Introduction
First described in 1952 by Christopherson et
al (1), alveolar soft-part
sarcoma (ASPS) is a malignant, highly vascular tumor with distinct
morphology compared with other soft-tissue sarcomas, and its cell
of origin and biology have remained unclear. Clinically, ASPS
constitutes <1% of all soft-tissue sarcomas (2) and occurs principally in individuals
between 15 and 35 years of age, with a notable female predominance,
particularly among patients <20 years of age (3). The tumor often originates in the muscle
and deep soft tissues of the extremities, but it has also been
found in tissues lacking skeletal muscle, such as those of the
lungs, breasts, stomach, female genital organs and bones (4,5). This
tumor type has an indolent clinical course, with a high tendency to
metastasize via hematogenous dissemination (6). Metastases to the lungs, bones and brain
are common and often asymptomatic, and may be detected after long
disease-free intervals, while cardiac metastases from ASPS are
rarely reported (7,8). Surgical excision of the primary and
pulmonary metastases is the mainstay of treatment, although it
remains rarely curative, and radiotherapy has a role in treating
visual or microscopic residual disease following resection
(9). Anthracycline-based chemotherapy
regimens rarely elicit responses, and in general, ASPS is
essentially impervious to well-established chemotherapy agents
(10). Overall, the prognosis of ASPS
is poor. The present study reports a rare case of ASPS with cardiac
metastasis.
Case report
On August 10th 2012, a 37-year-old man was admitted
to Shandong Provincial Hospital Affiliated to Shandong University
(Jinan, Shandong, China) with complaints of headaches, dizziness
and nausea over a period of 20 days. It was noted that 3 years
previously, the patient had undergone surgery in other institution
due to a progressively enlarging painless mass on the left forearm.
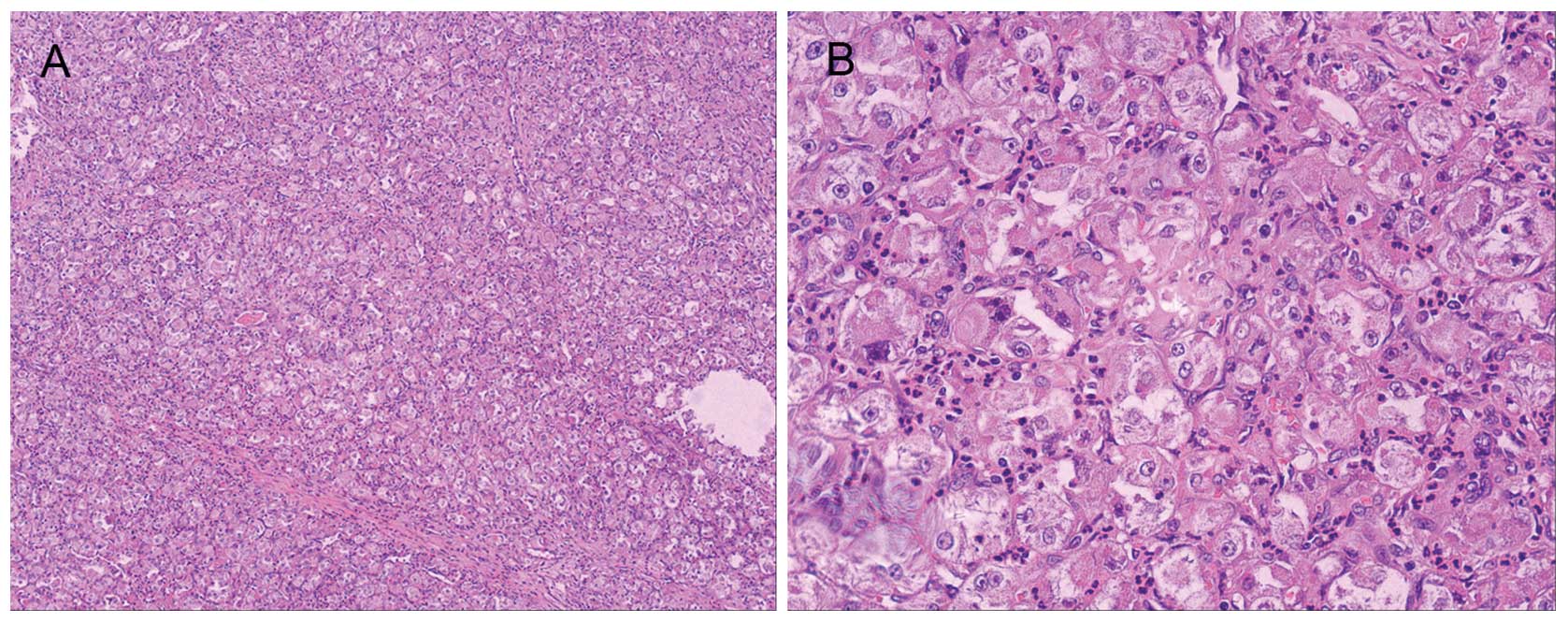
Histopathological examination of the specimen confirmed ASPS
(Fig. 1). During the current
admission, computed tomography (CT) scans showed metastatic brain,
lung and spleen lesions (Fig. 2),
with no cardiac lesion. The patient was administered palliative
whole-brain radiotherapy, which was delivered as 30 Gy in 10
fractions for 2 weeks. Chemotherapy consisting of 75
mg/m2 cisplatin on days 1–3, 40 mg/m2
pirarubicin on day 1, and 2 g/m2 ifosfamide on days 1–3,
every 3 weeks, was initiated during the palliative whole-brain
radiotherapy. Despite the fact that the symptoms associated with
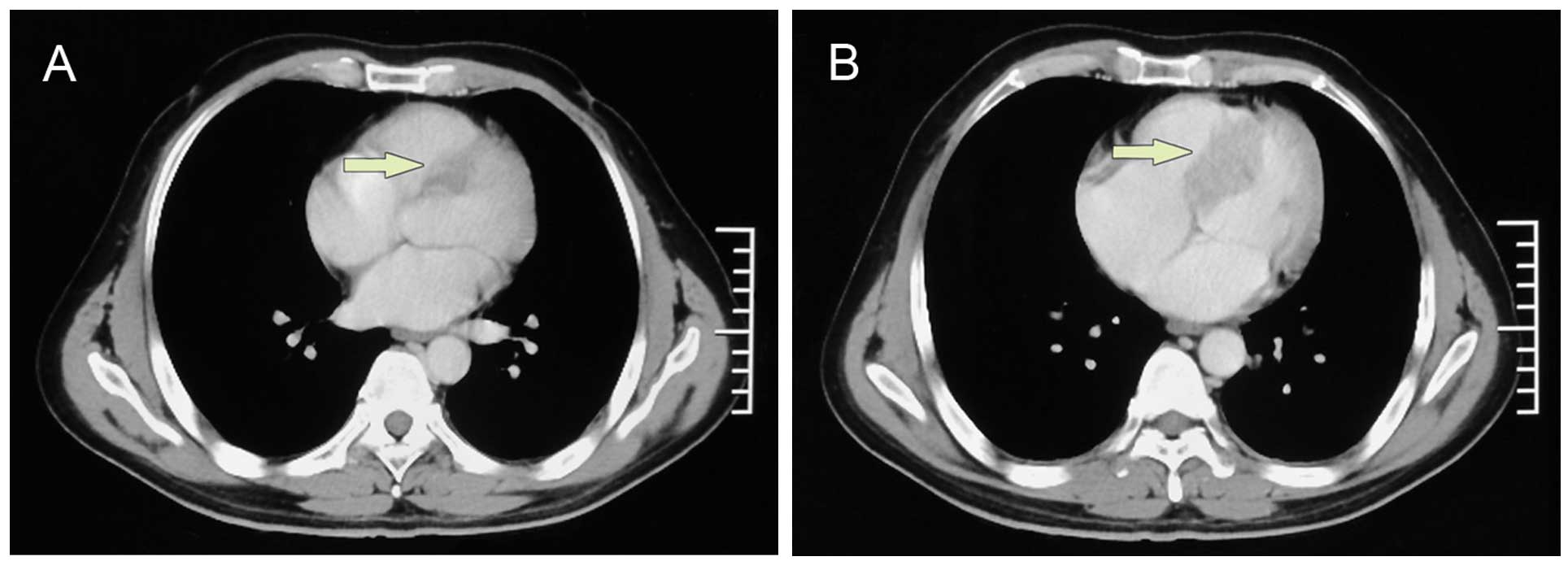
cerebral metastasis were alleviated, a repeat CT scan that was
performed after two cycles of chemotherapy demonstrated a new mass
located in the upper segment and extending to the middle of the
interventricular septum (Fig. 3),
with contrast enhancement of the rim of the lesion consistent with
cardiac metastasis. Transthoracic echocardiography was performed
and this lesion was identified as a mass measuring 40×21 mm within
the interventricular septum (Fig. 4),
without remarkable left ventricular outflow tract obstruction. The
cardiac mass appeared hypoechoic, with blood flow signals detected.
Ten days after the completion of two cycles of chemotherapy, the
patient had no symptoms associated with the cardiac lesion and
refused to continue further treatment. The patient subsequently
succumbed to cachexia and fast progression of the disease. Although
a histopathological specimen could not be obtained from the heart,
according to the CT scan and transthoracic echocardiography
demonstrative tools, we believed the cardiac lesion to be cardiac
metastasis of the ASPS from the forearm.
Discussion
ASPS is a rare soft-tissue malignancy that was
originally alluded to by Smetana and Scott (11) in 1951 as a malignant tumor of the
non-chromaffin paraganglia. In 1952, Christopherson et al
(1) described it under its present
name of ‘alveolar soft-part sarcoma’. Accounting for <1% of all
soft-tissue sarcomas, ASPS most typically occurs in adolescents and
young adults, with a female predominance (12,13). It is
known that the most common primary sites of the tumor are the lower
extremities, frequently the thigh or trunk (14). In the present case, a 37-year-old man
was diagnosed with ASPS with the primary location of the left
forearm. It is reported that ASPS is an indolent disease with
characteristic slow growth, but that is associated with an poor
overall outcome and a 5-year survival rate of only 20% in
unresectable metastatic patients (15,16).
Metastases to the lungs, brain and bones are common, and usually
lead to a low survival rate. However, ASPS metastases to the heart
are extremely unusual. In a review of the literature, only 2 papers
on cardiac metastasis were found: Akiyama et al (17) reported the case of a 29-year-old man
with ASPS of the brain and cardiac metastasis, while the case of a
13-year-old girl with metastases to the lungs and heart was
reported by Campbell et al (18). In the present case, the metastatic
mass was located in the septum, which was similar to the case by
Akiyama et al (17), while
Campbell et al (18) reported
a mass in the left ventricle. In spite of the lack of histology, we
hypothesized that the septal lesion in the present patient was a
metastatic tumor, as the incidence of primary cardiac tumors ranges
from 0.001 to 0.030% (19) and the
mass developed in a short time period.
The traditional treatments for ASPS consist of
surgery and radiotherapy (15,17).
Treatment should begin with mass resection; in particular, a
complete resection should be performed when possible. If only
partial excision or a questionable surgical margin or symptomatic
metastatic mass exist, radiotherapy should be added as a
supplementary procedure. In the present case, the patient presented
with headaches and nausea, which were produced by metastatic brain
tumors, so the patient underwent palliative radiotherapy. Following
the completion of radiotherapy, the symptoms were relieved and the
patient's quality of life improved. The role of adjuvant
chemotherapy in ASPS remains uncertain (6). However, in advanced cases, such as the
present patient with multiple metastases of the lungs, brain,
spleen and heart, chemotherapy may be considered as a treatment
option. Recently, a novel anti-tumor drug, cediranib, was used in a
phase II trial by Kummar et al (15). Cediranib (AZD2171) is a potent, oral,
small-molecule inhibitor of all three vascular endothelial growth
factor receptor (VEGFR-1, −2 and −3) tyrosine kinases, which
mediate angiogenesis and lymphangiogenesis (20,21). The
study evaluated 43 metastatic, unresectable ASPS patients who were
administered cediranib (30 mg) once daily in 28-day cycles, and it
was observed that cediranib has substantial single-agent activity,
producing an overall remission rate of 35% and a disease control
rate of 84% at 24 weeks (15). The
trial is still ongoing, and cediranib has not yet been approved for
use in China.
Patients who are diagnosed with widespread
metastases usually have a poor prognosis and ultimately succumb to
their condition. It is reported that the median survival time in
patients with these multiple metastases is 40 months (8,22). In the
present case, the coexistence of ASPS with heart, lung, brain and
spleen metastases in the 37-year-old male indicated a poor outcome.
The patient succumbed 44 months after the initial ASPS diagnosed,
which was similar to the aforementioned median survival time.
Magnetic resonance imaging (MRI) is an effective and
highly sensitive tool for the diagnosis of ASPS. Typically, the MRI
of ASPS features internal and external multilobulated signal
changes, also presenting with high signals on T1-weighted imaging
(T1WI) and T2WI. A low blood flow rate is observed in T1-weighted
high signal images, while a high blood flow rate is observed on
T1WI and T2WI, with multilobulated signal change (23,24).
However, no MRI was performed for the present patient prior to the
surgery three years previously.
In conclusion, the unusual primary location of ASPS
in the forearm of a male adult, and the recurrence of ASPS with
lung, brain, spleen and particularly cardiac metastasis, ensure
that the present case is an exceptional example compared with those
reported in the previous literature. It is difficult to make a
timely diagnosis of ASPS, as patients mostly presented with a
painless swelling, so that a histopathological examination is
required. MRI may be useful for the diagnosis when the symptoms of
ASPS are non-specific. The main treatment principle is a radical
resection, and radiation treatment is an adjuvant measure in
selected patients. Radiation therapy should be considered for
metastatic brain tumors, in order to relieve the symptoms and
improve the patient's quality of life. In patients with ASPS,
widespread metastases usually predict a poor prognosis. ASPS with
cardiac metastasis are extremely rare. In general, treatments of
cardiac metastasis are not found to be effective. In order to
detect cardiac metastasis early, a heart examination should be
performed in patients with ASPS.
Acknowledgements
This study is financially supported by The National
Natural Science Fund (grant no. 81201865).
References
|
1
|
Christopherson WM, Foote FW Jr and Stewart
FW: Alveolar soft-part sarcomas; Structurally characteristic tumors
of uncertain histogenesis. Cancer. 5:100–111. 1952. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Auerbach HE and Brooks JJ: Alveolar soft
part sarcoma: A clinicopathologic and immunohistochemical study.
Cancer. 60:66–73. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bu X and Bernstein L: A proposed
explanation for female predominance in alveolar soft part sarcoma.
Noninactivation of X; autosome translocation fusion gene? Cancer.
103:1245–1253. 2005.
|
|
4
|
Ordóñez NG: Alveolar soft part sarcoma: A
review and update. Adv Anat Pathol. 6:125–139. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Flieder DB, Moran CA and Suster S: Primary
alveolar soft-part sarcoma of the mediastinum: A
clinicopathological and immunohistochemical study of two cases.
Histopathology. 31:469–473. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hunter BC, Devaney KO, Ferlito A and
Rinaldo A: Alveolar soft partsarcoma of the head and neck region.
Ann Otol Rhinol Laryngol. 107:810–814. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lieberman PH, Brennan MF, Kimmel M,
Erlandson RA, Garin-Chesa P and Flehinger BY: Alveolar soft-part
sarcoma: A clinico-pathologic study of half a century. Cancer.
63:1–13. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Oniki T, Hashimoto Y, Fujinuma Y, Maruyama
Y, Namba K, Yajima M, Numano F and Maezawa H: Hypervascular
metastaticcardiac tumors: An unknown cause of mitral valve
prolapse. Intern Med. 31:78–81. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
O'Sullivan B, Davis AM, Turcotte R, Bell
R, Catton C, Chabot P, Wunder J, Kandel R, Goddard K, Sadura A, et
al: Preoperative versus postoperative radiotherapy in soft-tissue
sarcoma of the limbs: A randomised trial. The Lancet.
359:2235–2241. 2002. View Article : Google Scholar
|
|
10
|
Reichardt P, Lindner T, Pink D,
Thuss-Patience PC, Kretzschmar A and Dörken B: Chemotherapy in
alveolar soft part sarcomas. What do we know? Eur J Cancer.
39:1511–1516. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Smetana HF and Scott WF Jr: Malignant
tumors of nonchromaffin paraganglia. Mil Surg. 109:330–349.
1951.PubMed/NCBI
|
|
12
|
Zhang LL, Tang Q, Wang Z and Zhang XS:
Alveolar soft part sarcoma of the uterine corpus with pelvic lymph
node metastasis: Case report and literature review. Int J Clin Exp
Pathol. 5:715–719. 2012.PubMed/NCBI
|
|
13
|
Yavuz A, Göya C, Bora A and Beyazal M:
Primary alveolar soft part sarcoma of the scapula. Case Rep Oncol.
6:356–361. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mannan R, Bhasin TS, Kaur P, Manjari M and
Gill KS: Prominent intracytoplasmic crystals in alveolar soft part
sarcoma (ASPS): An aid in cytological diagnosis. J Clin Diagn Res.
8:145–146. 2014.PubMed/NCBI
|
|
15
|
Kummar S, Allen D, Monks A, Polley EC,
Hose CD, Ivy SP, Turkbey IB, Lawrence S, Kinders RJ, Choyke P, et
al: Cediranib for metastatic alveolar soft part sarcoma. J Clin
Oncol. 31:2296–2302. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cho YJ and Kim JY: Alveolar soft part
sarcoma: Clinical presentation, treatment and outcome in a series
of 19 patients. Clin Orthop Surg. 6:80–86. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Akiyama Y, Baba T, Ibayashi Y, Asai Y and
Houkin K: Alveolar soft part sarcoma in brain with cardiac
metastasis: A case report. Int J Cardiol. 114:e93–e95. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Campbell B, Seymour JF, Wheeler G and
Sexton M: Alveolar soft-part sarcoma: A cardiac metastasis as a
rare site of relapse. Am J Clin Oncol. 29:422–423. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Butany J, Nair V, Naseemuddin A, Nair GM,
Catton C and Yau T: Cardiac tumours: Diagnosis and management.
Lancet Oncol. 6:219–228. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Smith NR, James NH, Oakley I, Wainwright
A, Copley C, Kendrew J, Womersley LM, Jürgensmeier JM, Wedge SR and
Barry ST: Acute pharmacodynamic and antivascular effects of the
vascular endothelial growth factor signaling inhibitor AZD2171 in
Calu-6 human lung tumor xenografts. Mol Cancer Ther. 6:2198–2208.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wedge SR, Kendrew J, Hennequin LF,
Valentine PJ, Barry ST, Brave SR, Smith NR, James NH, Dukes M,
Curwen JO, et al: AZD2171: A highly potent, orally bioavailable,
vascular endothelial growth factor receptor-2 tyrosine kinase
inhibitor for the treatment of cancer. Cancer Res. 65:4389–4400.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Portera CA Jr, Ho V, Patel SR, Hunt KK,
Feig BW, Respondek PM, Yasko AW, Benjamin RS, Pollock RE and
Pisters PW: Alveolar soft part sarcoma: Clinical course and
patterns of metastasis in 70 patients treated at a single
institution. Cancer. 91:585–591. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Iwamoto Y, Morimoto N, Chuman H, Shinohara
N and Sugioka Y: The role of MR imaging in the diagnosis of
alveolar soft part sarcoma: A report of 10 cases. Skeletal Radiol.
24:267–270. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Suh JS, Cho J, Lee SH, Shin KH, Yang WI,
Lee JH, Cho JH, Suh KJ, Lee YJ and Ryu KN: Alveolar soft part
sarcoma: MR and angiographic findings. Skeletal Radiol. 29:680–689.
2000. View Article : Google Scholar : PubMed/NCBI
|