that the larger PLTs are more
reactive than the smaller ones and are more likely to aggregate,
leading to thrombosis. Large PLTs (LPLTs) are independent risk
factors for myocardial infarction, and PLT size is one predictor of
recurrent myocardial infarction and death (19). A high level of MPV, a marker of PLT
size, may indicate tendency towards thrombosis, and has been
demonstrated in the case of myocardial infarction and
cerebrovascular embolus (20). In
cancer, an increase in the percentage of large PLTs has been
observed, and because young, metabolically active PLTs appear in
the circulation, this may lead to an increase in MPV (21). Recent findings have suggested that the
MPV is a valuable biomarker for the diagnosis and follow up of
various types of cancer (12–15).
The association between PLT and cancer may be linked
by systemic inflammatory response (SIR) (22), which seems to play a critical role in
the development and progression of various types of cancer by
promoting cancer cell proliferation and survival, angiogenesis,
tumor metastasis and impacting tumor response to systemic therapies
(7). The mechanism involved in the
effect on PLT by inflammation may be due to the release of
pro-inflammatory cytokines, such as IL-1, IL-3 and IL-6, in many
types of cancer. These cytokines have been proven to be able to
promote the proliferation of megakaryocytes, resulting in PLT
activation and aggregation, which potentially lead to the gradual
establishment of thrombocytosis (11). This exact stimulation inevitably leads
to an increased detection of more primitive types of circulating
PLTs (11). This accelerated
coagulation of PLTs may promote the metastasis of cancer cells.
When covered with PLTs, cancer cells can overcome the stress in the
bloodstream, including attacks by the immune system and physical
factors (i.e., shear force and mechanical trauma due to passage
through the microvasculature) (8,23). Thus,
the alliance of PLTs and cancer united by inflammation presents a
positive feedback in the progression of malignancy.
Gastric cancer is usually located in the pyloric
antrum and in the pylorus, but in 25% of cases in the body (corpus)
and fundus of the stomach. Chronic inflammation of the stomach
caused by Helicobacter pylori often leads to neoplastic
transformation (21). Clinical and
epidemiological studies have shown that gastric cancer is an
inflammation-driven malignancy (24–26).
Elevated serum concentrations of pro-inflammatory cytokines such as
IL-6 were observed to be significantly higher in individuals with
gastric cancer, as well as in patients with other
inflammation-associated cancer types, such as colon and prostate
cancers (27,28). Therefore, we speculated that the
elevated MPV level in gastric cancer patients may be due to a
consequence of SIR. In patients with better chemotherapeutic
efficacy, decreased MPV level may be due to remission of SIR,
leading to a more favorable prognosis. By contrast, non-decreasing
MPV may reflect persistent SIR and worse outcomes.
The results of the present study indicate that MPV
may be used in the prediction of chemotherapy response and the
follow up of gastric cancer. Considering the high gastric cancer
morbidity and less developed economic conditions in China, this
non-invasive, convenient and cost-effective biomarker may be
beneficial in the treatment of gastric cancer.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81472296, 81101867,
81272542, 81200369 and 81372443), the China International Medical
Foundation (grant no. CIMF F H001 057), the Special Foundation of
Clinical Medicine of Jiangsu Provincial Bureau of Science and
Technology (grant no. BL2014039), the Scientific Research Project
of Jiangsu Provincial Bureau of Traditional Chinese Medicine (grant
no. L213236), the Medical Scientific Research Project of Jiangsu
Provincial Bureau of Health (grant no. Z201206), the Special
Foundation of Wu Jieping Medical Foundation for Clinical Scientific
Research (grant nos. 320.6753.1225 and 320.6750.12242), the Science
and Education for Health Foundation of Suzhou for Youth (grant nos.
SWKQ1003 and SWKQ1011), The Science and Technology Project
Foundation of Suzhou (grant nos. SYS201112, SYSD2012137, SYS201335
and SYS201504), The Science and Technology Foundation of Suzhou
Xiangcheng (grant nos. SZXC2012–70 and XJ201451) and a Project
Founded by the Priority Academic Program Development of Jiangsu
Higher Education Institutions.
References
|
1
|
Shen L, Shan YS, Hu HM, Price TJ, Sirohi
B, Yeh KH, Yang YH, Sano T, Yang HK, Zhang X, et al: Management of
gastric cancer in Asia: Resource-stratified guidelines. Lancet
Oncol. 14:e535–e547. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Leung WK, Wu MS, Kakugawa Y, et al: Asia
Pacific Working Group on Gastric Cancer: Screening for gastric
cancer in Asia: current evidence and practice. Lancet Oncol.
9:279–287. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang WH, Huang JQ, Zheng GF, Lam SK,
Karlberg J and Wong BC: Non-steroidal anti-inflammatory drug use
and the risk of gastric cancer: a systematic review and
meta-analysis. J Natl Cancer Inst. 95:1784–1791. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kılınçalp S, Ekiz F, Başar O, Ayte MR,
Coban S, Yılmaz B, Altınbaş A, Başar N, Aktaş B, Tuna Y, et al:
Mean platelet volume could be possible biomarker in early diagnosis
and monitoring of gastric cancer. Platelets. 25:592–594. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mroczko B, Wereszczyñska-Siemiatkowska U,
Groblewska M, Lukaszewicz M, Szmitkowski M, Gryko M and Kedra B:
The diagnostic value of hematopoietic cytokines measurement in the
sera of gastric cancer and gastric ulcer patients. Clin Chim Acta.
374:165–167. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mroczko B, Groblewska M, Łukaszewicz-Zajac
M, Bandurski R, Kedra B and Szmitkowski M: Pre-treatment serum and
plasma levels of matrix metalloproteinase 9 (MMP-9) and tissue
inhibitor of matrix metalloproteinases 1 (TIMP-1) in gastric cancer
patients. Clin Chem Lab Med. 47:1133–1139. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kemal Y, Yucel I, Ekiz K, Demirag G,
Yilmaz B, Teker F and Ozdemir M: Elevated serum neutrophil to
lymphocyte and platelet to lymphocyte ratios could be useful in
lung cancer diagnosis. Asian Pac J Cancer Prev. 15:2651–2654. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lian L, Li W, Li ZY, Mao YX, Zhang YT,
Zhao YM, Chen K, Duan WM and Tao M: Inhibition of MCF-7 breast
cancer cell-induced platelet aggregation using a combination of
antiplatelet drugs. Oncol Lett. 5:675–680. 2013.PubMed/NCBI
|
|
9
|
Egan K, Crowley D, Smyth P, O'Toole S,
Spillane C, Martin C, Gallagher M, Canney A, Norris L, Conlon N, et
al: Platelet adhesion and degranulation induce pro-survival and
pro-angiogenic signalling in ovarian cancer cells. PLoS One.
6:e261252011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tsuruo T and Fujita N: Platelet
aggregation in the formation of tumor metastasis. Proc Jpn Acad Ser
B Phys Biol Sci. 84:189–198. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Seretis C, Seretis F, Lagoudianakis E,
Politou M, Gemenetzis G and Salemis NS: Enhancing the accuracy of
platelet to lymphocyte ratio after adjustment for large platelet
count: a pilot study in breast cancer patients. Int J Surg Oncol.
2012:6536082012.PubMed/NCBI
|
|
12
|
Cho SY, Yang JJ, You E, Kim BH, Shim J,
Lee HJ, Lee WI, Suh JT and Park TS: Mean platelet volume/platelet
count ratio in hepatocellular carcinoma. Platelets. 24:375–377.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kemal Y, Demirag G, Ekiz K and Yucel I:
Mean platelet volume could be a useful biomarker for monitoring
epithelial ovarian cancer. J Obstet Gynaecol. 34:515–518. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mutlu H, Berk V, Karaca H, Erden A, Aslan
T and Akca Z: Treatment regimen with bevacizumab decreases mean
platelet volume in patients with metastatic colon cancer. Clin Appl
Thromb Hemost. 18:546–548. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Aksoy S, Kilickap S, Hayran M,
Harputluoglu H, Koca E, Dede DS, Erman M and Turker A: Platelet
size has diagnostic predictive value for bone marrow metastasis in
patients with solid tumors. Int J Lab Hematol. 30:214–219. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bazou D, Santos-Martinez MJ, Medina C and
Radomski MW: Elucidation of flow-mediated tumour cell-induced
platelet aggregation using an ultrasound standing wave trap. Br J
Pharmacol. 162:1577–1589. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Heras P, Hatzopoulos A, Kritikos N and
Kritikos K: Platelet count and tumor progression in gastric cancer
patients. Scand J Gastroenterol. 45:1005–1006. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Karagöz B, Bilgi O, Alacacioğlu A, Ozgün
A, Sayan O, Erikçi AA and Kandemir EG: Mean platelet volume
increase after tamoxifen, but not after anastrazole in adjuvant
therapy of breast cancer. Med Oncol. 27:199–202. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mutlu H, Artis TA, Erden A and Akca Z:
Alteration in mean platelet volume and platicrit values in patients
with cancer that developed thrombosis. Clin Appl Thromb Hemost.
19:331–333. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Matowicka-Karna J, Kamocki Z, Polińska B,
Osada J and Kemona H: Platelets and inflammatory markers in
patients with gastric cancer. Clin Dev Immunol. 2013:4016232013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
McMillan DC: Systemic inflammation,
nutritional status and survival in patients with cancer. Curr Opin
Clin Nutr Metab Care. 12:223–226. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shou LM, Zhang QY, Li W, et al:
Cantharidin and norcantharidin inhibit the ability of MCF-7 cells
to adhere to platelets via protein kinase C pathway-dependent
downregulation of α2 integrin. Oncol Rep. 30:1059–1066.
2013.PubMed/NCBI
|
|
24
|
Ilhan N, Ilhan N, Ilhan Y, Akbulut H and
Kucuksu M: C-reactive protein, procalcitonin, interleukin-6,
vascular endothelial growth factor and oxidative metabolites in
diagnosis of infection and staging in patients with gastric cancer.
World J Gastroenterol. 10:1115–1120. 2004.PubMed/NCBI
|
|
25
|
Lochhead P and El-Omar EM: Gastric cancer.
Br Med Bull. 85:87–100. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hussain SP and Harris CC: Inflammation and
cancer: an ancient link with novel potentials. Int J Cancer.
121:2373–2380. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Galizia G, Lieto E, De Vita F, et al:
Circulating levels of interleukin-10 and interleukin-6 in gastric
and colon cancer patients before and after surgery: relationship
with radicality and outcome. J Interferon Cytokine Res. 22:473–482.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shariat SF, Andrews B, Kattan MW, Kim J,
Wheeler TM and Slawin KM: Plasma levels of interleukin-6 and its
soluble receptor are associated with prostate cancer progression
and metastasis. Urology. 58:1008–1015. 2001. View Article : Google Scholar : PubMed/NCBI
|