Introduction
Lung cancer is the most common malignancy and the
leading cause of cancer-associated mortality worldwide (1–3). The
incidence of adenocarcinoma has gradually increased in the past
decades, and has become the most prevalent histological subtype of
primary lung cancer worldwide (4–6). Lung
adenocarcinoma is characterized by its diverse clinical,
radiological, pathological, histological and molecular
heterogeneity. This histological heterogeneity has led to
modifications in the classification of lung adenocarcinoma
(7,8).
According to the novel architectural classification of lung
adenocarcinoma proposed by the International Association for the
Study of Lung Cancer/American Thoracic Society/European Respiratory
Society (IASLC/ATS/ERS) in 2011, the previous morphological
classification of the different histological subtypes of lung
adenocarcinoma has been substituted by a comprehensive
multidisciplinary classification (9).
According to this novel classification, patients with
adenocarcinoma in situ (AIS) and minimally invasive
adenocarcinoma (MIA) are expected to present favorable five-year
survival, whereas the prognoses of patients with lung invasive
adenocarcinomas, including those with pathological stage IA, are
relatively poor (10–12). Clearly, a further understanding of the
mechanisms underlying the pathogenesis and progression of lung
invasive adenocarcinoma would promote the development of novel
prognostic markers and therapeutic targets that may improve the
treatments and clinical outcomes of patients with lung cancer
(13,14).
Metastasis-associated protein 1 (MTA1) has been
identified as a critical regulator of the carcinogenesis and
aggressiveness of a wide variety of human malignancies (15–19).
Previous studies by Li et al (20) demonstrated that high protein
expression levels of MTA1 are involved in tumor angiogenesis and
unfavorable prognosis in patients with early-stage non-small cell
lung cancer (NSCLC), and MTA1 acts as a proangiogenic factor by
promoting the migration, invasion and angiogenesis of NSCLC cells
in vitro (21), thus
contributing to the aggressive biological behavior and metastatic
propensity of this type of cancer. However, to the best of our
knowledge, the clinicopathological and prognostic roles of MTA1
protein expression, and its correlation with angiogenesis in lung
invasive adenocarcinoma, have not been investigated thus far.
To address these questions, the protein expression
levels of MTA1 were analyzed in the present study, and its
clinicopathological and prognostic significance, in addition to its
angiogenic activity in lung invasive adenocarcinoma, were evaluated
based on the 2011 IASLC/ATS/ERS classification of lung
adenocarcinoma (9).
Materials and methods
Patients
Medical records were reviewed to identify patients
with primary lung invasive adenocarcinoma who had undergone
complete lobectomy and systematic mediastinal lymph node dissection
consecutively between January 2006 and December 2008 at the
Department of Thoracic Surgery of Qilu Hospital, Shandong
University (Jinan, China). Patients who received preoperative
adjuvant chemotherapy and/or radiotherapy, succumbed to
perioperative complications or were not subjected to follow-up
examinations were excluded from the study. A total of 125 patients
were selected for the study. The histology slides of each patient
enrolled in the study were reviewed independently by two
pathologists, and the histological subtypes were classified
according to the criteria proposed by the 2011 IASLC/ATS/ERS
international multidisciplinary classification of lung
adenocarcinoma (9). The pathological
staging was determined based on the 7th edition of the Union for
International Cancer Control Tumor Node Metastasis classification
of malignant tumors (22). Informed
consent was obtained from all the individual participants included
in the study. The present study was approved by the institutional
review board of Qilu Hospital, Shandong University. The general
clinicopathological characteristics of the patients are presented
in Table I.
 | Table I.Correlation between the protein
expression levels of MTA1 and the clinicopathological factors of
the patients. |
Table I.
Correlation between the protein
expression levels of MTA1 and the clinicopathological factors of
the patients.
|
|
| MTA1 protein
expression levels |
|
|---|
|
|
|
|
|
|---|
| Variables | No. of
patients | Low | High |
P-valuea |
|---|
| Gender |
|
|
| 0.758 |
|
Man | 54 | 32 | 22 |
|
|
Woman | 71 | 44 | 27 |
|
| Age (years) |
|
|
| 0.204 |
|
<60 | 55 | 30 | 25 |
|
|
≥60 | 70 | 46 | 24 |
|
| Histological
subtypes |
|
|
| 0.351 |
|
Lepidic | 13 | 10 | 3 |
|
|
Acinar | 15 | 11 | 4 |
|
|
Papillary | 51 | 28 | 23 |
|
|
Solid | 35 | 19 | 16 |
|
|
Micropapillary | 11 | 8 | 3 |
|
|
Differentiation |
|
|
| 0.978 |
|
Well | 37 | 23 | 14 |
|
|
Moderate | 55 | 33 | 22 |
|
|
Poor | 33 | 20 | 13 |
|
| Pleural
invasion |
|
|
| 0.819 |
|
Absent | 52 | 31 | 21 |
|
|
Present | 73 | 45 | 28 |
|
| Tumor size
(cm) |
|
|
| 0.030 |
| ≤3 | 61 | 43 | 18 |
|
|
>3 | 64 | 33 | 31 |
|
| Lymph node
metastasis |
|
|
| 0.021 |
|
Absent | 67 | 47 | 20 |
|
|
Present | 58 | 29 | 29 |
|
| Pathological
stage |
|
|
| 0.054 |
| I | 64 | 45 | 19 |
|
| II | 40 | 22 | 18 |
|
|
III | 21 | 9 | 12 |
|
Follow-up
All patients were regularly followed up subsequently
to surgery. The patients were recommended to attend follow-up
visits every three months for the first two years following
surgery, and every six months thereafter. The follow-up protocol
consisted of physical examination, blood tests, sonography, chest
radiography, computed tomography (CT), magnetic resonance imaging,
whole body bone scans and positron emission tomography-CT scans, if
necessary. Recurrent disease was confirmed by fine-needle
aspiration or cytopathological diagnosis when clinically feasible.
All patients were followed up until mortality or last day of
follow-up. The deadline of follow-up was December 2013, and the
median clinical follow-up time was 58 months (range, 16–90
months).
Evaluation of MTA1 protein expression
and microvessel density (MVD)
Immunohistochemical staining for MTA1 and cluster of
differentiation (CD)105 was performed using an immunohistochemical
detection kit (cat no. SP-9000; ZSGB-BIO, Beijing, China) according
to the procedure previously described (20,23), using
a goat anti-human polyclonal antibody against MTA1 (sc-9446; Santa
Cruz Biotechnology, Inc., Dallas, TX, USA) and a rabbit anti-human
polyclonal antibody against CD105 (sc-20632; Santa Cruz
Biotechnology, Inc.), respectively. The slides were incubated at
60°C for 30 min, and deparaffinized in xylene, followed by
rehydration with graded alcohol. Antigen retrieval was performed in
citrate buffer for 15 min using a microwave oven. Once cooled down
to room temperature, the slides were immersed in 3% hydrogen
peroxide for 10 min, and incubated in blocking serum for 30 min to
reduce nonspecific binding. Upon discarding any excess of blocking
solution, primary goat anti-MTA1 polyclonal antibody (1:100) and
rabbit anti-CD105 polyclonal antibody (1:100) were applied to the
slides, and incubated overnight at 4°C. Next, the slides were
incubated at 37°C for 30 min with biotinylated antibodies and
streptavidin-peroxidase complex, followed by the addition of
3,3-diaminobenzidine solution to visualize the staining
corresponding to antibody-specific binding. Subsequently, the
slides were counterstained with hematoxylin (ZLI-9609; ZSGB-BIO),
and mounted with neutral balsam.
Semiquantitative determination of MTA1 protein
expression was performed based on the staining intensity, as
follows: i) A value of 0 was assigned to negative staining; ii) a
score of 1 was assigned to weak staining; iii) 2 indicated moderate
staining; and 3, intense staining. The proportion of positively
stained cancer cells was 0, 0~5%; 1, 6~25%; 2, 26~50%; 3, 51~75%;
and 4, ≥76%. The sum of the scores corresponding to the staining
intensity and the percentage of positively stained cells was used
to classify the different cases, and those tumors that displayed a
final staining score of ≥4 were defined as exhibiting high protein
expression levels of MTA1.
CD105 was observed to be expressed in the cytoplasm
and membrane of endothelial cells, and the MVD count was performed
as previously described (23,24). Briefly, the number of CD105+
microvessels was counted in a ×200 microscopic field, and the mean
value of the microvessels counted in five different vascular ‘hot
spots’ was regarded as the final value for each case. All the
immunostained slides were independently evaluated by two
pathologists blinded to the clinicopathological and prognostic
information of the patients. If disagreement emerged on the same
slide, the reviewers would together use a multihead microscope, and
discussed until a consensus score was achieved.
Statistical analysis
All data was statistically analyzed with SPSS
statistical software version 18.0 (SPSS Inc., Chicago, IL, USA).
The χ2 test was used to examine the association between
the protein expression levels of MTA1 and the clinicopathological
characteristics of the patients. The correlation between the
protein expression levels of MTA1 and intratumoral MVD was analyzed
by the nonparametric Mann-Whitney U test. Survival curves were
plotted by the Kaplan-Meier method, and survival differences were
compared by the log-rank test. Multivariate analysis was performed
to identify significantly independent prognostic factors. P<0.05
was considered to indicate a statistically significant
difference.
Results
Correlation between MTA1 protein
expression and clinicopathological factors
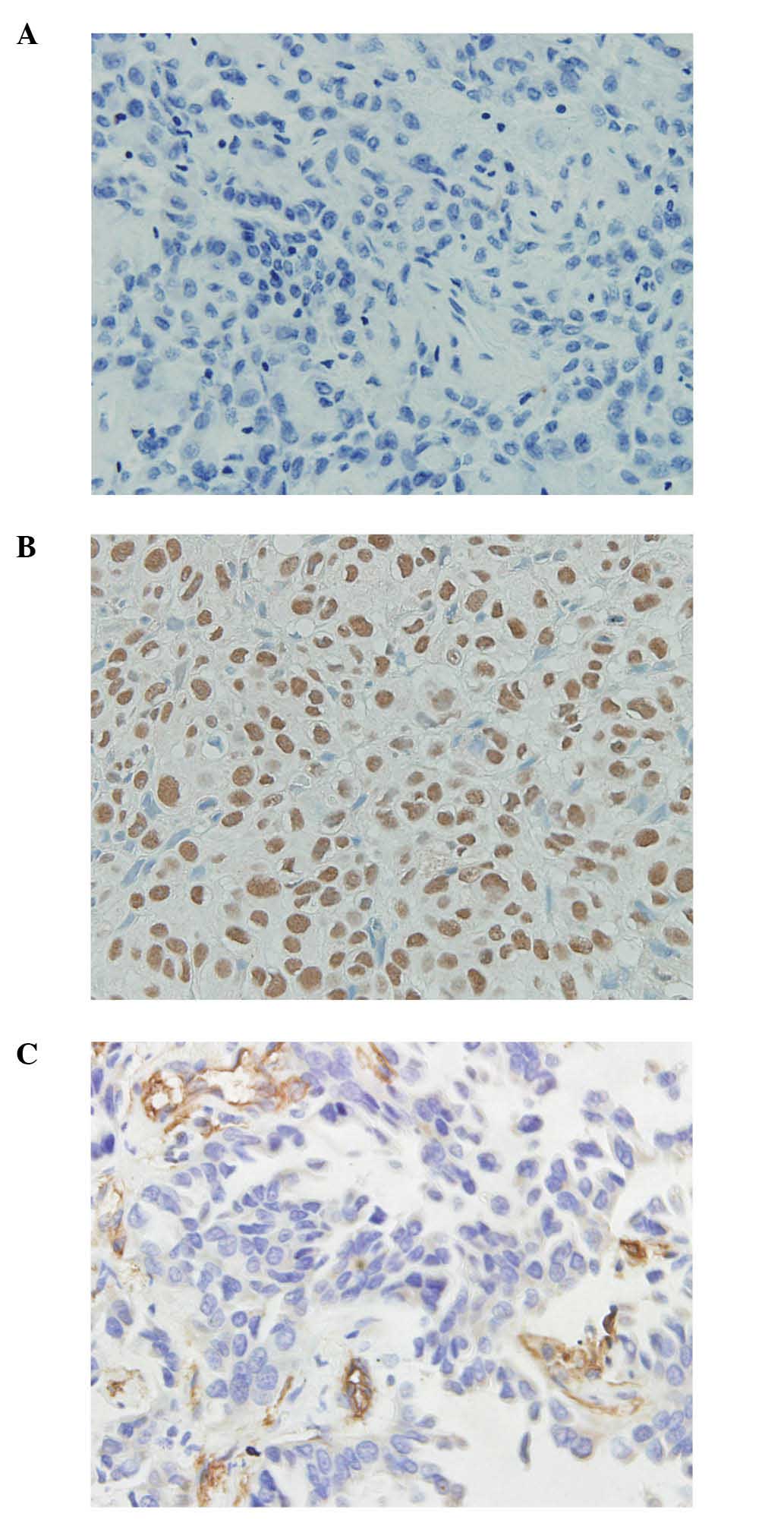
Immunohistochemical analysis revealed positive
immunostaining for MTA1 in the nuclei of the cancer cells, and
different immunoreactivity for MTA1 protein was detected in regards
to the percentage of positive cells stained and the intensity of
the nuclear staining (Fig. 1A and B).
Of the 125 primary lung invasive adenocarcinoma specimens analyzed,
high protein expression levels of MTA1 were detected in 49 cases,
and the association between the protein expression levels of MTA1
and the clinicopathological characteristics of the patients was
analyzed by the χ2 test. As indicated in Table I, high protein expression levels of
MTA1 were significantly correlated with the size of the tumor
(P=0.030) and lymph node metastasis (P=0.021), whereas no
significant differences were detected among the protein expression
levels of MTA1 on the basis of the patients' gender (P=0.758), age
(P=0.204), histological subtypes (P=0.351), differentiation
(P=0.978), pleural invasion (P=0.819) and pathological stage
(P=0.054, which may be considered borderline significance).
Correlation between MTA1 protein
expression and tumor angiogenesis
Intratumoral MVD was quantified by counting the
number of CD105+ endothelial cells present in the cancer
tissues (Fig. 1C). The staining
intensity of MVD varied from 6.6 to 49.6, with a median of
32.2/high power field (HPF). Double-staining of MTA1 and CD105 in
the same serial sections of cancer tissues revealed that low
protein expression levels of MTA1 were generally associated with
few microvessels (6.6–49.4; median, 29.3/HPF), whereas high protein
expression levels of MTA1 were generally associated with abundant
microvessels (9.0–49.6; median, 37.0/HPF). Statistical analysis
further demonstrated a significantly higher MVD in tumors with high
protein expression levels of MTA1 than in those with low protein
expression levels of MTA1 (P=0.015, Mann-Whitney U test; Fig. 2).
Follow-up results and analysis of
prognostic factors
During the follow-up period, tumor relapse developed
in 76 patients (60.8%), and 63 patients (50.4%) succumbed to the
disease. The median survival time of the subgroup of patients
exhibiting high protein expression levels of MTA1 was 48.0 months,
while the median survival time of the subgroup of patients
displaying low protein expression levels of MTA1 was 61.5 months.
As indicated in Table II, the
results of the log-rank test demonstrated that the protein
expression levels of MTA1 were significantly associated with the
five-year disease-free survival (P=0.001; Fig. 3A). Other parameters such as tumor
size, lymph node metastasis and pathological stage were also
observed to be significantly associated with five-year disease-free
survival (P<0.001). Furthermore, the protein expression levels
of MTA1 were significantly associated with the five-year overall
survival (P=0.005; Fig. 3B), in
addition to tumor size, lymph node metastasis and pathological
stage (P<0.001). The significantly independent prognostic
factors were further analyzed by a multivariate Cox regression
model, and the results indicated that MTA1 protein expression,
tumor size, lymph node metastasis and pathological stage were
independent prognostic factors for five-year disease-free survival
(P=0.024, 0.044, 0.029 and 0.001, respectively), whereas only the
pathological stage (P=0.028) was identified as a significantly
independent prognostic factor for five-year overall survival.
 | Table II.Results of univariate and
multivariate survival analyses. |
Table II.
Results of univariate and
multivariate survival analyses.
|
| Disease-free
survival | Overall
survival |
|---|
|
|
|
|
|---|
|
|
| Multivariate
analysis |
| Multivariate
analysis |
|---|
|
|
|
|
|
|
|---|
| Variables | Univariate analysis
P-value | 95% CI | P-value | Univariate analysis
P-value | 95% CI | P-value |
|---|
| Gender |
0.893 | 0.549–1.494 | 0.699 |
0.956 | 0.528–1.545 | 0.710 |
| Age |
0.664 | 0.745–2.060 | 0.409 |
0.304 | 0.676–2.023 | 0.575 |
| Histological
subtypes |
0.172 | 0.973–1.517 | 0.086 |
0.221 | 0.956–1.525 | 0.114 |
|
Differentiation |
0.087 | 0.975–1.819 | 0.072 |
0.075 | 0.961–1.900 | 0.083 |
| Pleural
invasion |
0.374 | 0.622–2.047 | 0.690 |
0.279 | 0.602–2.227 | 0.660 |
| Tumor size | <0.001 | 1.017–3.809 | 0.044 | <0.001 | 0.883–3.893 | 0.103 |
| Lymph node
metastasis | <0.001 | 0.112–0.888 | 0.029 | <0.001 | 0.268–2.350 | 0.676 |
| Pathological
stage | <0.001 | 1.682–6.686 | 0.001 | <0.001 | 1.088–4.368 | 0.028 |
| MTA1 protein
levels |
0.001 | 1.076–2.899 | 0.024 |
0.005 | 0.773–2.256 | 0.309 |
Discussion
Major advances in the treatment of lung
adenocarcinoma are the result of the combined therapy that is
currently administered to patients with lung cancer. This combined
therapy consists of surgical resection, chemotherapy, radiotherapy
and molecular targeting agents based on epidermal growth factor
receptor (EGFR) (8). However, due to
the high heterogeneity of lung adenocarcinoma, the overall
five-year survival of patients affected by this disease is <30%
(25–28). This heterogeneity has led to
modifications in the histological categories employed to classify
the different types of lung adenocarcinoma, as described in the
2011 IASLC/ATS/ERS classification of lung adenocarcinoma (9). According to this novel classification,
patients with AIS and MIA present favorable five-year disease-free
survival, whereas the prognoses of patients with lung invasive
adenocarcinoma, including those with pathological stage IA, are
relatively poor (11,12,29). The
aim of the present study was to further investigate the underlying
mechanisms involved in the invasive ability and metastatic
properties of lung invasive adenocarcinoma, and to identify
possible targets for novel treatments, including individualized
therapy, which may improve the clinical outcomes of patients with
this type of cancer.
MTA1 is a component of the nucleosome remodeling and
histone deacetylation complex, which functions in histone
deacetylation, alteration of chromatin structure and control of
transcription (30,31). As a vital regulator, MTA1 has been
observed to be aberrantly expressed in various human malignant
tumors, and its expression levels are positively correlated with
aggressive phenotypes characterized by their invasiveness and
metastatic potential (15). Thus,
MTA1 may be a target for overcoming tumor progression (15,17). The
association between MTA1 expression, angiogenesis and unfavorable
prognosis in patients with early-stage NSCLC has been previously
reported (20). Thus, it is
clinically required to further assess the protein expression levels
of MTA1 and analyze its clinicopathological and prognostic
significance in human lung invasive adenocarcinoma. A total of 125
patients with primary lung invasive adenocarcinoma were enrolled in
the present study, which aimed to elucidate the angiogenic
activities and the clinicopathological and prognostic significance
of MTA1 protein expression. The results revealed that high protein
expression levels of MTA1 were significantly associated with tumor
size and lymph node metastasis, and markedly associated with
pathological stage, all factors known to contribute to aggressive
phenotypes.
Antiangiogenesis is a pivotal strategy for treating
malignancies (32), and MTA1 has been
defined as a proangiogenic factor (15,21,33,34).
A previous study demonstrated that high intratumoral protein
expression levels of MTA1 were significantly associated with
angiogenesis in NSCLC (20). In
addition, RNA interference-mediated downregulation of MTA1 protein
expression in the lung adenocarcinoma cell line 95D was able to
substantially inhibit the formation of capillary tube-like
structures in vitro (21).
However, the correlation of MTA1 protein expression with tumor
angiogenesis in lung invasive adenocarcinoma has not been
investigated thus far. In the present study, the expression of
CD105, a homodimeric cell membrane glycoprotein, was evaluated in
order to quantify tumor angiogenesis, since this marker is able to
discriminate immature neovascularization from mature and
established blood vessels (35,36), thus
indicating the presence of active angiogenesis in the tumor
(37–39). The results demonstrated that high
protein expression levels of MTA1 were significantly associated
with increased angiogenic activity, as measured by the number of
CD105-associated intratumoral microvessels, suggesting that MTA1
may be involved in tumor progression by participating in the
process of angiogenesis in lung invasive adenocarcinoma. Tumor
angiogenesis is a complex process, and the mechanism by which MTA1
modulates angiogenesis remains unknown (22,30–34).
Therefore, further studies are required in order to elucidate the
mechanisms by which MTA1 induces angiogenesis.
With regard to prognosis, the results of the
univariate survival analysis conducted in the present study
demonstrated that patients with high protein expression levels of
MTA1 presented a significantly shorter five-year disease-free and
overall survival than those patients with low protein expression
levels of MTA1. Subsequent multivariate analysis demonstrated that
high protein expression levels of MTA1 were an independent
prognostic factor for unfavorable disease-free survival, but not
for overall survival. Clinically, the long-term survival of
patients with cancer may be influenced by multiple factors,
including postoperative chemotherapy with different regimens and
cycles, radiotherapy and EGFR-tyrosine kinase inhibitor therapy,
which may cause statistical bias, thus resulting in the inability
to objectively and adequately evaluate the prognostic significance
of MTA1. Nevertheless, the subgroup of patients with high protein
expression levels of MTA1 may require further oncologic evaluation
and clinical attention following surgical resection than those
patients with low protein expression levels of MTA1, due to their
higher risk of relapse.
Taken together, the results of the present study
demonstrated for the first time that aberrantly high protein
expression levels of MTA1 are involved in the malignant phenotype
and unfavorable prognoses of patients with lung invasive
adenocarcinoma, possibly due to its potent angiogenic activity.
However, the stepwise progression and facilitated neoangiogenesis
is too complicated to be clearly elucidated by a simple clinical
study like the present one. Therefore, future studies will
contribute to a further understanding of the underlying mechanisms
targeting MTA1, and provide a greater insight into the aggressive
phenotype of lung invasive adenocarcinoma, which may aid the design
of novel drugs targeting MTA1 and the development of more efficient
antiangiogenic therapies aimed to improve the survival of certain
subgroups of patients affected by lung cancer.
Acknowledgements
The present study was supported by the Independent
Innovation Foundation of Shandong University (Jinan, China) (grant
no. 2012DX005) and the National Natural Science Foundation of China
(Beijing, China) (grant no. 81301832).
Glossary
Abbreviations
Abbreviations:
|
MTA1
|
metastasis-associated protein 1
|
|
IASLC/ATS/ERS
|
International Association for the
Study of Lung Cancer/American Thoracic Society/European Respiratory
Society
|
|
AIS
|
adenocarcinoma in situ
|
|
MIA
|
minimally invasive adenocarcinoma
|
|
NSCLC
|
non-small cell lung cancer
|
|
MVD
|
microvessel density
|
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Scarpa A, Sikora K, Fassan M, et al:
Molecular typing of lung adenocarcinoma on cytological samples
using a multigene next generation sequencing panel. PLoS One.
8:e804782013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fan Z and Schraeder R: The changing
pathology of lung cancer. Surg Oncol Clin N Am. 20:637–653. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bremer RE, Scoggin TS, Somers EB,
OShannessy DJ and Tacha DE: Interobserver agreement and assay
reproducibility of folate receptor α expression in lung
adenocarcinoma: A prognostic marker and potential therapeutic
target. Arch Pathol Lab Med. 137:1747–1752. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sunaga N, Kaira K, Tomizawa Y, et al:
Clinicopathological and prognostic significance of interleukin-8
expression and its relationship to KRAS mutation in lung
adenocarcinoma. Br J Cancer. 110:2047–2053. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shim HS, Lee da H, Park EJ and Kim SH:
Histopathologic characteristics of lung adenocarcinomas with
epidermal growth factor receptor mutations in the International
Association for the Study of Lung Cancer/American Thoracic
Society/European Respiratory Society lung adenocarcinoma
classification. Arch Pathol Lab Med. 135:1329–1334. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shimada Y, Saji H, Nomura M, Matsubayashi
J, Yoshida K, Kakihana M, Kajiwara N, Ohira T and Ikeda N: Cancer
stem cell-related marker expression in lung adenocarcinoma and
relevance of histologic subtypes based on IASLC/ATS/ERS
classification. Onco Targets Ther. 6:1597–1604. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Travis WD, Brambilla E, Noguchi M,
Nicholson AG, Geisinger KR, Yatabe Y, Beer DG, Powell CA, Riely GJ,
Van Schil PE, et al: International association for the study of
lung cancer/american thoracic society/european respiratory society
international multidisciplinary classification of lung
adenocarcinoma. J Thorac Oncol. 6:244–285. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Maeda R, Yoshida J, Ishii G, Hishida T,
Nishimura M and Nagai K: Prognostic impact of histology on
early-stage non-small cell lung cancer. Chest. 140:135–145. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang J, Wu J, Tan Q, Zhu L and Gao W: Why
do pathological stage IA lung adenocarcinomas vary from prognosis?
A clinicopathologic study of 176 patients with pathological stage
IA lung adenocarcinoma based on the IASLC/ATS/ERS classification. J
Thorac Oncol. 8:1196–1202. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ha SY and Roh MS: The new 2011
international association for the study of lung cancer/american
thoracic society/european respiratory society classification of
lung adenocarcinoma in resected specimens: Clinicopathologic
relevance and emerging issues. Korean J Pathol. 47:316–325. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cagle PT and Chirieac LR: Advances in
treatment of lung cancer with targeted therapy. Arch Pathol Lab
Med. 136:504–509. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Huang JY, Cui SY, Chen YT, Song HZ, Huang
GC, Feng B, Sun M, De W, Wang R and Chen LB: MicroRNA-650 was a
prognostic factor in human lung adenocarcinoma and confers the
docetaxel chemoresistance of lung adenocarcinoma cells via
regulating Bcl-2/Bax expression. PLoS One. 8:e726152013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Toh Y and Nicolson GL: The role of the MTA
family and their encoded proteins in human cancers: Molecular
functions and clinical implications. Clin Exp Metastasis.
26:215–227. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li Y, Chao Y, Fang Y, et al: MTA1 promotes
the invasion and migration of non-small cell lung cancer cells by
downregulating miR-125b. J Exp Clin Cancer Res. 32:332013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang H, Fan L, Wei J, et al: Akt mediates
metastasis-associated gene 1 (MTA1) regulating the expression of
E-cadherin and promoting the invasiveness of prostate cancer cells.
PLoS One. 7:e468882012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhou H, Xu X, Xun Q, et al: microRNA-30c
negatively regulates endometrial cancer cells by targeting
metastasis-associated gene-1. Oncol Rep. 27:807–812.
2012.PubMed/NCBI
|
|
19
|
Song Q, Li Y, Zheng X, Fang Y, Chao Y, Yao
K and Zhu X: MTA1 contributes to actin cytoskeleton reorganization
and metastasis of nasopharyngeal carcinoma by modulating Rho
GTPases and Hedgehog signaling. Int J Biochem Cell Biol.
45:1439–1446. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li SH, Tian H, Yue WM, Li L, Li WJ, Chen
ZT, Hu WS, Zhu YC and Qi L: Overexpression of metastasis-associated
protein 1 is significantly correlated with tumor angiogenesis and
poor survival in patients with early-stage non-small cell lung
cancer. Ann Surg Oncol. 18:2048–2056. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li S, Tian H, Yue W, Li L, Gao C, Si L, Li
W, Hu W, Qi L and Lu M: Down-regulation of MTA1 protein leads to
the inhibition of migration, invasion and angiogenesis of
non-small-cell lung cancer cell line. Acta Biochim Biophys Sin
(Shanghai). 45:115–122. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rami-Porta R, Crowley JJ and Goldstraw P:
The revised TNM staging system for lung cancer. Ann Thorac
Cardiovasc Surg. 15:4–9. 2009.PubMed/NCBI
|
|
23
|
Deng X, Du L, Wang C, Yang Y, Li J, Liu H,
Zhang J, Wang L, Zhang X, Li W, et al: Close association of
metastasis-associated protein 1 overexpression with increased
angiogenesis and poor survival in patients with histologically
node-negative gastric cancer. World J Surg. 37:792–798. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Vermeulen PB, Gasparini G, Fox SB,
Colpaert C, Marson LP, Gion M, Beliën JA, de Waal RM, Van Marck E,
Magnani E, et al: Second international consensus on the methodology
and criteria of evaluation of angiogenesis quantification in solid
human tumours. Eur J Cancer. 38:1564–1579. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xu C, Gui Q, Chen W, Wu L, Sun W, Zhang N,
Xu Q, Wang J and Fu X: Small interference RNA targeting tissue
factor inhibits human lung adenocarcinoma growth in vitro
and in vivo. J Exp Clin Cancer Res. 30:632011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ma Q, Li P, Xu M, Yin J, Su Z, Li W and
Zhang J: Ku80 is highly expressed in lung adenocarcinoma and
promotes cisplatin resistance. J Exp Clin Cancer Res. 31:992012.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kauffmann M, Krüger T and Aebert H:
Surgery on extracorporeal circulation in early and advanced
non-small cell lung cancer. Thorac Cardiovasc Surg. 61:103–108.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Huang J, Song H, Liu B, Yu B, Wang R and
Chen L: Expression of Notch-1 and its clinical significance in
different histological subtypes of human lung adenocarcinoma. J Exp
Clin Cancer Res. 32:842013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yoshizawa A, Motoi N, Riely GJ, Sima CS,
Gerald WL, Kris MG, Park BJ, Rusch VW and Travis WD: Impact of
proposed IASLC/ATS/ERS classification of lung adenocarcinoma:
Prognostic subgroups and implications for further revision of
staging based on analysis of 514 stage I cases. Mod Pathol.
24:653–664. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang Y, Ng HH, Erdjument-Bromage H,
Tempst P, Bird A and Reinberg D: Analysis of the NuRD subunits
reveals a histone deacetylase core complex and a connection with
DNA methylation. Genes Dev. 13:1924–1935. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Manavathi B, Peng S, Rayala SK, Talukder
AH, Wang MH, Wang RA, Balasenthil S, Agarwal N, Frishman LJ and
Kumar R: Repression of Six3 by a corepressor regulates rhodopsin
expression. Proc Natl Acad Sci USA. 104:13128–13133. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Jang KS, Paik SS, Chung H, Oh YH and Kong
G: MTA1 overexpression correlates significantly with tumor grade
and angiogenesis in human breast cancers. Cancer Sci. 97:374–379.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kai L, Wang J, Ivanovic M, Chung YT,
Laskin WB, Schulze-Hoepfner F, Mirochnik Y, Satcher RL Jr and
Levenson AS: Targeting prostate cancer angiogenesis through
metastasis-associated protein 1 (MTA1). Prostate. 71:268–280. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Minhajat R, Mori D, Yamasaki F, Sugita Y,
Satoh T and Tokunaga O: Organ-specific endoglin (CD105) expression
in the angiogenesis of human cancers. Pathol Int. 56:717–723. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Paschoal JP, Bernardo V, Canedo NH,
Ribeiro OD, Caroli-Bottino A and Pannain VL: Microvascular density
of regenerative nodule to small hepatocellular carcinoma by
automated analysis using CD105 and CD34 immunoexpression. BMC
Cancer. 14:722014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Tanaka F, Otake Y, Yanagihara K, Kawano Y,
Miyahara R, Li M, Yamada T, Hanaoka N, Inui K and Wada H:
Evaluation of angiogenesis in non-small cell lung cancer:
Comparison between anti-CD34 antibody and anti-CD105 antibody. Clin
Cancer Res. 7:3410–3415. 2001.PubMed/NCBI
|
|
38
|
Wikström P, Lissbrant IF, Stattin P,
Egevad L and Bergh A: Endoglin (CD105) is expressed on immature
blood vessels and is a marker for survival in prostate cancer.
Prostate. 51:268–275. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yang W, Zhang Y, Fu Z, Sun X, Mu D and Yu
J: Imaging proliferation of 18F-FLT PET/CT correlated
with the expression of microvessel density of tumour tissue in
non-small-cell lung cancer. Eur J Nucl Med Mol Imaging.
39:1289–1296. 2012. View Article : Google Scholar : PubMed/NCBI
|